Abstract
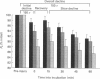
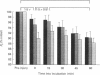
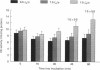
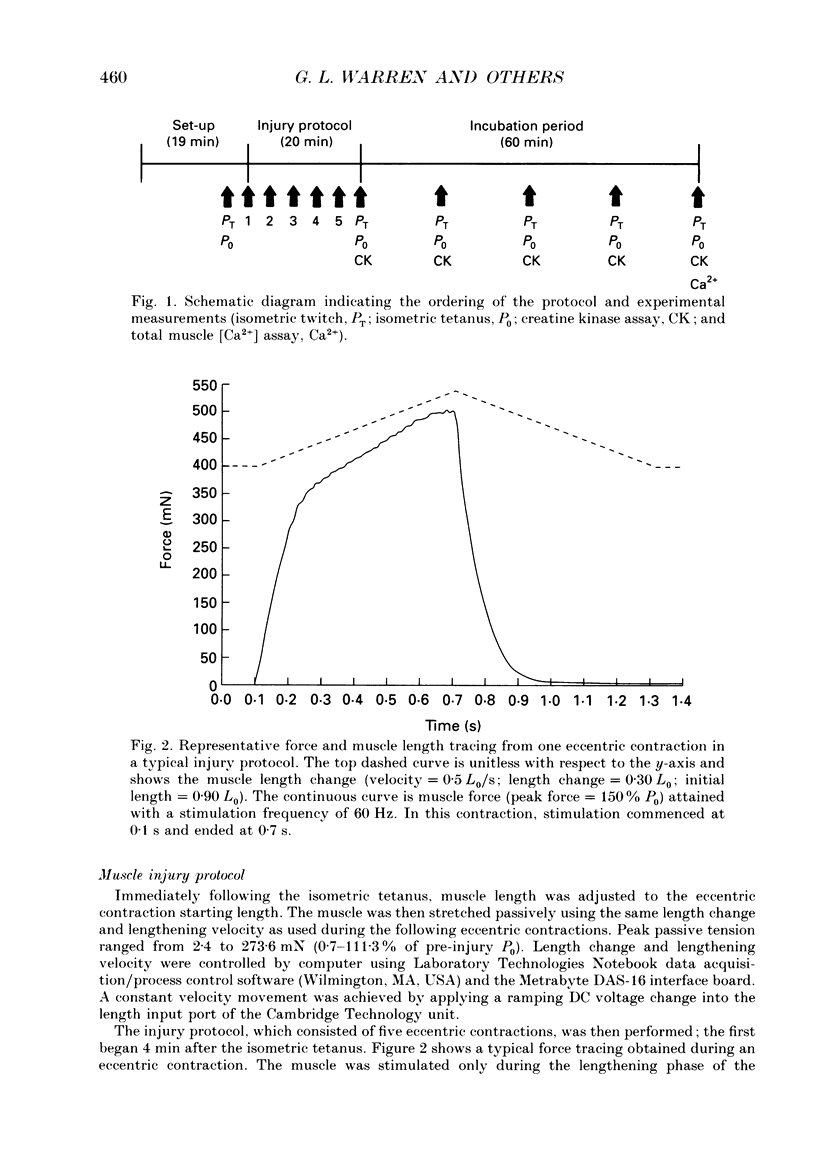
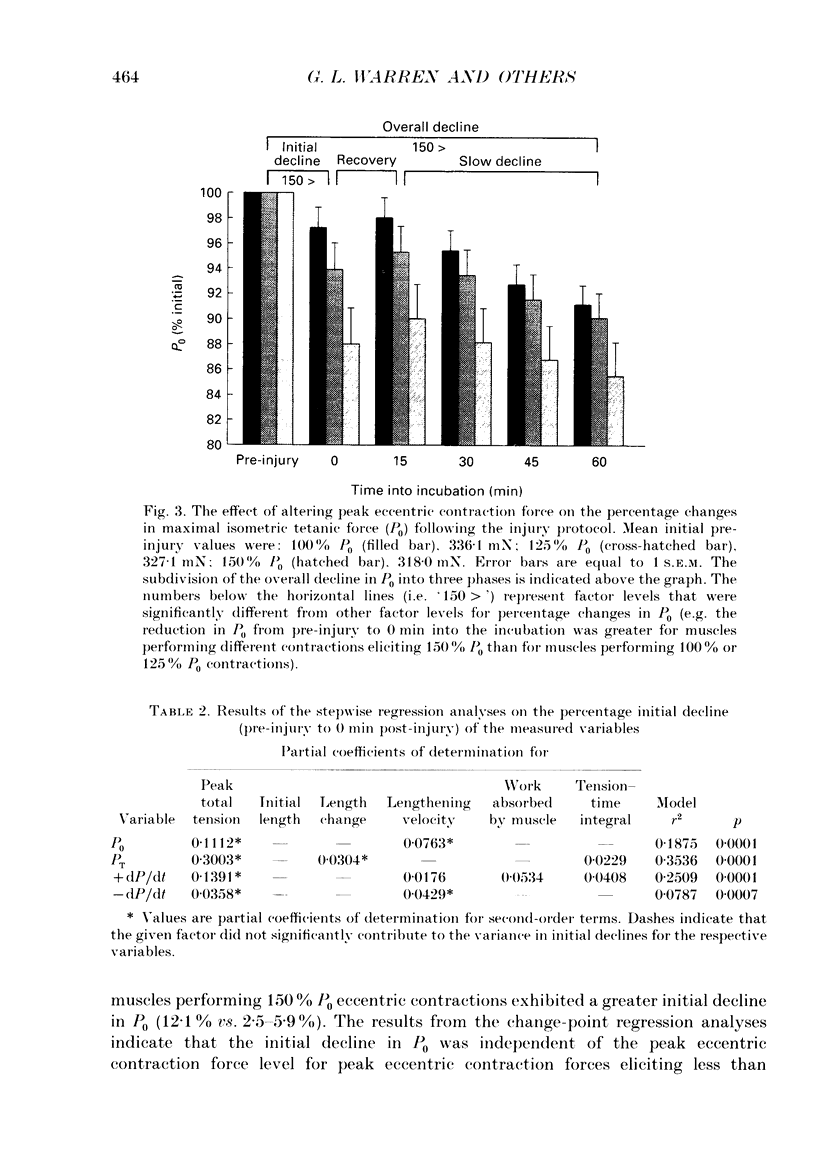
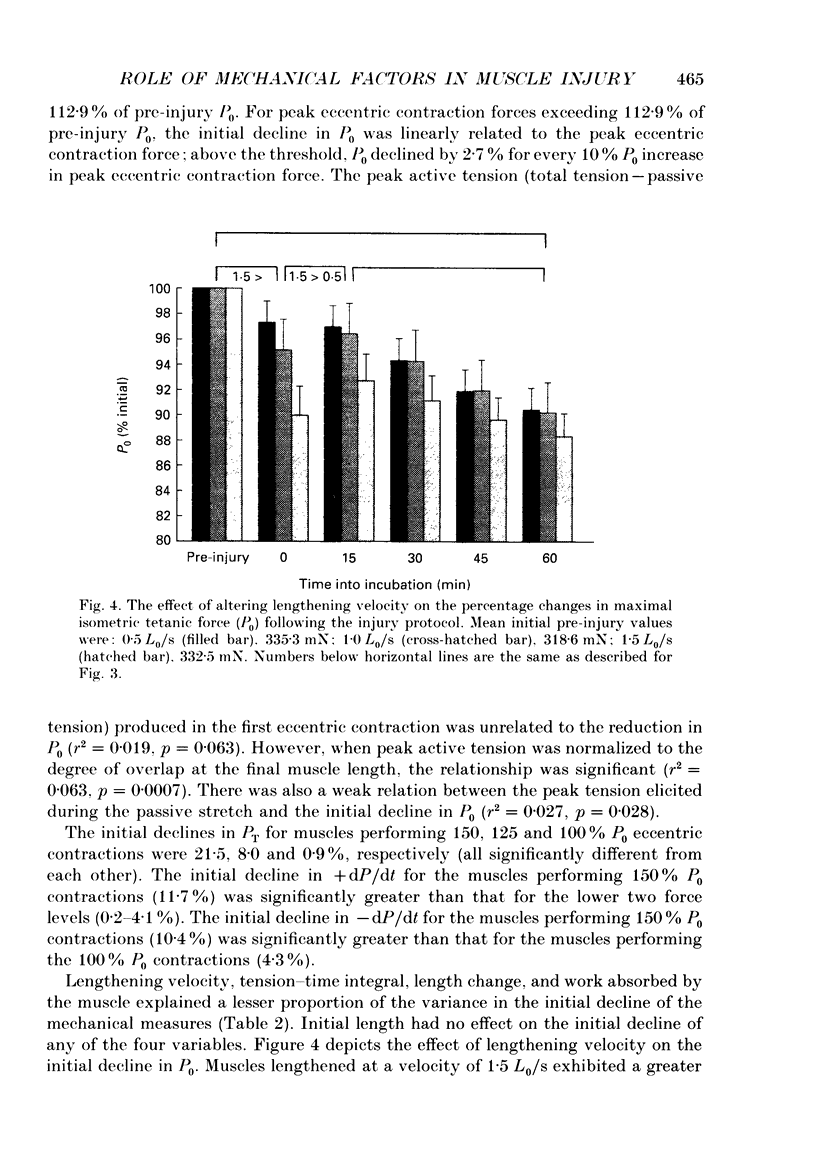
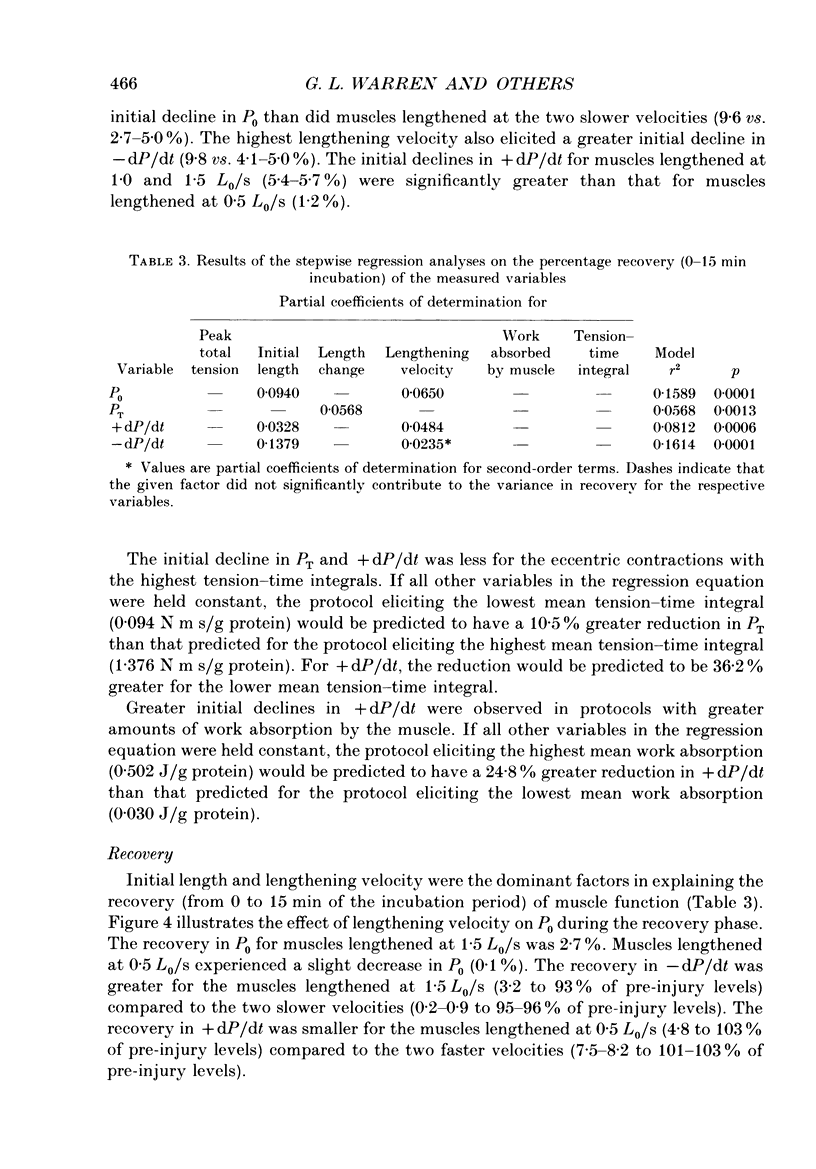
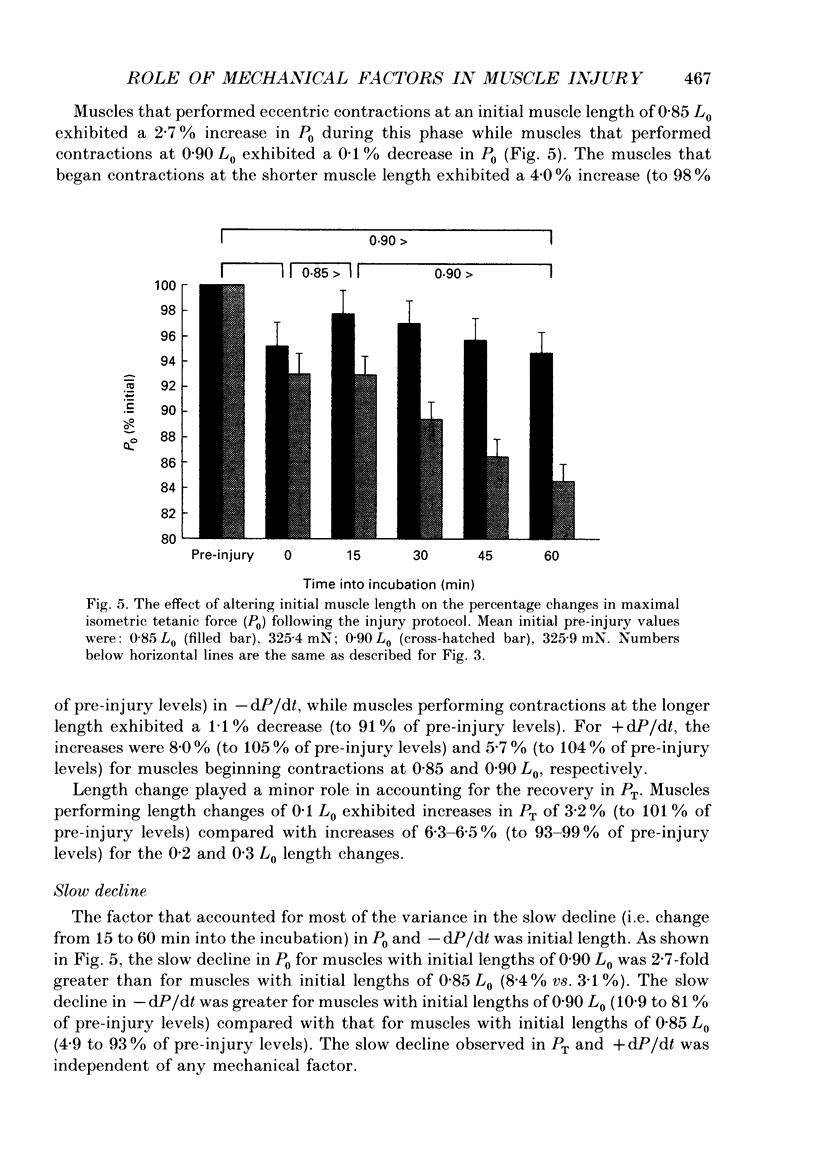
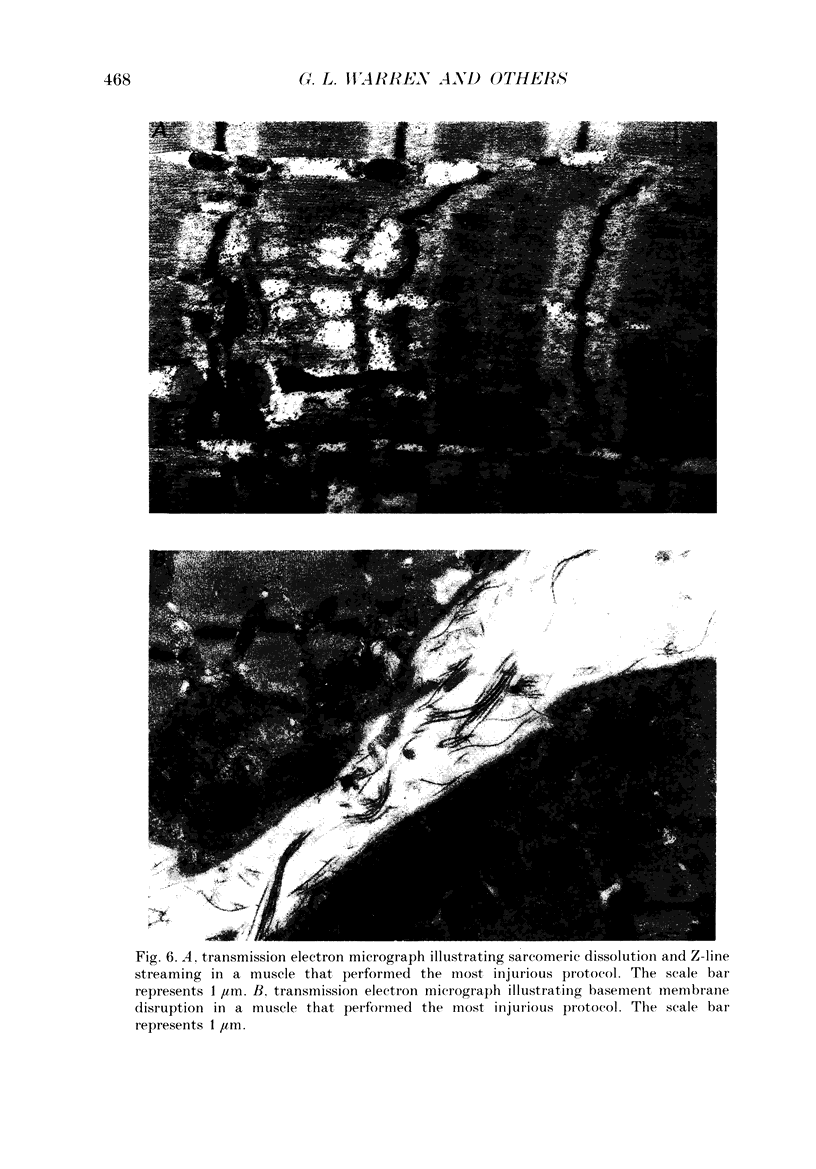
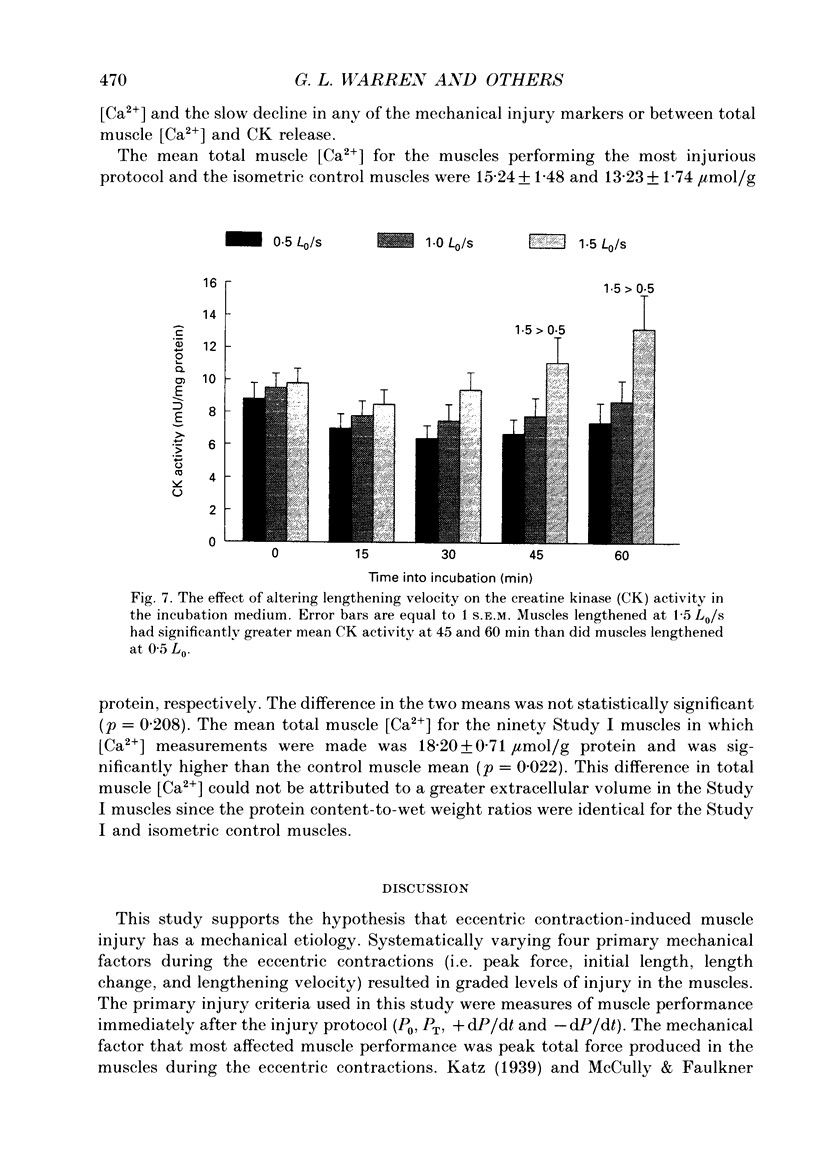
1. Mechanical factor(s) associated with the initiation of eccentric contraction-induced muscle injury were investigated in isolated rat soleus muscles (n = 180; 42 protocols with 4-6 muscles per protocol). Five eccentric contractions were performed with 4 min between contractions. Three levels of peak eccentric contraction force (100, 125 and 150% of pre-injury maximal isometric tetanic tension, P0), length change (0.1, 0.2 and 0.3 muscle length, L0) and lengthening velocity (0.5, 1.0 and 1.5 L0/s) were utilized. Force was varied with stimulation frequency (10-150 Hz). The eccentric contractions were initiated at muscle lengths of 0.85 or 0.90 L0. Following the fifth eccentric contraction, the muscle was incubated in Krebs-Ringer buffer for 60 min. Peak isometric twitch tension (PT), P0, maximal rate of tension development (+ dP/dt), maximal rate of relaxation (-dP/dt), and creatine kinase (CK) release were measured prior to the five eccentric contractions and at 15 min intervals during the incubation period. Total muscle [Ca2+] was measured after 60 min incubation. 2. The mean (+/- S.E.M.) initial decline in P0 for the muscles performing the most injurious protocol was 13.6 +/- 4.8% (n = 6); P0 in control muscles immediately following performance of five isometric contractions was elevated 1.2 +/- 1.0% (n = 8). These means were different at probability, p = 0.005. Mean [ATP] in muscles immediately following the isometric control and most injurious protocols, respectively, were 16.30 +/- 1.49 and 19.84 +/- 1.38 mumol/g dry wt (p = 0.229). 3. Decrements in P0, PT, +dP/dt, and -dP/dt immediately after the injury protocol were related most closely to the peak forces produced during the eccentric contractions; greater initial declines in P0, +dP/dt and -dP/dt were also observed at higher lengthening velocities independent of peak force. Slow declines in P0 and -dP/dt during the 60 min incubation following the injury protocol were greatest for muscles performing contractions at the longer initial length. CK release was independent of all mechanical factors with the exception of lengthening velocity. CK activity at 45 and 60 min into the incubation period was greater for muscles lengthened at the highest velocity used (1.5 L0/s). Mean total muscle [Ca2+] for muscles performing the eccentric contractions was elevated by 38% over isometric control muscles but the elevation was unrelated to any of the four mechanical factors. 