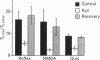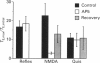Abstract
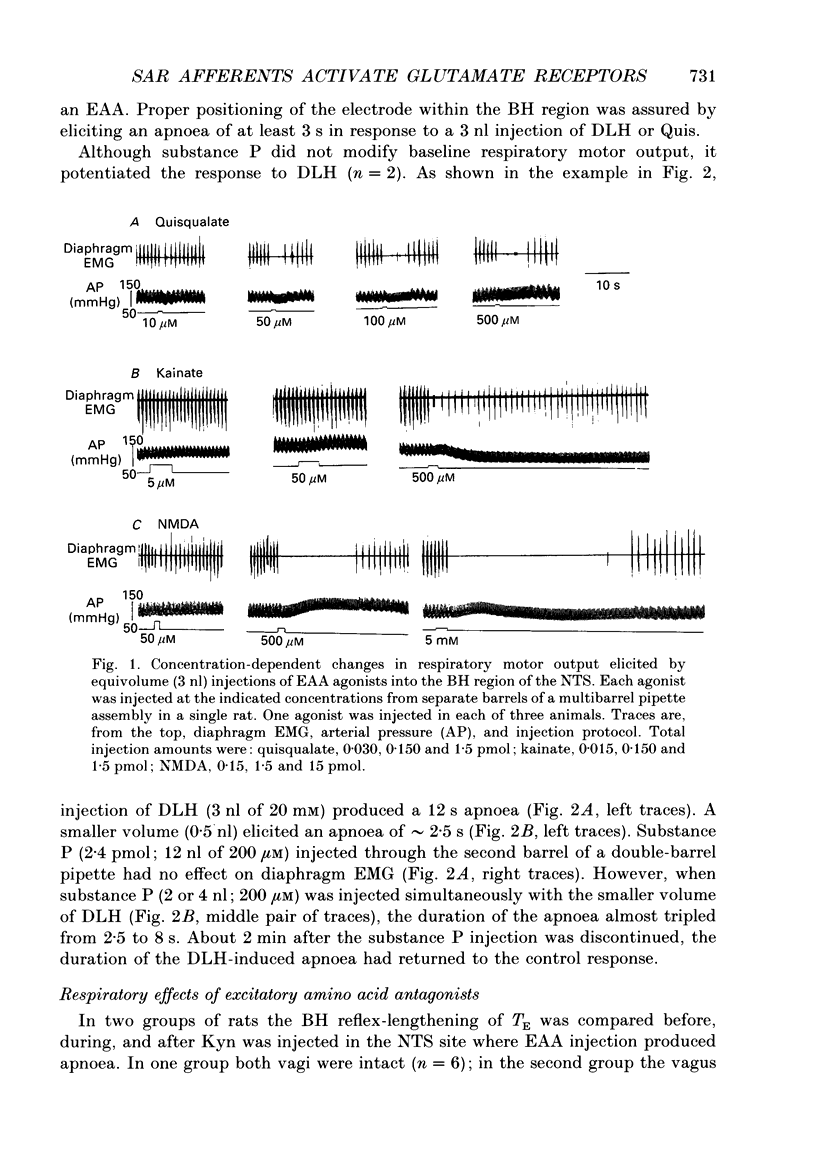
1. The goal of the present study was to identify potential neurotransmitter candidates in the Breuer-Hering (BH) reflex pathway, specifically at synapses between the primary afferents and probable second-order neurones (pump cells) within the nucleus tractus solitarii (NTS). We hypothesized that if activation of specific receptors in the NTS is required for production of the BH reflex, then (1) injection of the receptor agonist(s) would mimic the reflex response (apnoea), (2) injection of appropriate antagonists would impair the apnoea produced by either lung inflation or agonist injection, and (3) second-order neurones in the pathway would be excited by either lung inflation or agonists while antagonists would prevent the response to either. 2. Studies were carried out either in spontaneously breathing or in paralysed, thoracotomized and ventilated rats in which either diaphragm EMG or phrenic nerve activity, expired CO2 concentration and arterial pressure were continuously monitored. The BH reflex was physiologically activated by inflating the lungs. 3. Pressure injections (0.03-15 pmol) of selective excitatory amino acid (EAA) receptor agonists, quisqualic acid (Quis) and N-methyl-D-aspartic acid (NMDA) into an area of the NTS shown previously to contain neurones required for production of the BH reflex produced dose-dependent apnoeas that mimicked the response to lung inflation. Injection of substance P (0.03-4 pmol) did not alter baseline respiratory pattern. 4. Injections of the EAA antagonists, kynurenic acid (Kyn; 0.6-240 pmol), 6-cyano-7-nitro-quinoxaline-2,3-dione (CNQX) or 6,7-dinitroquinoxaline-2,3-dione (DNQX) into the BH region of the NTS reversibly impaired the apnoea produced by lung inflation. All three antagonists reduced or abolished the apnoeas resulting from injection of Quis or NMDA, and slowed baseline respiratory frequency. In contrast, injections of the highly selective NMDA receptor antagonist, D-2-amino-5-phosphonovaleric acids (AP5), in doses sufficient to block the apnoeic response to NMDA, neither altered the reflex apnoea evoked by lung inflation nor the baseline respiratory pattern. 5. Pump cells located within the BH region were excited by pressure injections of the broad spectrum EAA agonist, DL-homocysteic acid (DLH). Kyn reversibly blocked the excitation of pump cells in response to either lung inflation or DLH injection. 6. These findings suggest that EAAs mediate primary afferent excitation of second-order neurones in the Breuer-Hering reflex pathway, primarily through the activation of non-NMDA EAA receptor subtypes.
