Abstract
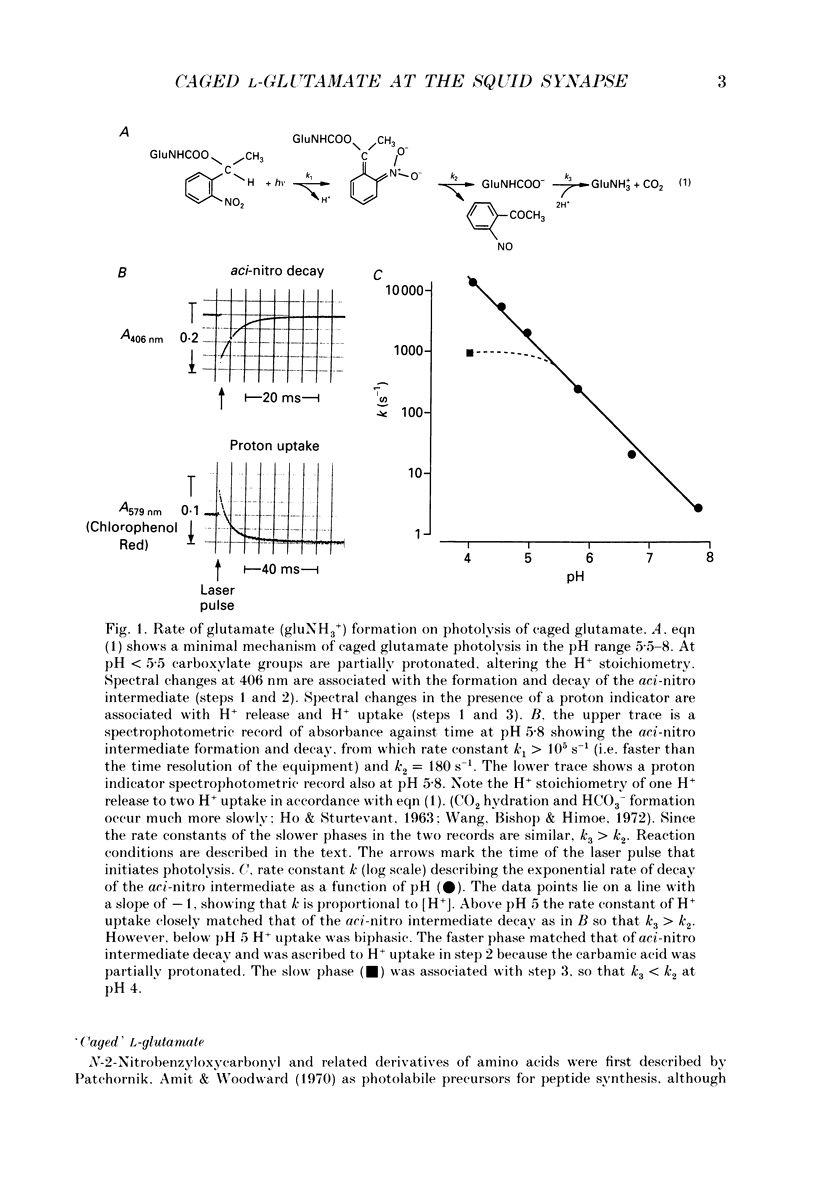
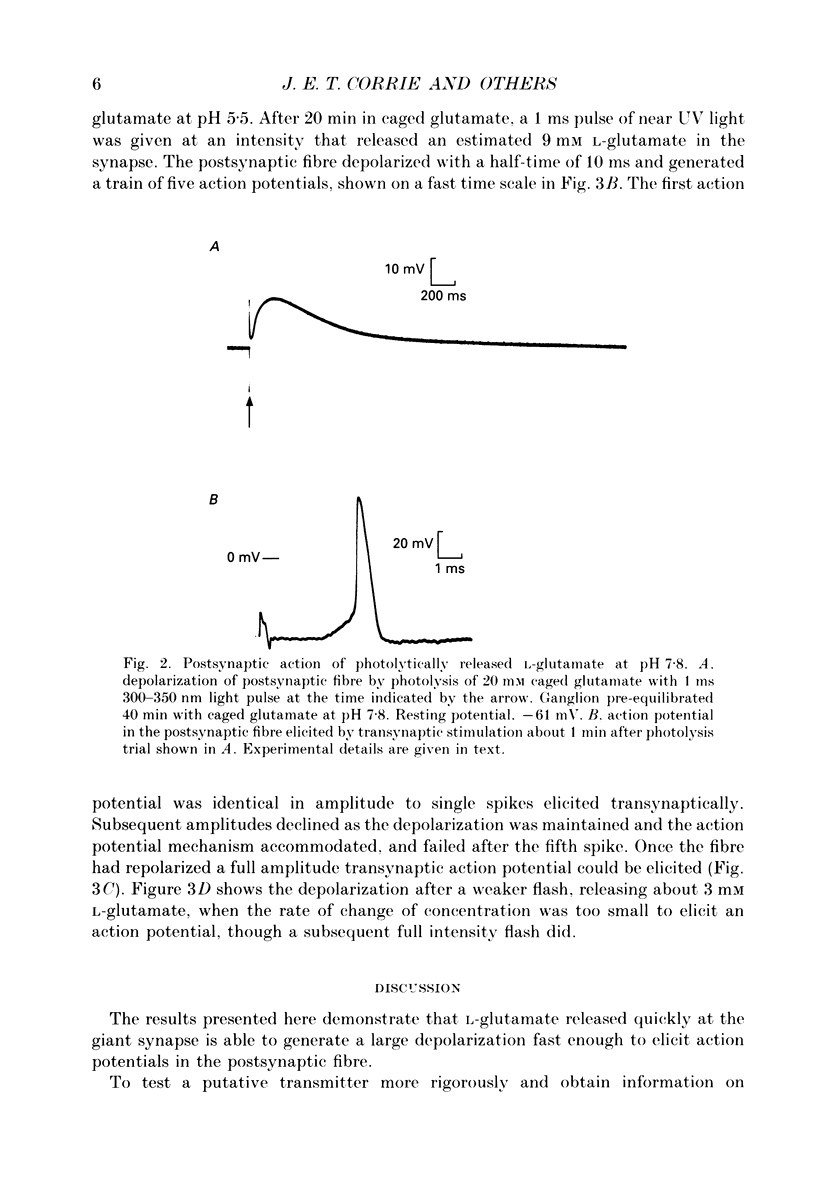
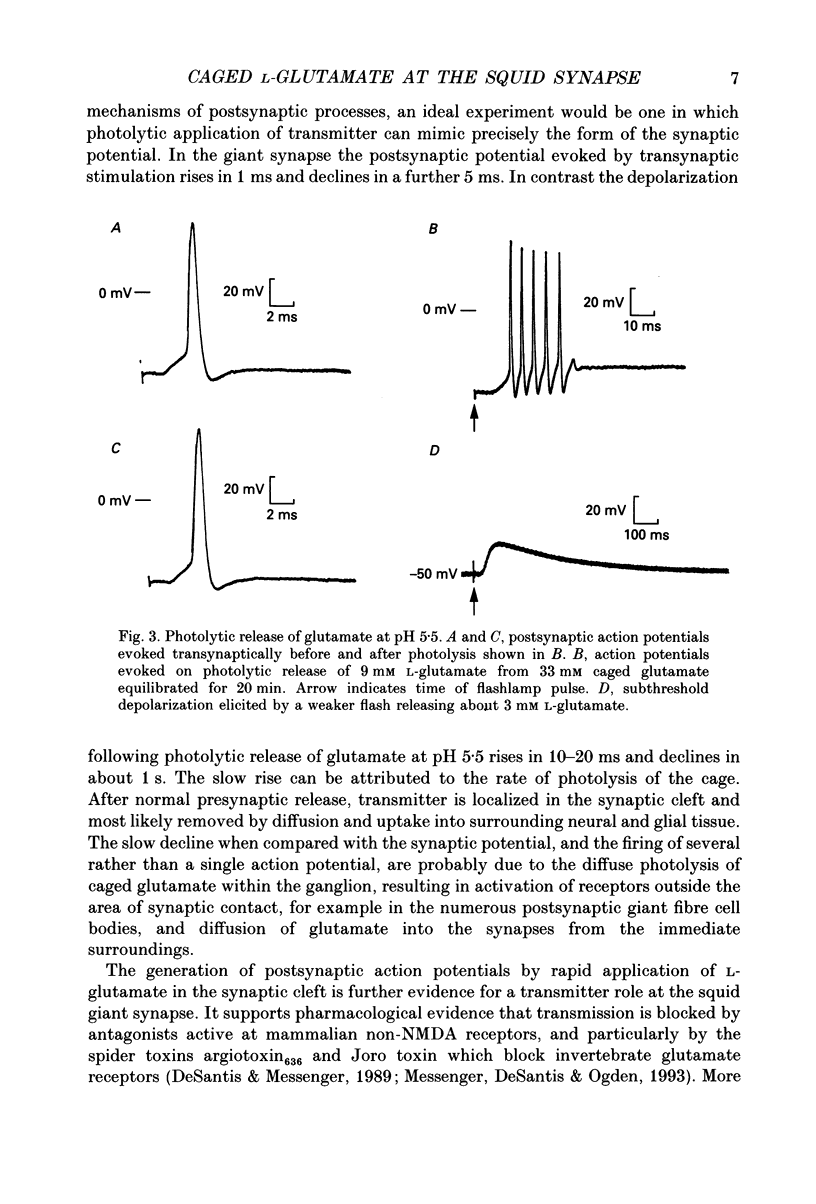
1. Pharmacological evidence suggests L-glutamate is a strong candidate as a transmitter at the giant synapse of the squid. Postsynaptic activation at the giant synapse cannot be effected by conventional application of putative neurotransmitters by iontophoresis or perfusion, apparently because the complex structure of the synapse prevents a sufficiently rapid change in concentration at the postsynaptic membrane. Flash photolytic release of L-glutamate from a pharmacologically inert 'caged' L-glutamate pre-equilibrated in the stellate ganglion of Alloteuthis or Loligo was used to determine whether L-glutamate can produce postsynaptic activation when released rapidly in the synaptic clefts. 