Abstract
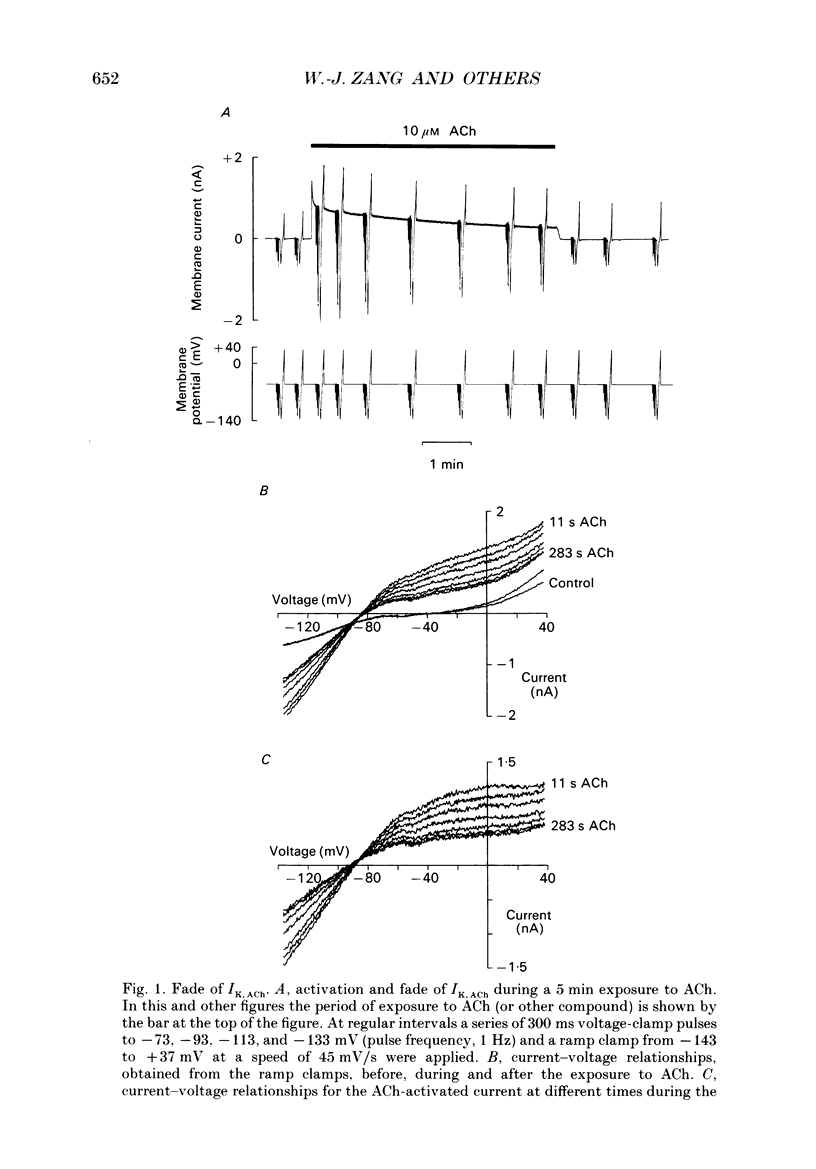
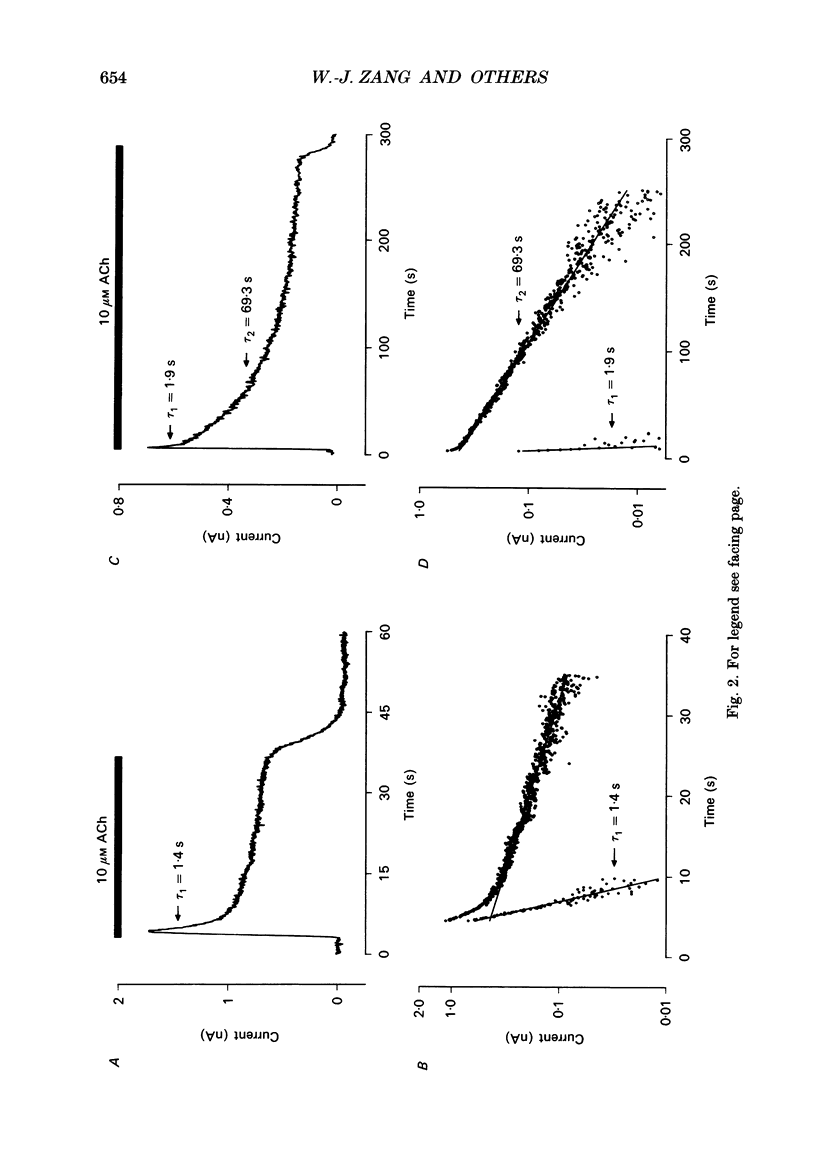
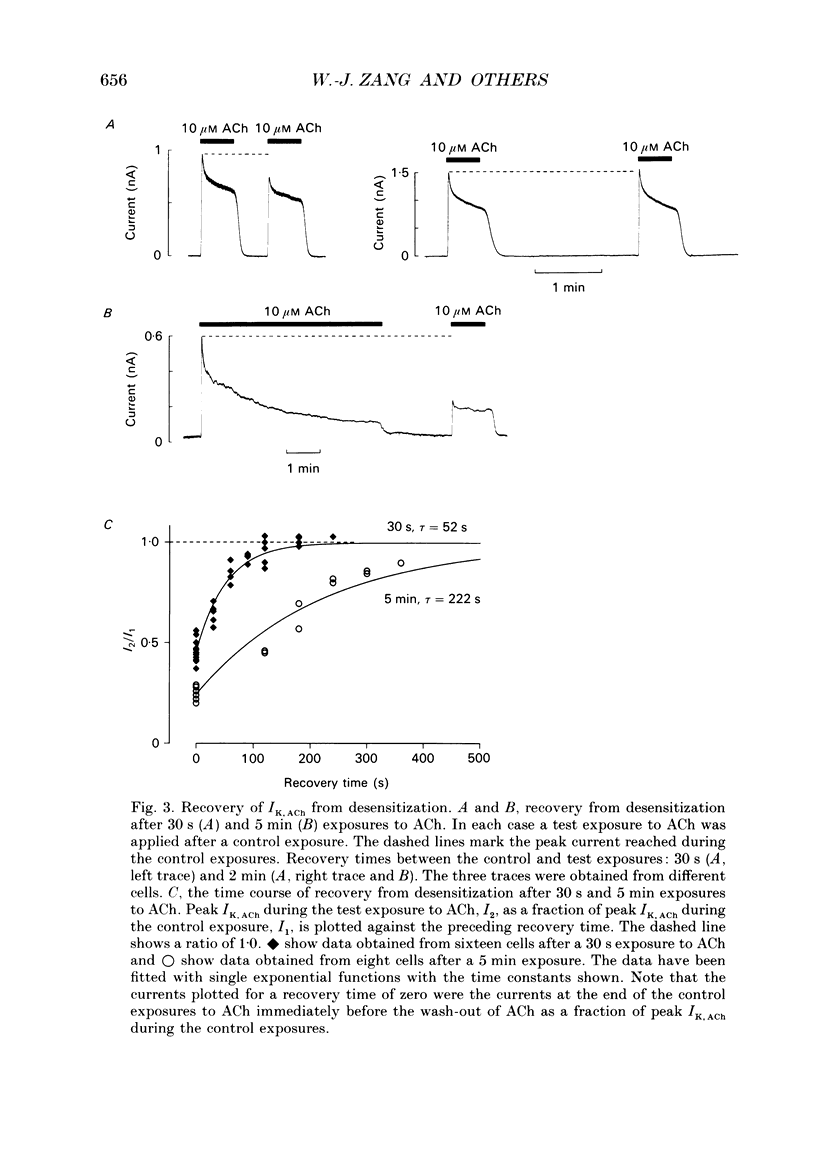
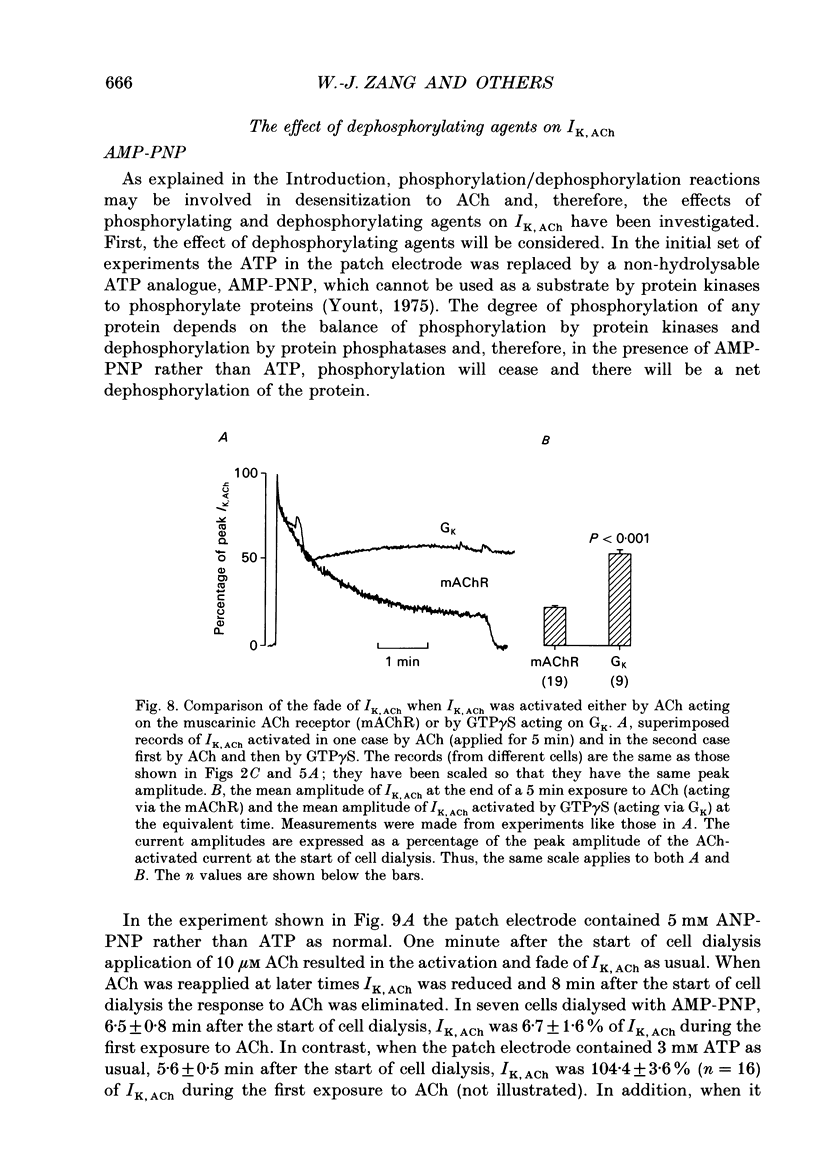
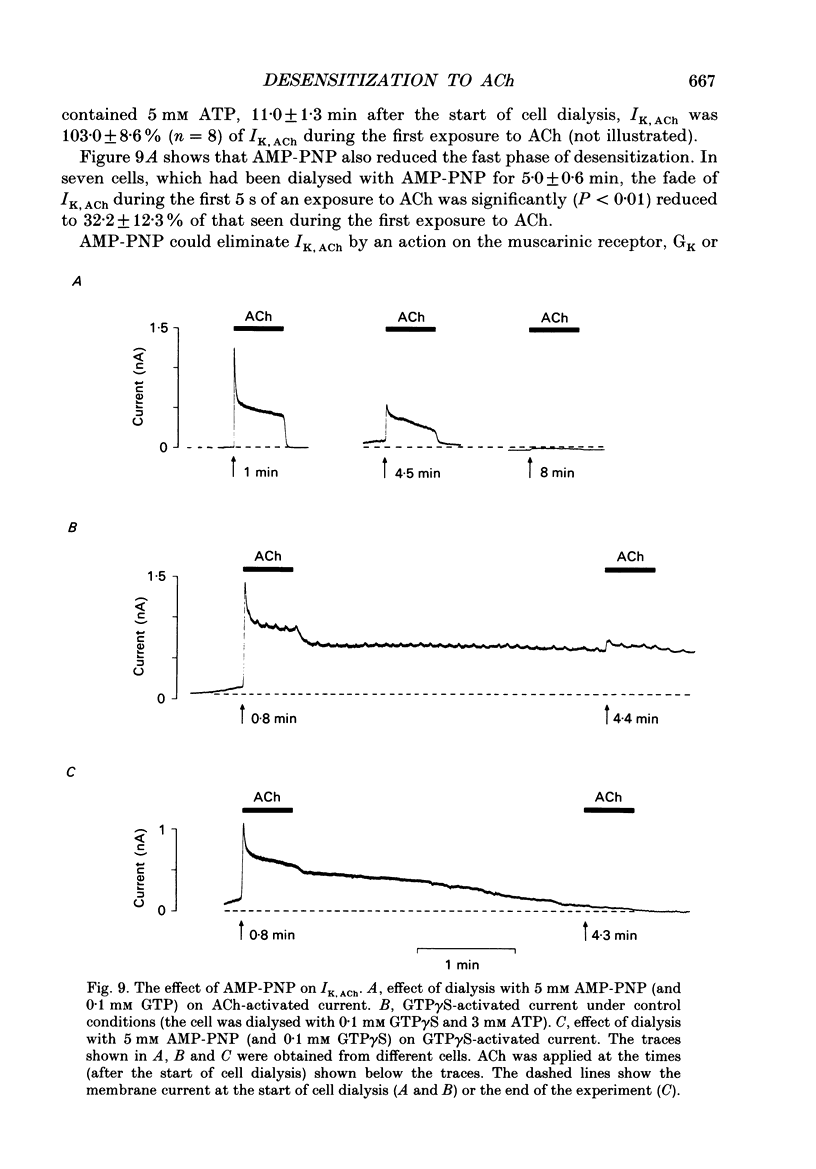
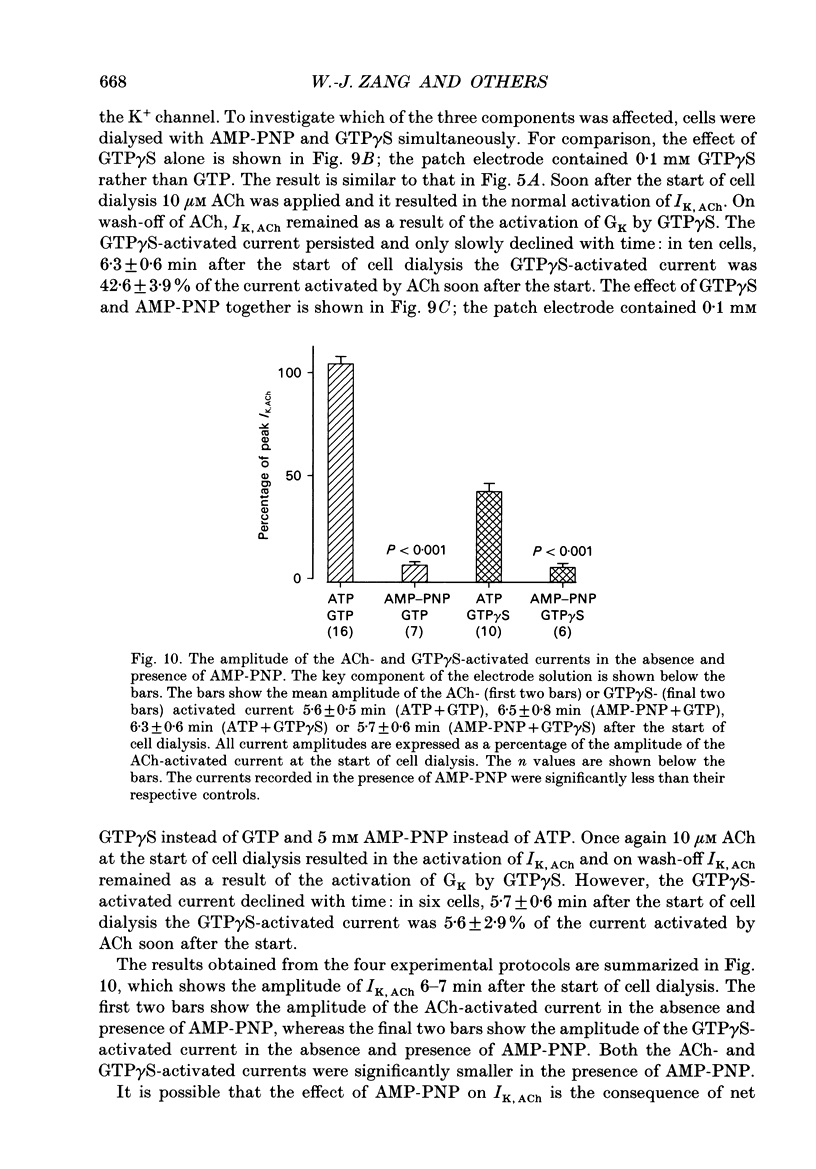
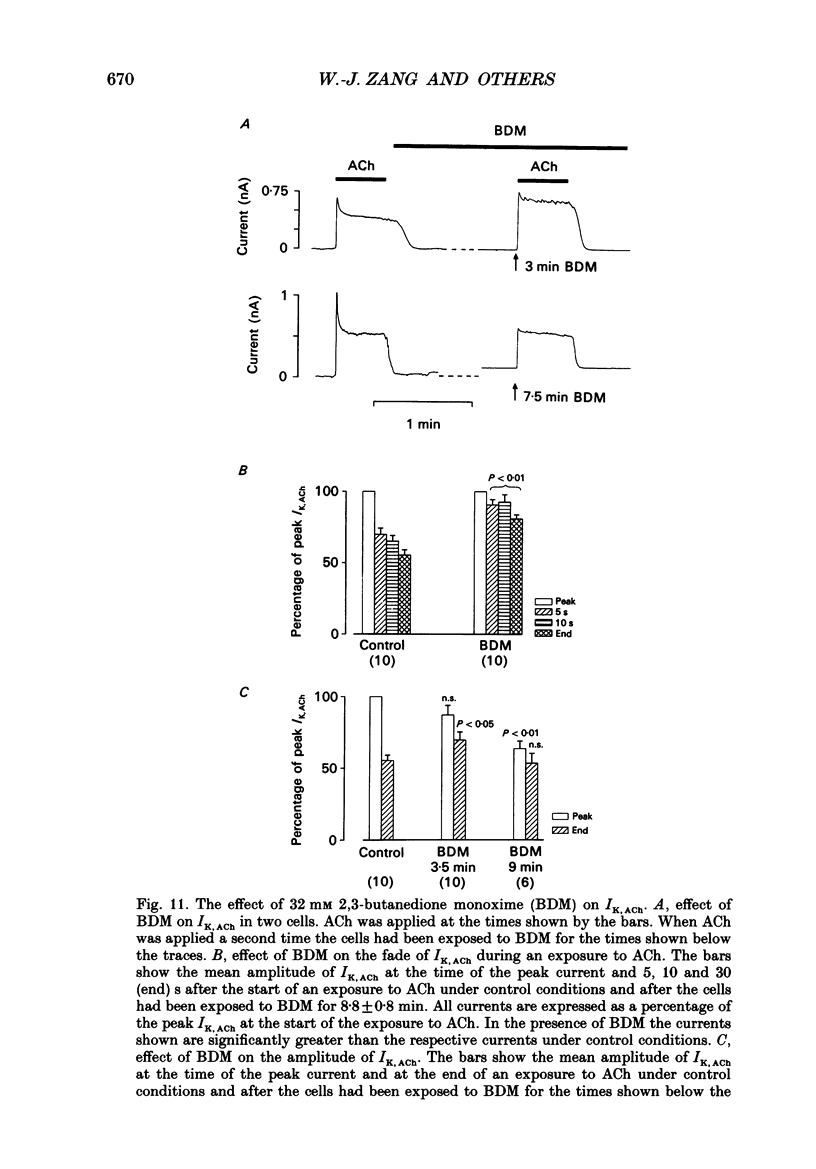
1. The ACh-activated K+ current (IK,ACh) has been investigated in guinea-pig atrial cells at 36 degrees C using the whole-cell patch-clamp technique. 2. During an exposure to ACh, IK,ACh faded as a result of desensitization. Throughout the fade of the current, the current reversed at EK and showed inward-going rectification. The fade was, therefore, the result of a genuine decrease in IK,ACh. 3. The onset of desensitization (as judged by the fade of IK,ACh) was biphasic and the time constants of the fast and slow phases of desensitization were 1.58 +/- 0.14 (n = 16) and 148.2 +/- 12.8 s (n = 18) respectively. Recovery from the fast and slow