Full text
PDF





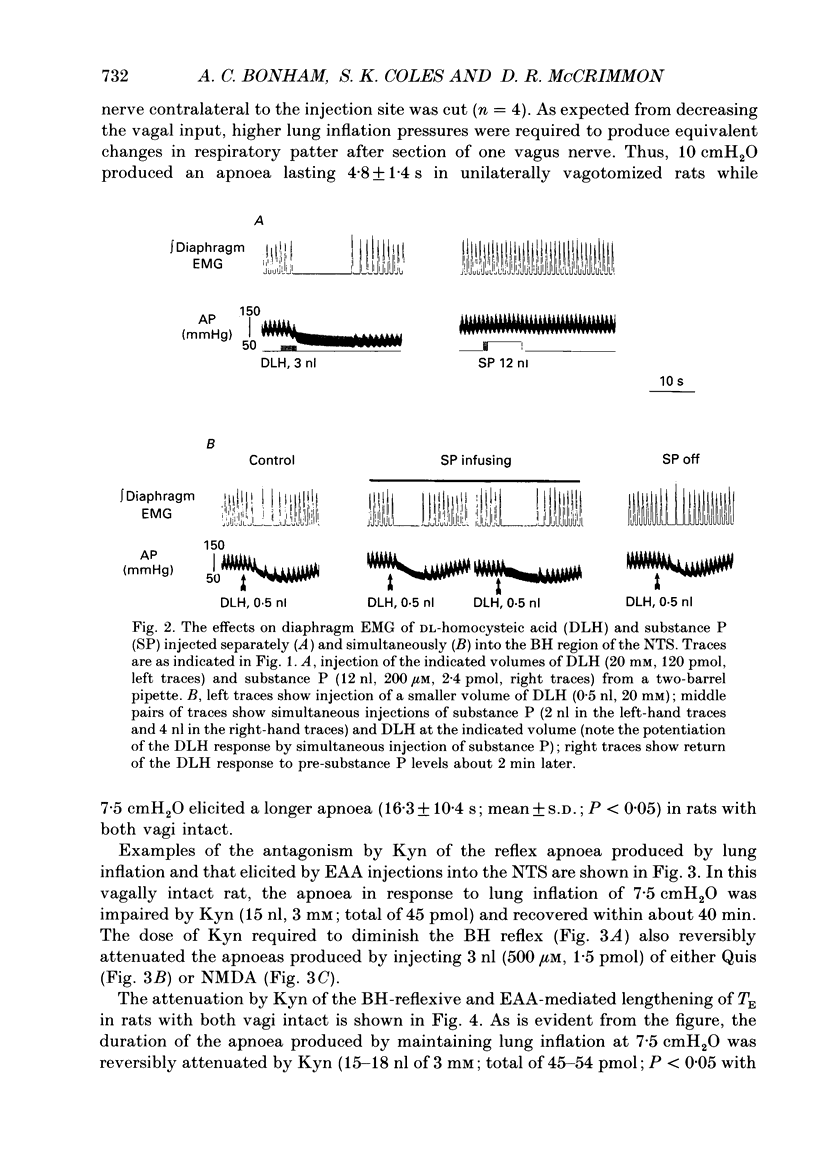
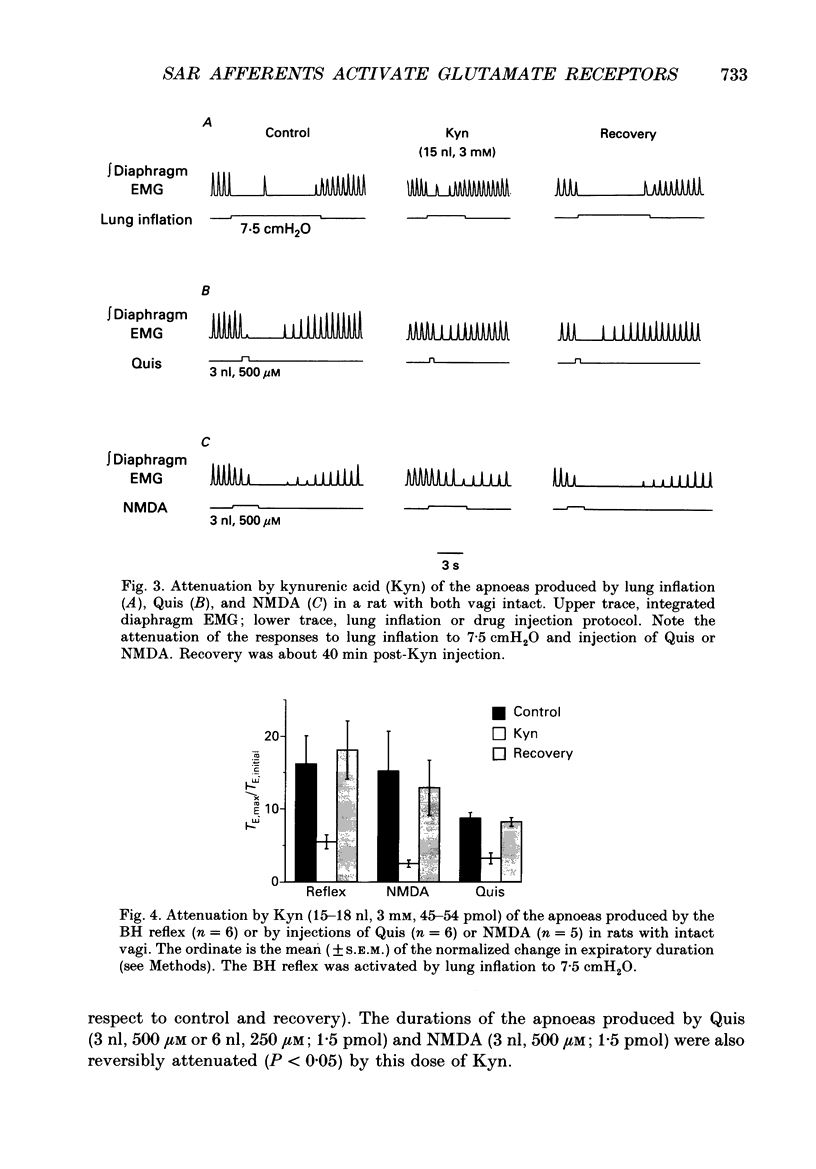
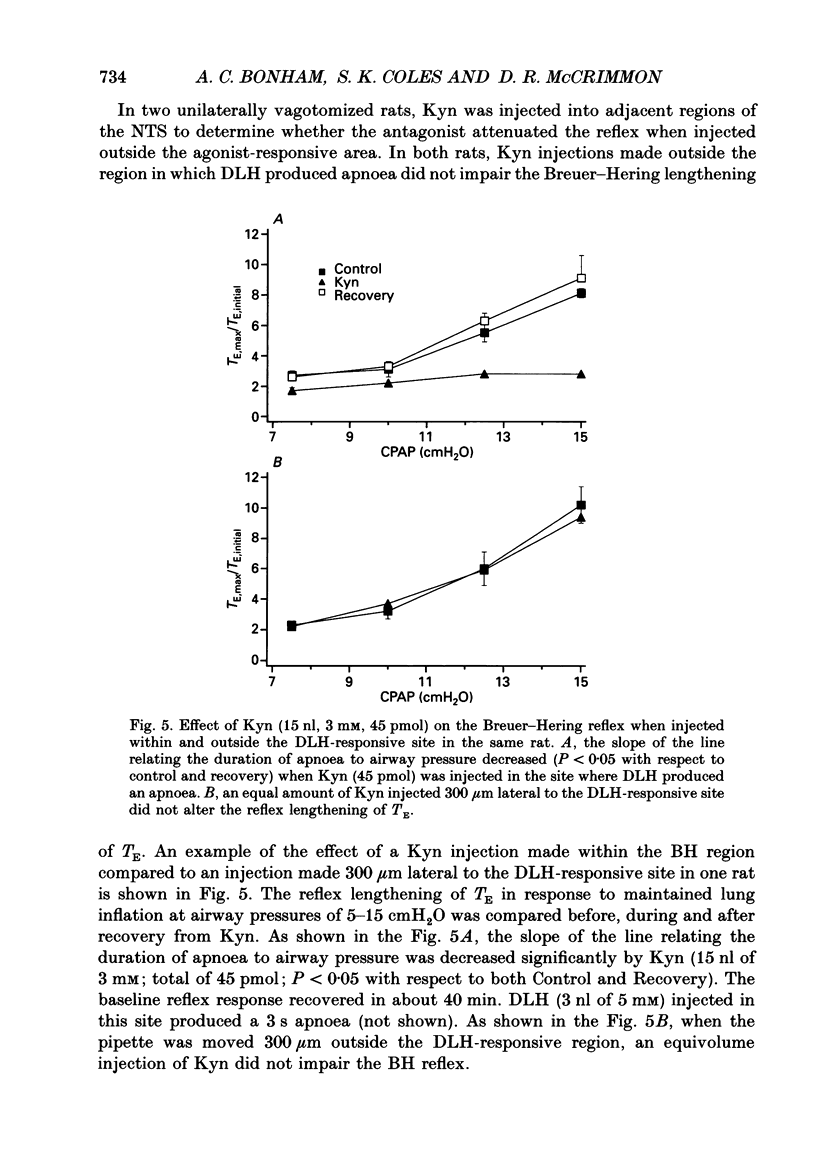

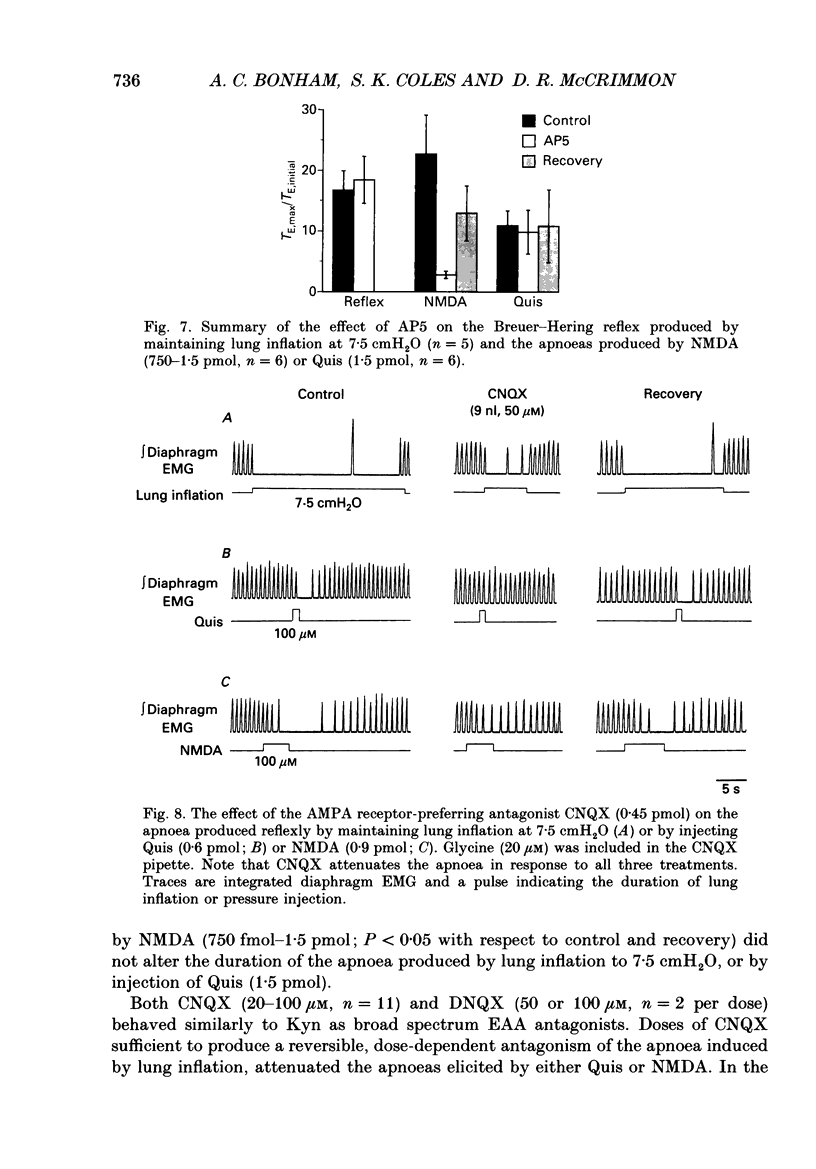
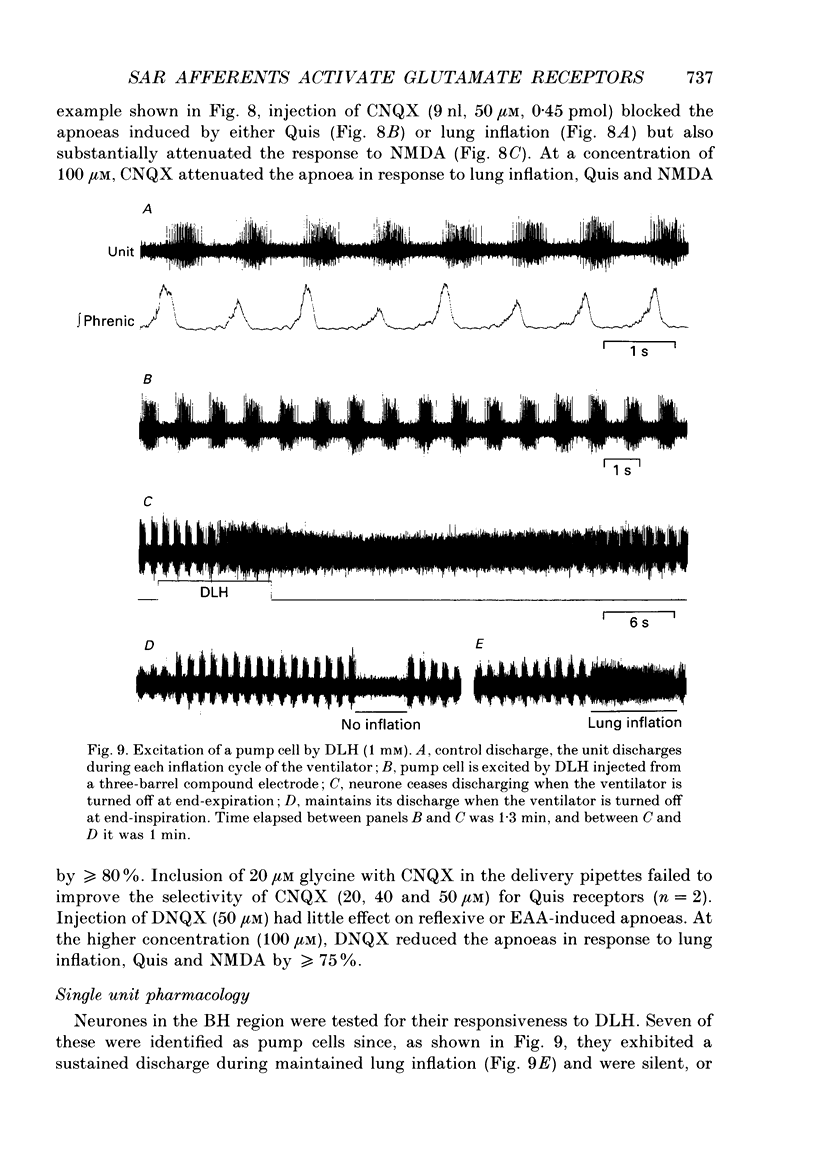
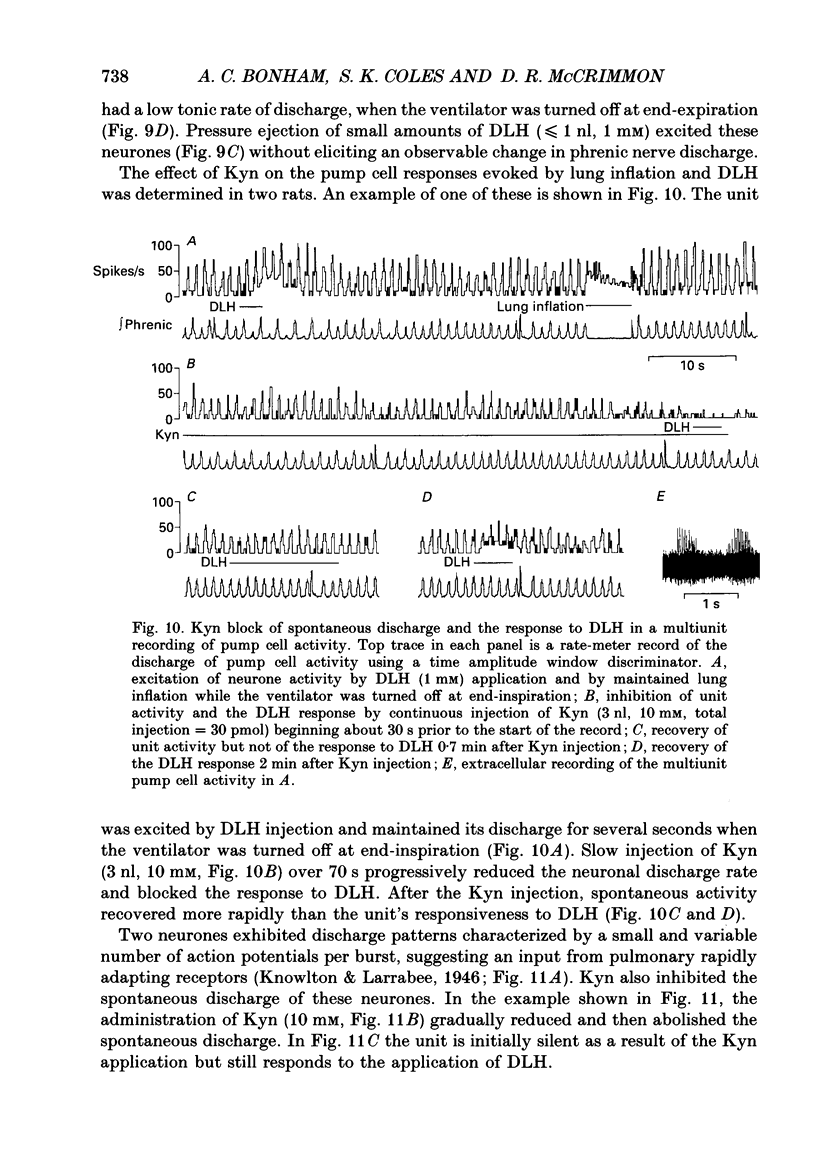
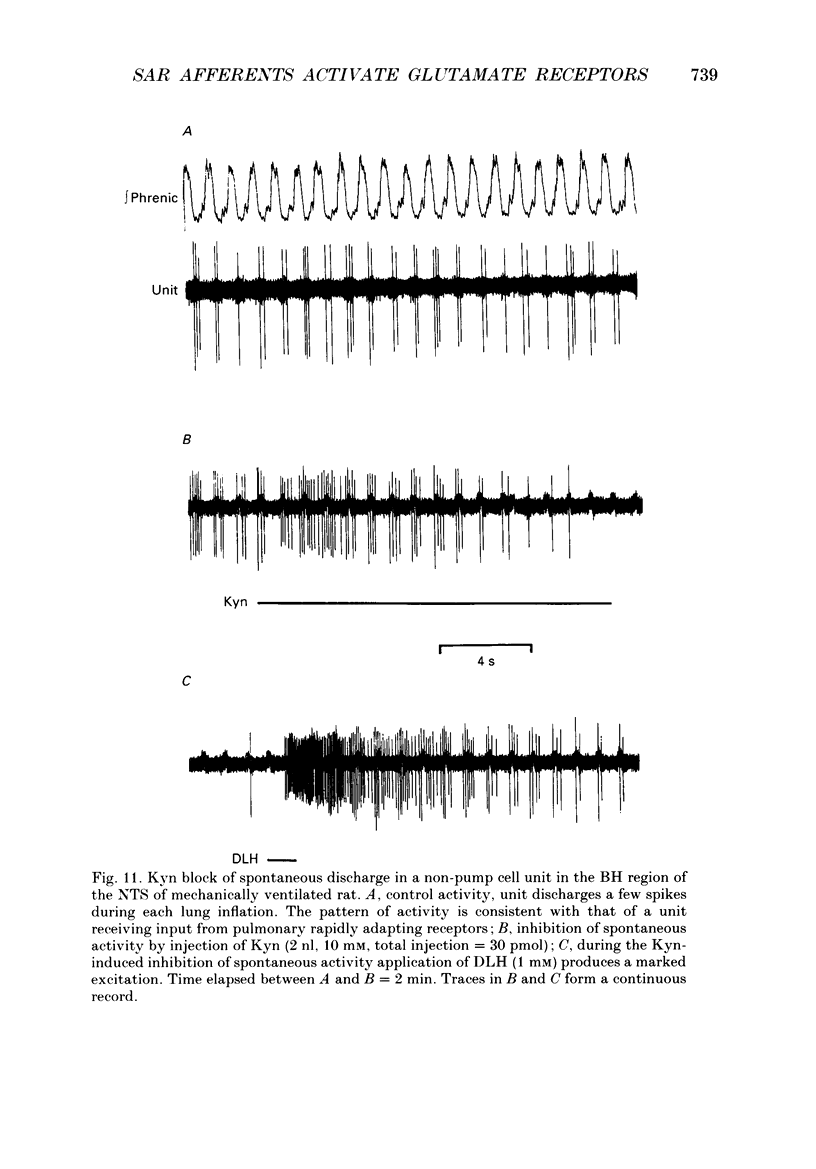






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen M. C., Yang M. Y. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990 Oct;259(4 Pt 2):H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- Backman S. B., Anders C., Ballantyne D., Röhrig N., Camerer H., Mifflin S., Jordan D., Dickhaus H., Spyer K. M., Richter D. W. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Arch. 1984 Oct;402(2):129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- Bartoli A., Cross B. A., Guz A., Huszczuk A., Jeffries R. The effect of varying tidal volume on the associated phrenic motoneurone output:studies of vagal and chemical feedback. Respir Physiol. 1975 Nov;25(2):135–155. doi: 10.1016/0034-5687(75)90093-6. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Dick T. E. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. J Neurophysiol. 1987 Dec;58(6):1259–1274. doi: 10.1152/jn.1987.58.6.1259. [DOI] [PubMed] [Google Scholar]
