Abstract
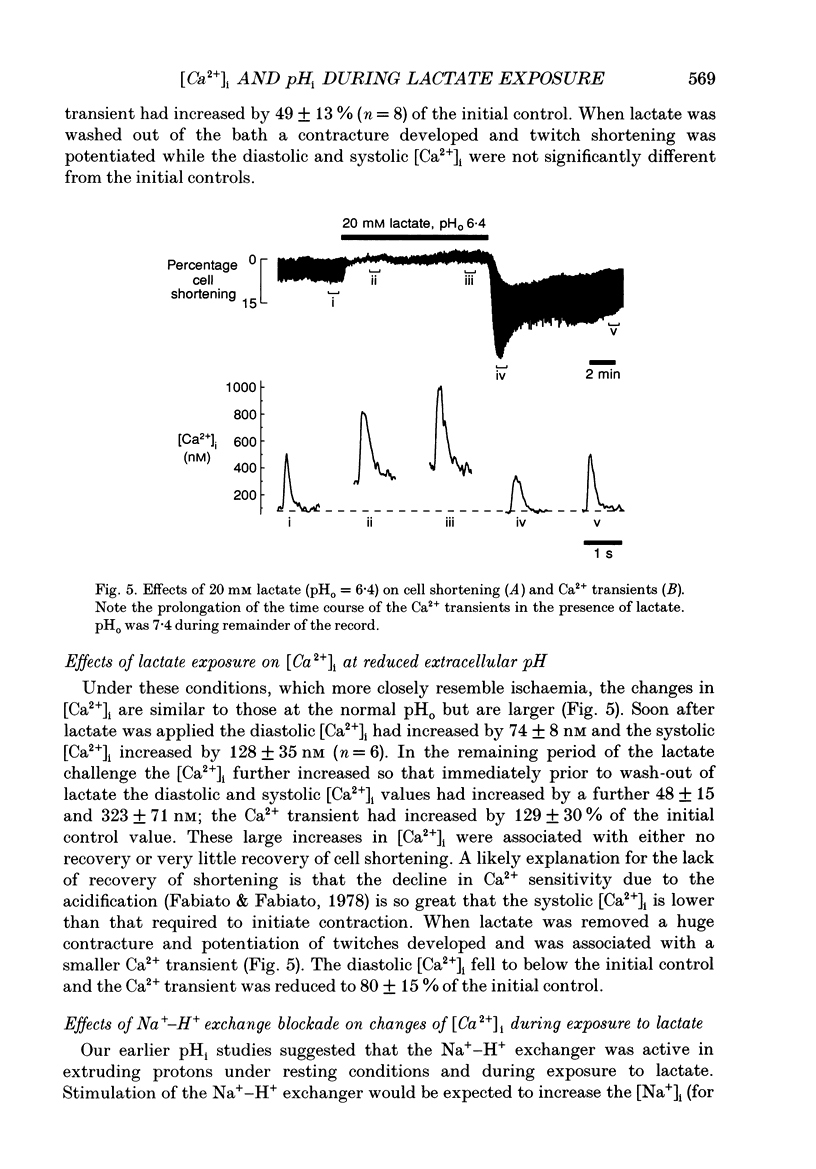
1. We investigated the mechanisms involved in the rise of myoplasmic calcium concentration ([Ca2+]i) when isolated rat ventricular myocytes were exposed to lactate. The intracellular pH (pHi) and [Ca2+]i were measured using the fluorescent indicators 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF) and fura-2, respectively. Cell shortening was used as a measure of contractile performance. 2. Exposure to 20 mM lactate at the normal extracellular pH (pHo 7.4) for 10 min caused the pHi to fall rapidly by 0.24 pH units and cell shortening was reduced. Thereafter, pHi partially recovered by 0.16 pH units, which was paralleled by a recovery of shortening. 3. Exposure to lactate at a reduced extracellular pH (pHo 6.4) induced a very large acidosis of 0.70 pH units and cell shortening was abolished. During maintained exposure to lactate the pHi remained constant and cell shortening did not recover. 4. Application of Na(+)-H+ exchanger inhibitors, amiloride or ethylisopropyl-amiloride (EIPA), abolished the recovery of pHi and shortening during maintained exposure to lactate at pHo 7.4 and caused an additional acidosis during maintained application of lactate at pHo 6.4. 5. Application of lactate at both the normal and reduced pHo resulted in a rapid, followed by a slower, rise in [Ca2+]i. The diastolic and systolic [Ca2+]i and the amplitude of the systolic rise in the [Ca2+]i (the Ca2+ transient) all increased in both the rapid and the slow phase. 6. When lactate was applied at pHo 7.4, in the presence of EIPA, the initial rise of [Ca2+]i still occurred but the slower increase was abolished. This suggests an involvement of the Na(+)-H+ exchanger in the slower rise of [Ca2+]i. 7. In conclusion, the Na(+)-H+ exchanger is an important regulator of pHi during a lactate-induced intracellular acidosis. The rise of [Ca2+]i involves at least two mechanisms: (i) a rapid component which may represent reduced myoplasmic Ca2+ buffering, impaired Ca2+ removal by the sarcoplasmic reticulum or a direct inhibitory effect of protons on the Na(+)-Ca2+ exchanger; (ii) a slower component linked to stimulation of Na(+)-H+ exchanger which causes an increased [Na+]i and stimulates the Na(+)-Ca2+ exchanger, resulting in an enhanced Ca2+ influx.
