Abstract
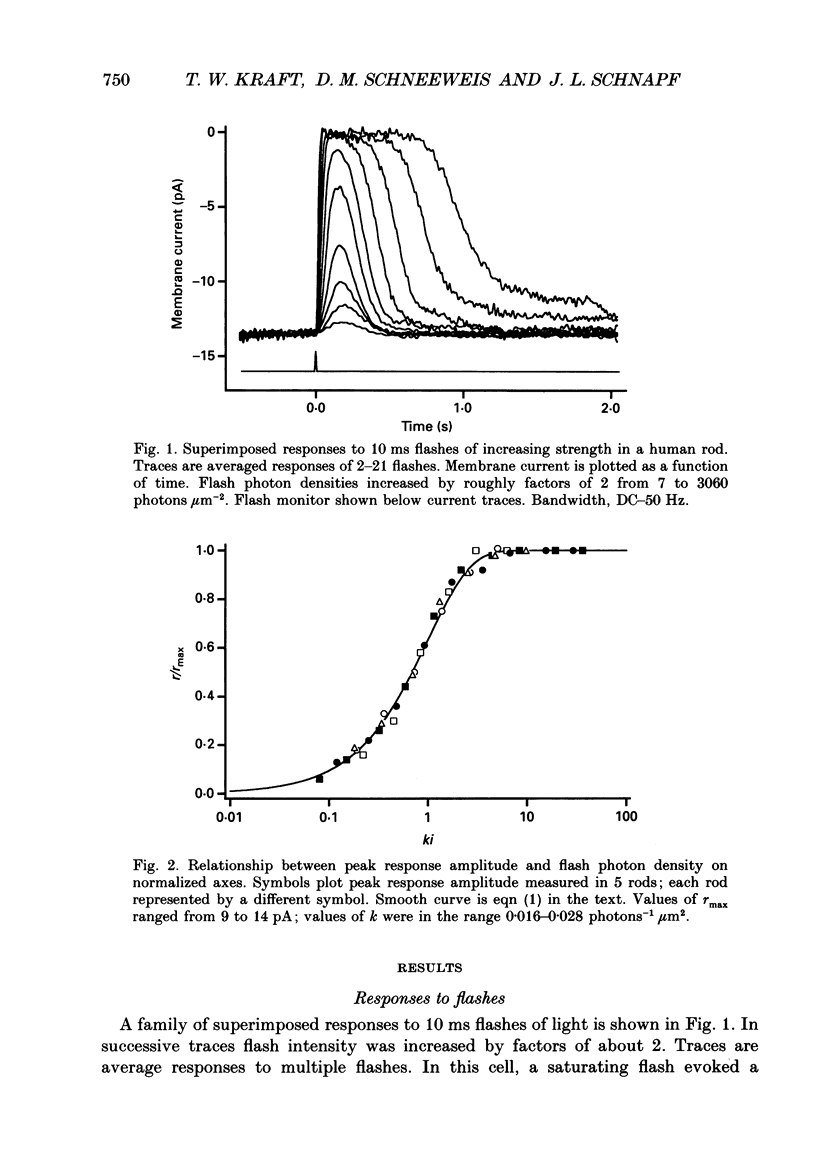
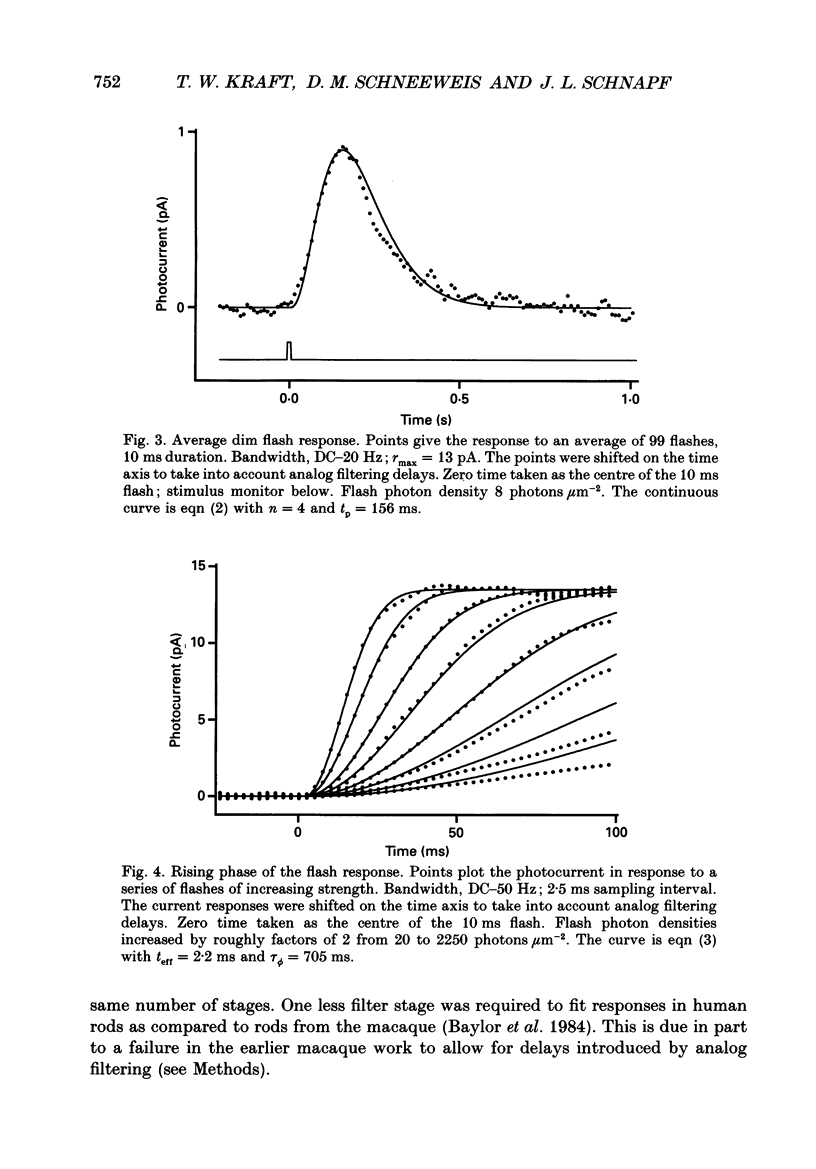
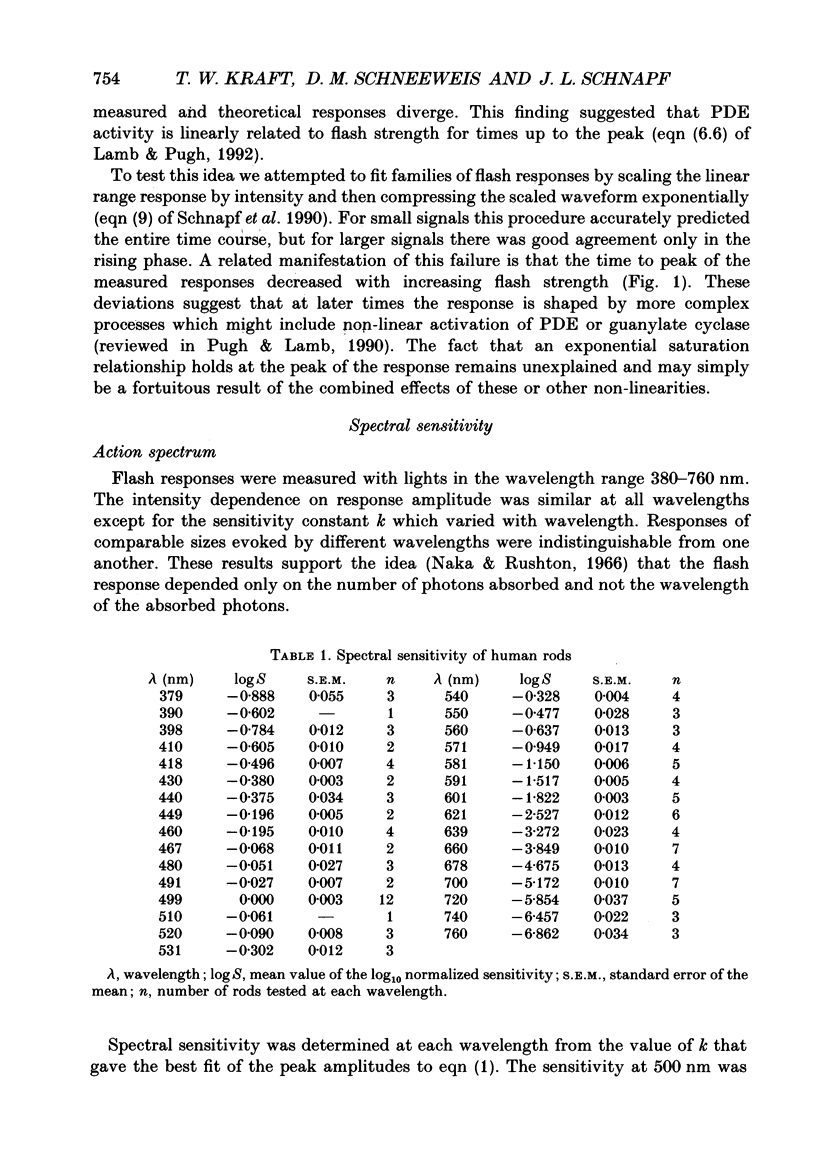
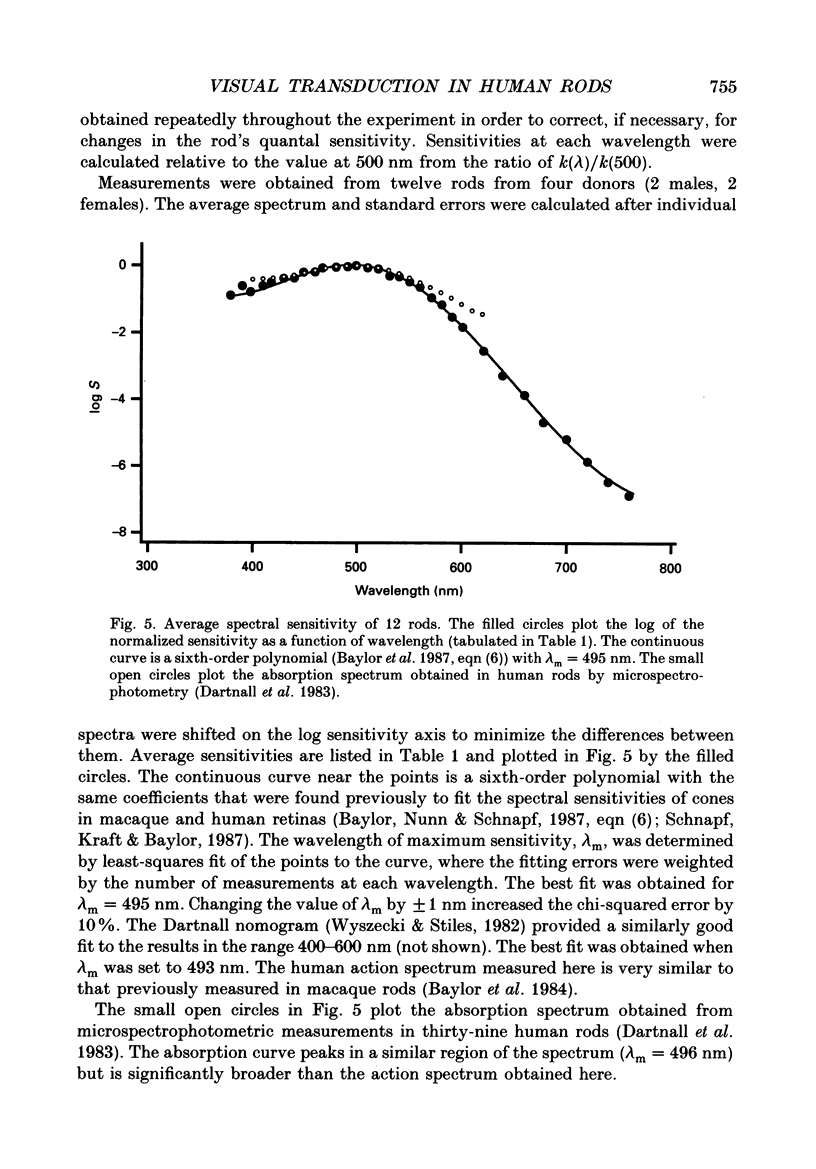
1. Photocurrents were recorded with suction electrodes from rod photoreceptors of seven humans. 2. Brief flashes of light evoked transient outward currents of up to 20 pA. With increasing light intensity the peak response amplitude increased along an exponential saturation function. A half-saturating peak response was evoked by approximately sixty-five photoisomerizations. 3. Responses to brief dim flashes rose to a peak in about 200 ms. The waveform was roughly like the impulse response of a series of four to five low-pass filters. 4. The rising phases of the responses to flashes of increasing strength were found to fit with a biochemical model of phototransduction with an 'effective delay time' and 'characteristic time' of about 2 and 800 ms, respectively. 5. Spectral sensitivities were obtained over a wavelength range from 380 to 760 nm. The action spectrum, which peaked at 495 nm, followed the template described for photoreceptors in the macaque retina. Variation between rods in the position of the spectrum on the wavelength axis was small. 