- Bonham A. C., Joad J. P. Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol. 1991 Sep;441:95–112. doi: 10.1113/jphysiol.1991.sp018740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham A. C., McCrimmon D. R. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990 Aug;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. A., Lightman S. L. Cardio-respiratory actions of substance P, TRH and 5-HT in the nucleus tractus solitarius of rats: evidence for functional interactions of neuropeptides and amine neurotransmitters. Neuropeptides. 1985 Sep;6(5):425–436. doi: 10.1016/0143-4179(85)90141-6. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J Neurophysiol. 1984 Apr;51(4):753–776. doi: 10.1152/jn.1984.51.4.753. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Jones P. W., Guz A. The role of vagal afferent information during inspiration in determining phrenic motoneurone output. Respir Physiol. 1980 Feb;39(2):149–167. doi: 10.1016/0034-5687(80)90042-0. [DOI] [PubMed] [Google Scholar]
- Davies R. O., Kubin L., Pack A. I. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol. 1987 Feb;383:571–585. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. D., Lowry O. H., Loewy A. D. The distribution of glutamate, GABA and aspartate in the nucleus tractus solitarius of the cat. Brain Res. 1982 Apr 8;237(1):254–260. doi: 10.1016/0006-8993(82)90576-5. [DOI] [PubMed] [Google Scholar]