Full text
PDF



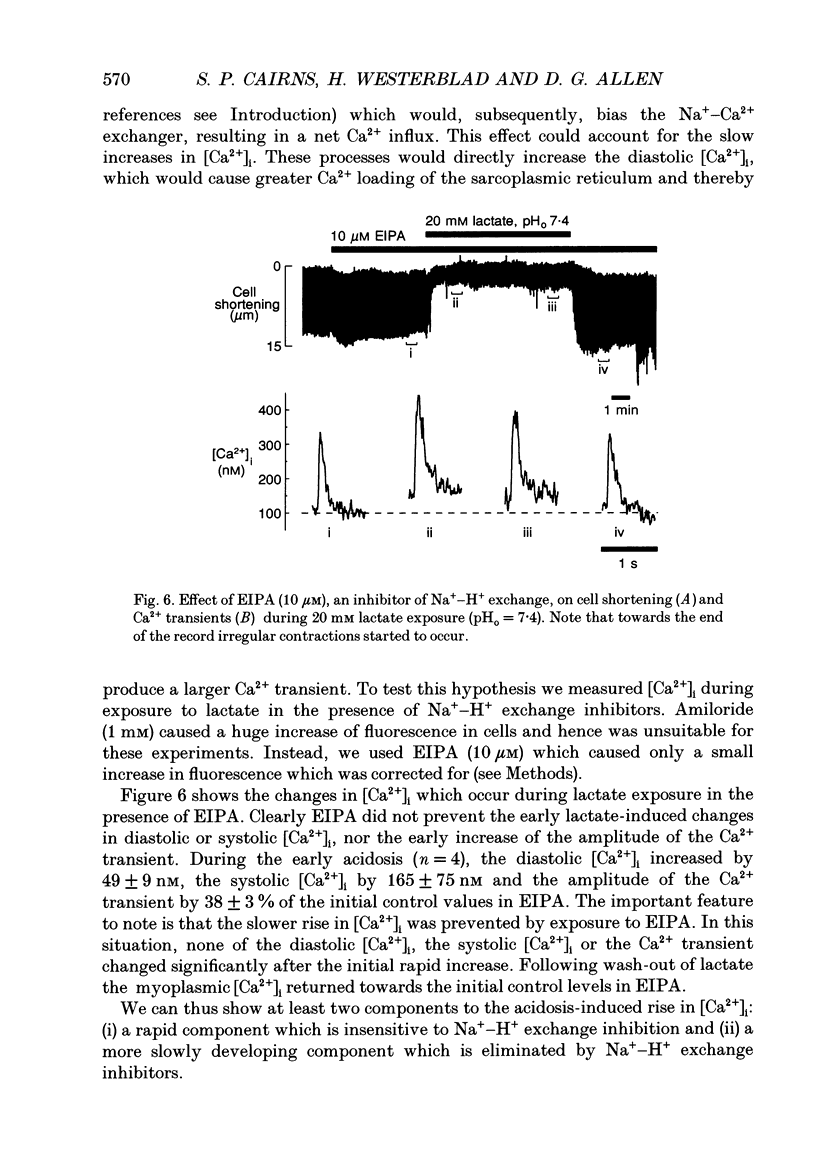




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Blanchard E. M., Solaro R. J. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ Res. 1984 Sep;55(3):382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Buchbinder M., Harrison D. C. Calcium overload, "injury" current, and early ischaemic cardiac arrhythmias--a direct connection. Lancet. 1983 Feb 5;1(8319):272–274. doi: 10.1016/s0140-6736(83)91688-4. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earm Y. E., Irisawa H. Effects of pH on the Na-Ca exchange current in single ventricular cells of the guinea pig. Jpn Heart J. 1986 Nov;27 (Suppl 1):153–158. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harrison S. M., Frampton J. E., McCall E., Boyett M. R., Orchard C. H. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992 Feb;262(2 Pt 1):C348–C357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Cellular mechanisms underlying calcium-proton interactions in cultured chick ventricular cells. J Physiol. 1988 Apr;398:391–410. doi: 10.1113/jphysiol.1988.sp017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Kohmoto O., Spitzer K. W., Movsesian M. A., Barry W. H. Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res. 1990 Mar;66(3):622–632. doi: 10.1161/01.res.66.3.622. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Frelin C., Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985 Nov;17(11):1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Mechanisms of acute ischemic contractile failure of the heart. Role of intracellular calcium. J Clin Invest. 1991 Aug;88(2):361–367. doi: 10.1172/JCI115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel F., Kranias E. G., Grassi de Gende A., Sumida M., Schwartz A. The effect of pH on the transient-state kinetics of Ca2+-Mg2+-ATPase of cardiac sarcoplasmic reticulum. A comparison with skeletal sarcoplasmic reticulum. Circ Res. 1982 Feb;50(2):310–317. doi: 10.1161/01.res.50.2.310. [DOI] [PubMed] [Google Scholar]