4. These data support the hypothesis that eccentric contraction-induced injury is initiated by mechanical factors, with muscle tension playing the dominant role.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990 Aug;22(4):429–435. [PubMed] [Google Scholar]
- Armstrong R. B. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984 Dec;16(6):529–538. [PubMed] [Google Scholar]
- Armstrong R. B., Warren G. L., Warren J. A. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991 Sep;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- CASELLA C. Tensile force in total striated muscle, isolated fibre and sarcolemma. Acta Physiol Scand. 1950 Dec;21(4):380–401. doi: 10.1111/j.1748-1716.1950.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol. 1985 Feb;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbeling C. B., Clarkson P. M. Exercise-induced muscle damage and adaptation. Sports Med. 1989 Apr;7(4):207–234. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Umazume Y. Lattice shrinkage with increasing resting tension in stretched, single skinned fibers of frog muscle. Biophys J. 1986 Sep;50(3):385–389. doi: 10.1016/S0006-3495(86)83474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Molitoris B. A. A statistical method for determining the breakpoint of two lines. Anal Biochem. 1984 Aug 15;141(1):287–290. doi: 10.1016/0003-2697(84)90458-5. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi T. G., Fielding R. A., O'Reilly K. P., Meredith C. N., Lee H. Y., Evans W. J. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med Sci Sports Exerc. 1991 Sep;23(9):1028–1034. [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol (1985) 1986 Jul;61(1):293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol (1985) 1985 Jul;59(1):119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Morgan D. L. New insights into the behavior of muscle during active lengthening. Biophys J. 1990 Feb;57(2):209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Ghosh G., Aurora P. Muscle fatigue and pain after eccentric contractions at long and short length. Clin Sci (Lond) 1988 May;74(5):553–557. doi: 10.1042/cs0740553. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I. Mechanical properties of the sarcolemma and myoplasm in frog muscle as a function of sarcomere length. J Gen Physiol. 1972 May;59(5):559–585. doi: 10.1085/jgp.59.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985 Mar;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Stauber W. T. Eccentric action of muscles: physiology, injury, and adaptation. Exerc Sport Sci Rev. 1989;17:157–185. [PubMed] [Google Scholar]
- Taylor R. G., Abresch R. T., Lieberman J. S., Fowler W. M., Jr, Portwood M. M. Effect of pentobarbital on contractility of mouse skeletal muscle. Exp Neurol. 1984 Feb;83(2):254–263. doi: 10.1016/S0014-4886(84)90096-7. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Chan M. Adhesive strength of single muscle cells to basement membrane at myotendinous junctions. J Appl Physiol (1985) 1989 Sep;67(3):1063–1069. doi: 10.1152/jappl.1989.67.3.1063. [DOI] [PubMed] [Google Scholar]
- Tidball J. G. Energy stored and dissipated in skeletal muscle basement membranes during sinusoidal oscillations. Biophys J. 1986 Dec;50(6):1127–1138. doi: 10.1016/S0006-3495(86)83557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meulen J. H., Kuipers H., Drukker J. Relationship between exercise-induced muscle damage and enzyme release in rats. J Appl Physiol (1985) 1991 Sep;71(3):999–1004. doi: 10.1152/jappl.1991.71.3.999. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Hodgson J. A., Burke R. E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978 Sep;41(5):1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Warren J. A., Jenkins R. R., Packer L., Witt E. H., Armstrong R. B. Elevated muscle vitamin E does not attenuate eccentric exercise-induced muscle injury. J Appl Physiol (1985) 1992 Jun;72(6):2168–2175. doi: 10.1152/jappl.1992.72.6.2168. [DOI] [PubMed] [Google Scholar]