- Ezure K., Manabe M., Yamada H. Distribution of medullary respiratory neurons in the rat. Brain Res. 1988 Jul 12;455(2):262–270. doi: 10.1016/0006-8993(88)90085-6. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Windhorst U., Anders K., Richter D. W. Synaptic interaction between medullary respiratory neurones during apneusis induced by NMDA-receptor blockade in cat. J Physiol. 1992 May;450:303–323. doi: 10.1113/jphysiol.1992.sp019128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 1989 Oct 23;500(1-2):199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Gallagher P. J., Paxinos G., White S. W. The role of substance P in arterial chemoreflex control of ventilation. J Auton Nerv Syst. 1985 Feb-Mar;12(2-3):195–210. doi: 10.1016/0165-1838(85)90061-x. [DOI] [PubMed] [Google Scholar]
- Gillis R. A., Helke C. J., Hamilton B. L., Norman W. P., Jacobowitz D. M. Evidence that substance P is a neurotransmitter of baro- and chemoreceptor afferents in nucleus tractus solitarius. Brain Res. 1980 Jan 13;181(2):476–481. doi: 10.1016/0006-8993(80)90633-2. [DOI] [PubMed] [Google Scholar]
- Granata A. R., Sved A. F., Reis D. J. In vivo release by vagal stimulation of L-[3H] glutamic acid in the nucleus tractus solitarius preloaded with L-[3H] glutamine. Brain Res Bull. 1984 Jan;12(1):5–9. doi: 10.1016/0361-9230(84)90207-7. [DOI] [PubMed] [Google Scholar]