phases of desensitization (after 30 s and 5 min exposures to ACh respectively) occurred with time constants of 52 and 222 s respectively. This suggests that two processes are involved in desensitization. 4. The Q10 of the rate constant of the fast phase of desensitization was 2.2 +/- 0.3 (n = 6). 5. Intracellular perfusion with guanosine 5'-O-(3-thiotriphosphate) (GTP gamma S) or extracellular perfusion with AlF4- were used to bypass the muscarinic receptor and trigger IK,ACh by directly activating the G protein, GK, that links the muscarinic receptor to the K+ channel. Both GTP gamma S and AlF4- activated a current with the same reversal potential and the same degree of inward-going rectification as the ACh-activated current. 6. Desensitization still occurred when the muscarinic receptor was bypassed and IK,ACh was triggered by direct activation of GK with either GTP gamma S or AlF4-. This suggests that desensitization is, in part, the result of a modification of either GK or the K+ channel. 7. Activation of the muscarinic receptor by ACh resulted in greater desensitization than direct activation of GK; at the end of a 5 min exposure to ACh, current was only 22 +/- 1% (n = 19) of its peak value, whereas, after direct activation of GK by GTP gamma S for 5 min, current was 42 +/- 6% (n = 5) of its peak value. This suggests that desensitization also involves the muscarinic receptor. 8. When cells were perfused with GTP gamma S, the fast phase of desensitization could still occur, but the slow phase was reduced. This suggests that the fast phase involves GK or the K+ channel, whereas the slow phase involves the muscarinic receptor.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





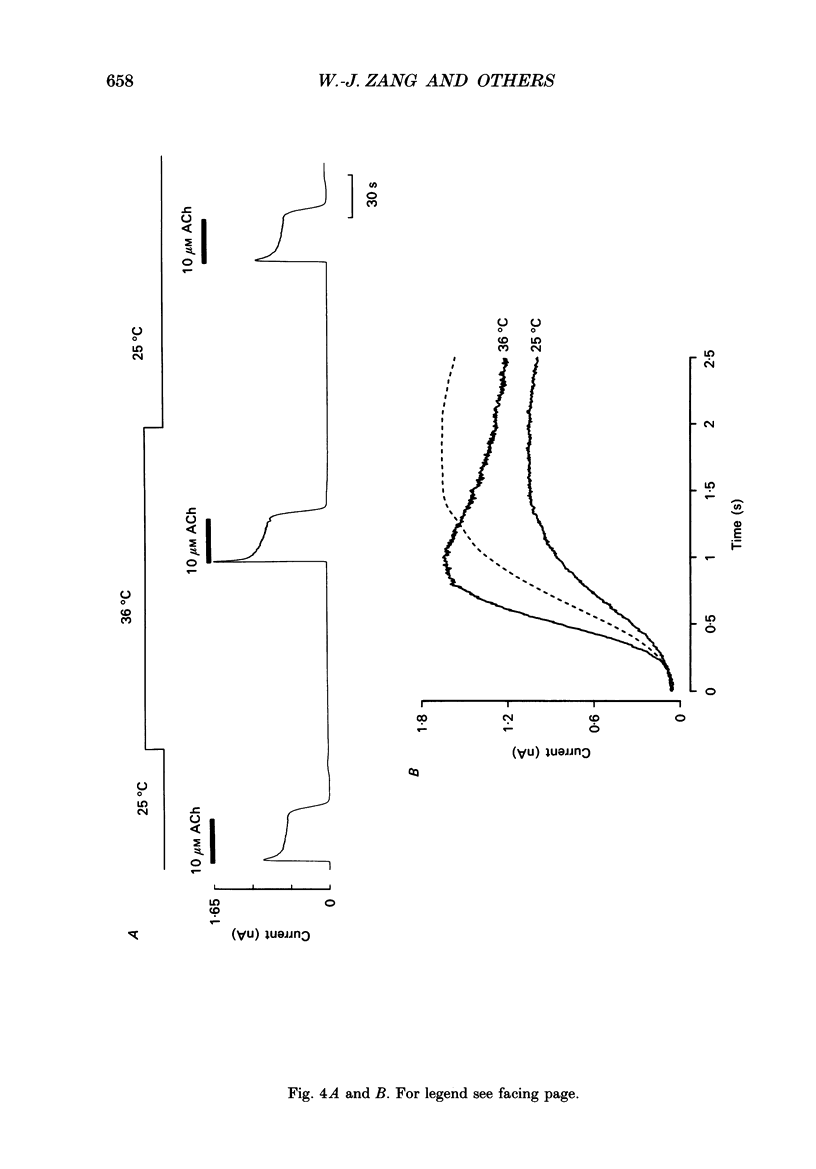
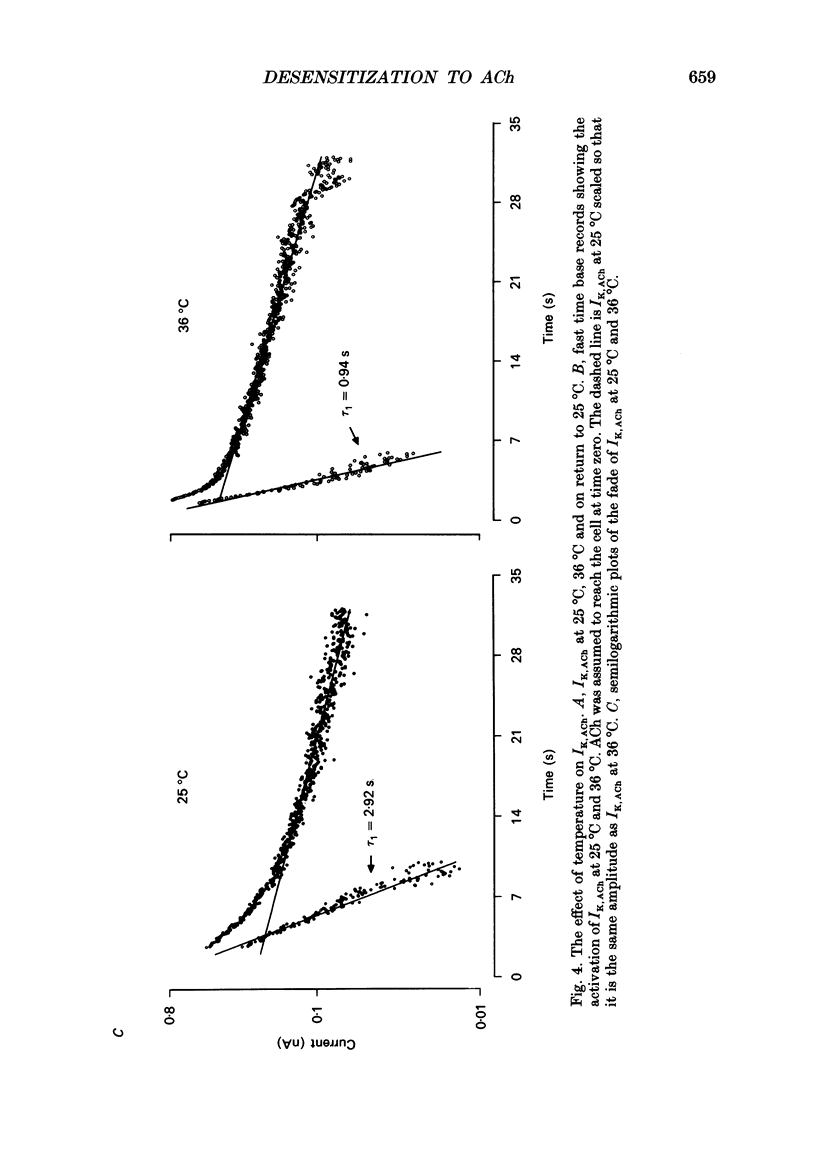

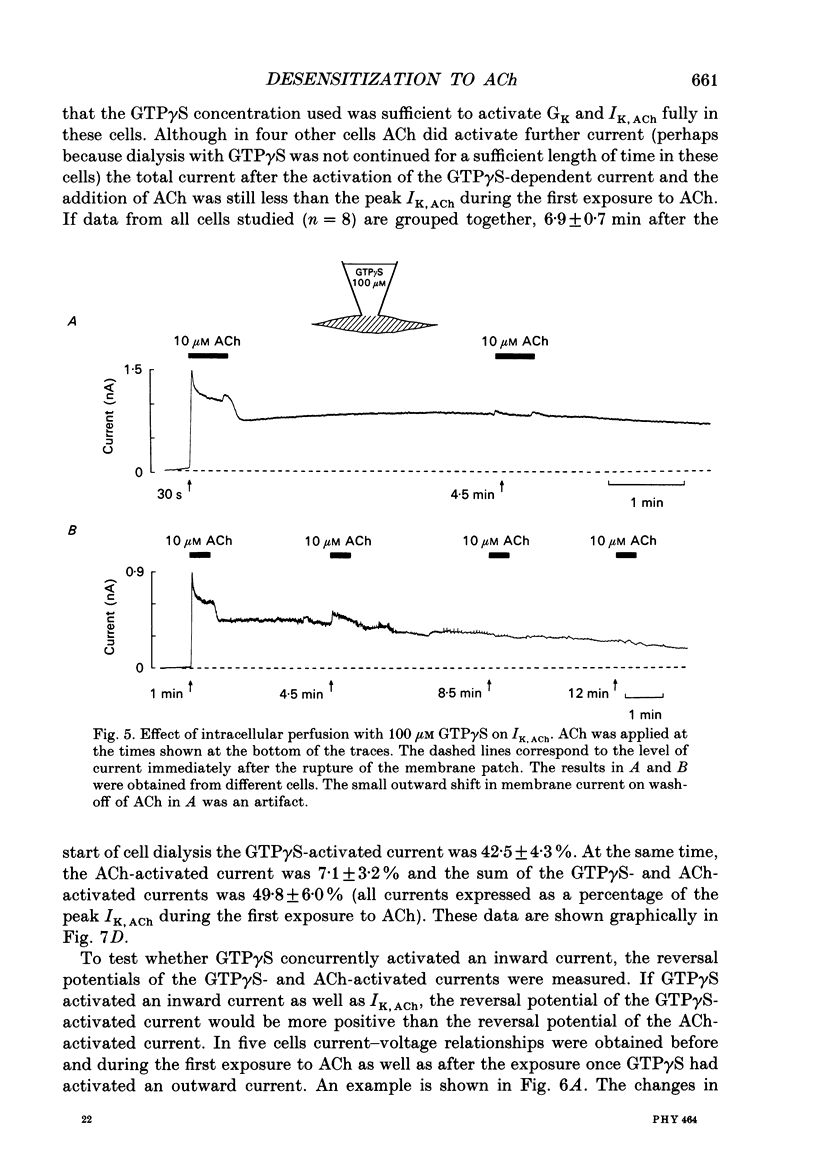
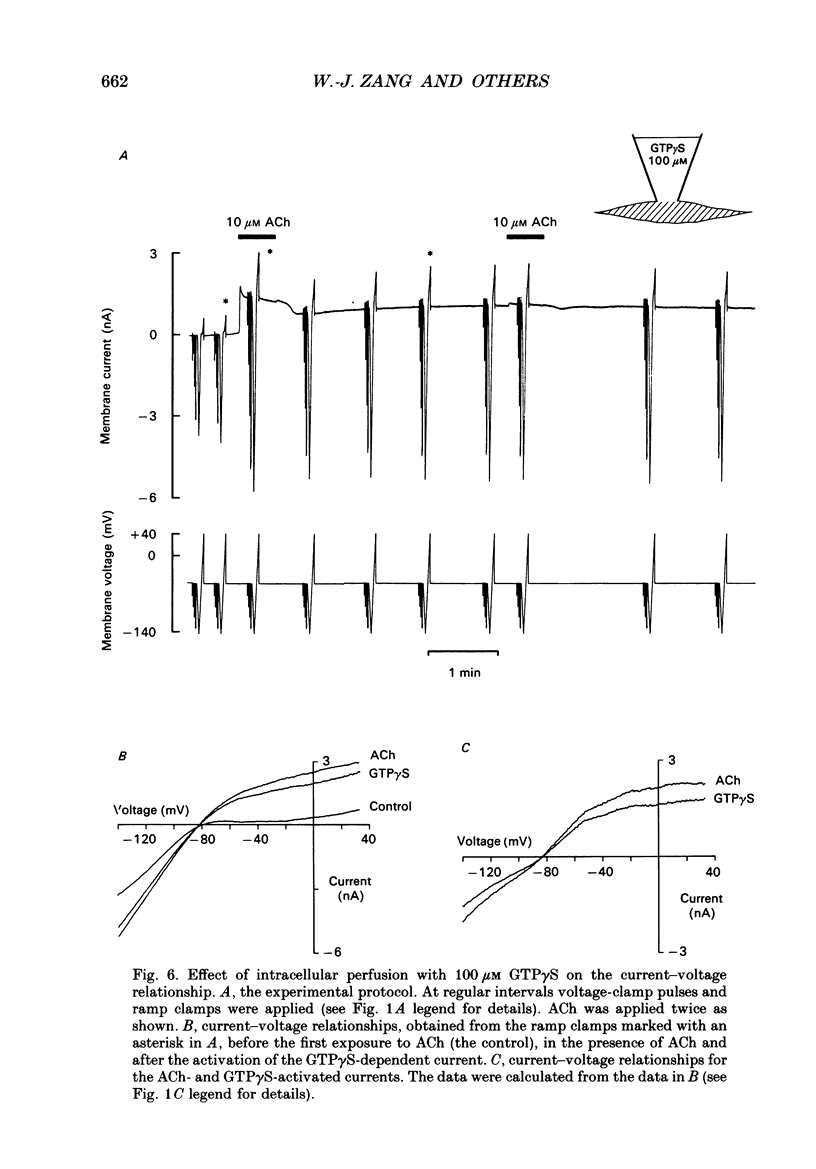