6. The scotopic luminosity function derived from human psychophysical experiments was found to agree well with the measured rod action spectrum after adjustments were made for lens absorption and photopigment self-screening in the intact eye. 7. Responses to steps of light rose monotonically to a maintained level, showing little or no relaxation. Nevertheless, the relationship between light intensity and steady-state response amplitude was shallower than that expected from simple response saturation. This is consistent with an adaptation mechanism acting on a rapid time scale. 8. Flash sensitivity fell with increasing intensities of background light according to Weber's law. Sensitivity was reduced twofold by lights evoking about 120 photoisomerizations per second. Background lights decreased the time to peak and the integration time of the flash response by up to 20%.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelson E. H. Saturation and adaptation in the rod system. Vision Res. 1982;22(10):1299–1312. doi: 10.1016/0042-6989(82)90143-2. [DOI] [PubMed] [Google Scholar]
- Alpern M. A note on the action spectrum of human rod vision. Vision Res. 1987;27(9):1471–1480. doi: 10.1016/0042-6989(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Alpern M., Pugh E. N., Jr The density and photosensitivity of human rhodopsin in the living retina. J Physiol. 1974 Mar;237(2):341–370. doi: 10.1113/jphysiol.1974.sp010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987 Sep;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J., Mollon J. D. Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. J Physiol. 