- Green J. F., Schmidt N. D., Schultz H. D., Roberts A. M., Coleridge H. M., Coleridge J. C. Pulmonary C-fibers evoke both apnea and tachypnea of pulmonary chemoreflex. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):562–567. doi: 10.1152/jappl.1984.57.2.562. [DOI] [PubMed] [Google Scholar]
- Greer J. J., Smith J. C., Feldman J. L. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991 Jun;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippi M. A., Pack A. I., Davies R. O., Fishman A. P. Adaptation to reflex effects of prolonged lung inflation. J Appl Physiol (1985) 1985 Apr;58(4):1360–1371. doi: 10.1152/jappl.1985.58.4.1360. [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Filtz T. M., Donaldson S. R. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res. 1987 Mar 31;407(2):272–284. doi: 10.1016/0006-8993(87)91105-x. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Miller R. J. CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) antagonizes NMDA-evoked [3H]GABA release from cultured cortical neurons via an inhibitory action at the strychnine-insensitive glycine site. Brain Res. 1989 Jun 5;489(1):185–189. doi: 10.1016/0006-8993(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Helke C. J., O'Donohue T. L., Jacobowitz D. M. Substance P as a baro- and chemoreceptor afferent neurotransmitter: immunocytochemical and neurochemical evidence in the rat. Peptides. 