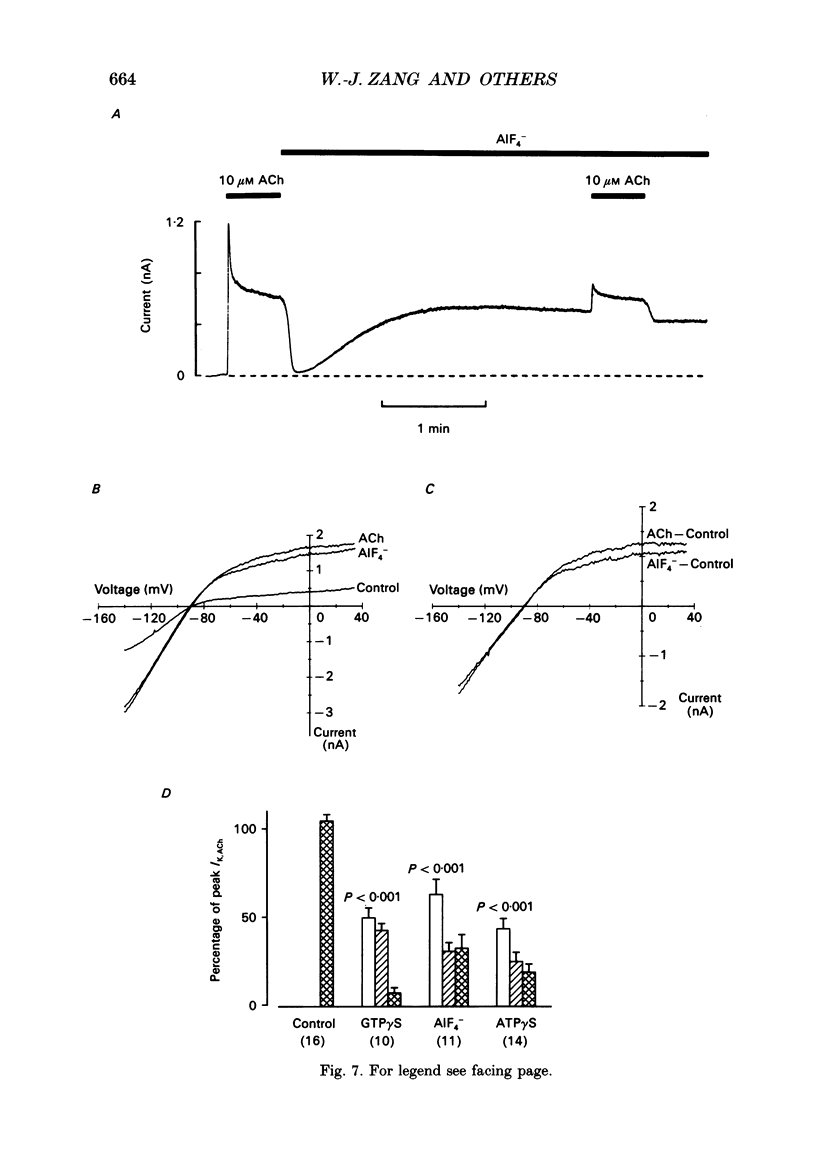










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKEW B. M. Oximes and hydroxamic acids as antidotes in anticholinesterase poisoning. Br J Pharmacol Chemother. 1956 Dec;11(4):417–423. doi: 10.1111/j.1476-5381.1956.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Stone W. C., Caron M. G., Lefkowitz R. J. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989 Apr 25;264(12):6707–6710. [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986 Aug 25;261(24):11056–11063. [PubMed] [Google Scholar]
- Boyd N. D. Two distinct kinetic phases of desensitization of acetylcholine receptors of clonal rat PC12 cells. J Physiol. 1987 Aug;389:45–67. doi: 10.1113/jphysiol.1987.sp016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H., Roberts A. The negative inotropic effect of acetylcholine on ferret ventricular myocardium. J Physiol. 