1980 Jan;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Dartnall H. J. Visual pigments of rods and cones in a human retina. J Physiol. 1980 Jan;298:501–511. doi: 10.1113/jphysiol.1980.sp013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Green D. G. The absence of spread of adaptation between rod photoreceptors in turtle retina. J Physiol. 1985 Dec;369:161–181. doi: 10.1113/jphysiol.1985.sp015894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson D. W., Cornwall M. C., MacNichol E. F., Jin J., Johnson R., Derguini F., Crouch R. K., Nakanishi K. Sensitization of bleached rod photoreceptors by 11-cis-locked analogues of retinal. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6823–6827. doi: 10.1073/pnas.87.17.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J., Bowmaker J. K., Mollon J. D. Human visual pigments: microspectrophotometric results from the eyes of seven persons. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):115–130. doi: 10.1098/rspb.1983.0091. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. C., Birch D. G. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci. 1990 Oct;5(4):379–387. doi: 10.1017/s0952523800000468. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R. Incorporation of chelator into guinea-pig rods shows that calcium mediates mammalian photoreceptor light adaptation. J Physiol. 1991 May;436:93–105. doi: 10.1113/jphysiol.1991.sp018541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Tamura T., Yau K. W. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991 Mar;97(3):413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 1990;30(12):1923–1948. doi: 10.1016/0042-6989(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Pugh E. N. Rhodopsin flash photolysis in man. J Physiol. 1975 Jun;248(2):393–412. doi: 10.1113/jphysiol.1975.sp010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf J. L., Kraft T. W., Baylor D. A. Spectral sensitivity of human cone photoreceptors. 1987 Jan 29-Feb 4Nature. 325(6103):439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., Nunn B. J., Meister M., Baylor D. A. Visual transduction in cones of the monkey Macaca fascicularis. J Physiol. 1990 Aug;427:681–713. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol. 1991 Jul;98(1):95–130. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Nakatani K., Yau K. W. Light adaptation in cat retinal rods. Science. 1989 Aug 18;245(4919):755–758. doi: 10.1126/science.2772634. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. Human rhodopsin. Science. 1958 Jan 31;127(3292):222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- Winderickx J., Lindsey D. T., Sanocki E., Teller D. Y., Motulsky A. G., Deeb S. S. Polymorphism in red photopigment underlies variation in colour matching. Nature. 1992 Apr 2;356(6368):431–433. doi: 10.1038/356431a0. [DOI] [PubMed] [Google Scholar]