1980 Spring;1(1):1–9. doi: 10.1016/0196-9781(80)90027-3. [DOI] [PubMed] [Google Scholar]
- Kubo T., Kihara M. Evidence of N-methyl-D-aspartate receptor-mediated modulation of the aortic baroreceptor reflex in the rat nucleus tractus solitarii. Neurosci Lett. 1988 Apr 22;87(1-2):69–74. doi: 10.1016/0304-3940(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Lawson E. E., Richter D. W., Czyzyk-Krzeska M. F., Bischoff A., Rudesill R. C. Respiratory neuronal activity during apnea and other breathing patterns induced by laryngeal stimulation. J Appl Physiol (1985) 1991 Jun;70(6):2742–2749. doi: 10.1152/jappl.1991.70.6.2742. [DOI] [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988 Dec;26(2):169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- McCrimmon D. R., Feldman J. L., Speck D. F. Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain stem. J Neurosci. 1986 Aug;6(8):2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon D. R., Smith J. C., Feldman J. L. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989 Jun;9(6):1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. D., Felder R. B. Excitatory amino acid receptors intrinsic to synaptic transmission in nucleus tractus solitarii. Brain Res. 1988 Jul 26;456(2):333–343. doi: 10.1016/0006-8993(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Cross B. A., Hiramoto T., Scheid P. Effects of intrapulmonary CO2 and airway pressure on phrenic activity and pulmonary stretch receptor discharge in dogs. Respir Physiol. 1980 Jul;41(1):29–48. doi: 10.1016/0034-5687(80)90021-3. [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Vidruk E. H. Effects of hypercapnia on phrenic and stretch receptor responses to lung inflation. Respir Physiol. 1987 Jun;68(3):319–330. doi: 10.1016/s0034-5687(87)80017-8. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Monteau R., Gauthier P., Rega P., Hilaire G. Effects of N-methyl-D-aspartate (NMDA) antagonist MK-801 on breathing pattern in rats. Neurosci Lett. 1990 Feb 5;109(1-2):134–139. doi: 10.1016/0304-3940(90)90551-j. [DOI] [PubMed] [Google Scholar]
- Morilak D. A., Morris M., Chalmers J. Release of substance P in the nucleus tractus solitarius measured by in vivo microdialysis: response to stimulation of the aortic depressor nerves in rabbit. Neurosci Lett. 1988 Nov 22;94(1-2):131–137. doi: 10.1016/0304-3940(88)90283-2. [DOI] [PubMed] [Google Scholar]