1988 Oct;404:613–635. doi: 10.1113/jphysiol.1988.sp017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Roberts A. The fade of the response to acetylcholine at the rabbit isolated sino-atrial node. J Physiol. 1987 Dec;393:171–194. doi: 10.1113/jphysiol.1987.sp016818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J Clin Invest. 1984 Jan;73(1):1–4. doi: 10.1172/JCI111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbüchel H., Vereecke J., Carmeliet E. Atrial membranes contain nucleoside diphosphate kinase (NDPK) activity: its role in regulation of muscarinic K+ channels. Pacing Clin Electrophysiol. 1991 Nov;14(11 Pt 2):1721–1727. doi: 10.1111/j.1540-8159.1991.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Huang G. J., McArdle J. J. Novel suppression of an L-type calcium channel in neurones of murine dorsal root ganglia by 2,3-butanedione monoxime. J Physiol. 1992 Feb;447:257–274. doi: 10.1113/jphysiol.1992.sp019001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara M., Nakajima T., Irisawa H., Giles W. Regulation of spontaneous opening of muscarinic K+ channels in rabbit atrium. J Physiol. 1991 Feb;433:589–613. doi: 10.1113/jphysiol.1991.sp018445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J Physiol. 1991 Jun;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Hosey M. M. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. J Biol Chem. 1986 Sep 25;261(27):12429–12432. [PubMed] [Google Scholar]
- Kwatra M. M., Leung E., Maan A. C., McMahon K. K., Ptasienski J., Green R. D., Hosey M. M. Correlation of agonist-induced phosphorylation of chick heart muscarinic receptors with receptor desensitization. J Biol Chem. 1987 Dec 5;262(34):16314–16321. [PubMed] [Google Scholar]
- Magnússon M. K., Halldórsson H., Kjeld M., Thorgeirsson G. Endothelial inositol phosphate generation and prostacyclin production in response to G-protein activation by AlF4-. Biochem J. 1989 Dec 15;264(3):703–711. doi: 