- Mohabir R., Lee H. C., Kurz R. W., Clusin W. T. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991 Dec;69(6):1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- Murphy E., Perlman M., London R. E., Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ Res. 1991 May;68(5):1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Orchard C. H. The role of the sarcoplasmic reticulum in the response of ferret and rat heart muscle to acidosis. J Physiol. 1987 Mar;384:431–449. doi: 10.1113/jphysiol.1987.sp016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. M., Kitakaze M., Marban E. 23Na-NMR measurements of intracellular sodium in intact perfused ferret hearts during ischemia and reperfusion. Am J Physiol. 1990 Dec;259(6 Pt 2):H1767–H1773. doi: 10.1152/ajpheart.1990.259.6.H1767. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Na/H exchange in cultured chick heart cells. pHi regulation. J Gen Physiol. 1985 Jan;85(1):43–64. doi: 10.1085/jgp.85.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Tani M., Neely J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989 Oct;65(4):1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- Uto A., Arai H., Ogawa Y. Reassessment of Fura-2 and the ratio method for determination of intracellular Ca2+ concentrations. Cell Calcium. 1991 Jan;12(1):29–37. doi: 10.1016/0143-4160(91)90082-p. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Acidification and intracellular sodium ion activity during stimulated myocardial ischemia. Am J Physiol. 1990 Jul;259(1 Pt 1):C169–C179. doi: 10.1152/ajpcell.1990.259.1.C169. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne A., Marrannes R., Vanheel B. Influence of organic acids on intracellular pH. Am J Physiol. 1983 Sep;245(3):C178–C183. doi: 10.1152/ajpcell.1983.245.3.C178. [DOI] [PubMed] [Google Scholar]