- Morin-Surun M. P., Jordan D., Champagnat J., Spyer K. M., Denavit-Saubie M. Excitatory effects of iontophoretically applied substance P on neurons in the nucleus tractus solitarius of the cat: lack of interaction with opiates and opioids. Brain Res. 1984 Jul 30;307(1-2):388–392. doi: 10.1016/0006-8993(84)90502-x. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985 May 6;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Randić M., Hećimović H., Ryu P. D. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurones. Neurosci Lett. 1990 Sep 4;117(1-2):74–80. doi: 10.1016/0304-3940(90)90122-p. [DOI] [PubMed] [Google Scholar]
- Saether K., Hilaire G., Monteau R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res. 1987 Sep 1;419(1-2):87–96. doi: 10.1016/0006-8993(87)90571-3. [DOI] [PubMed] [Google Scholar]
- Talman W. T., Perrone M. H., Reis D. J. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980 Aug 15;209(4458):813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Respiratory reflexes in man and other mammalian species. Clin Sci. 1961 Oct;21:163–170. [PubMed] [Google Scholar]
- Zheng Y., Barillot J. C., Bianchi A. L. Patterns of membrane potentials and distributions of the medullary respiratory neurons in the decerebrate rat. Brain Res. 1991 Apr 19;546(2):261–270. doi: 10.1016/0006-8993(91)91490-r. [DOI] [PubMed] [Google Scholar]