10.1042/bj2640703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Levy M. N., Matsuda Y. Fade of cardiac responses during tonic vagal stimulation. Am J Physiol. 1982 Aug;243(2):H219–H225. doi: 10.1152/ajpheart.1982.243.2.H219. [DOI] [PubMed] [Google Scholar]
- Martin P. Secondary AV conduction responses during tonic vagal stimulation. Am J Physiol. 1983 Oct;245(4):H584–H591. doi: 10.1152/ajpheart.1983.245.4.H584. [DOI] [PubMed] [Google Scholar]
- Otero A. D. Transphosphorylation and G protein activation. Biochem Pharmacol. 1990 May 1;39(9):1399–1404. doi: 10.1016/0006-2952(90)90420-p. [DOI] [PubMed] [Google Scholar]
- Otero A. S., Breitwieser G. E., Szabo G. Activation of muscarinic potassium currents by ATP gamma S in atrial cells. Science. 1988 Oct 21;242(4877):443–445. doi: 10.1126/science.3051383. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Foster K. A. Role of G proteins in the regulation of the cardiovascular system. Annu Rev Physiol. 1989;51:229–244. doi: 10.1146/annurev.ph.51.030189.001305. [DOI] [PubMed] [Google Scholar]
- Seargeant L. E., Stinson R. A. Inhibition of human alkaline phosphatases by vanadate. Biochem J. 1979 Jul 1;181(1):247–250. doi: 10.1042/bj1810247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G., Otero A. S. G protein mediated regulation of K+ channels in heart. Annu Rev Physiol. 1990;52:293–305. doi: 10.1146/annurev.ph.52.030190.001453. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- WILSON I. B., GINSBURG B. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim Biophys Acta. 1955 Sep;18(1):168–170. doi: 10.1016/0006-3002(55)90040-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Dixon C. J., Cuthbertson K. S., Cobbold P. H. Fluoroaluminate mimics agonist application in single rat hepatocytes. Biochem J. 1990 Jan 15;265(2):613–615. doi: 10.1042/bj2650613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]