2. The preparation, reaction mechanism and properties of the caged L-glutamate, N-1-(2-nitrophenyl)ethoxycarbonyl-L-glutamate, are described. The product quantum yield on photolysis was 0.65 (+/- 0.05). On flash photolysis glutamate release followed a single exponential time-course in the pH range 5.5-7.8. The rate constant was proportional to [H+] and was 93 s-1 at pH 5.5 and 16 degrees C in artificial sea water (ionic strength, I = 0.68 M). 3. At pH 7.8 flash photolysis of caged glutamate pre-equilibrated in the synapse caused only a slow depolarization. A second photolytic release of L-glutamate or transsynaptic activation produced no further depolarization, suggesting desensitization and inactivation of postsynaptic mechanisms by the initial pulse of L-glutamate. 4. Synaptic transmission in the giant synapse was normal at pH 5.5. Flash photolysis at pH 5.5 caused rapid production of L-glutamate within the synaptic cleft and a fast postsynaptic depolarization which generated postsynaptic action potentials.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gillespie J. I. The actions of L-glutamate at the postsynaptic membrane of the squid giant synapse. J Exp Biol. 1988 Nov;140:535–548. doi: 10.1242/jeb.140.1.535. [DOI] [PubMed] [Google Scholar]
- De Santis A., Messenger J. B. New evidence that L-glutamate is a transmitter at the squid giant synapse. Q J Exp Physiol. 1989 Mar;74(2):219–222. doi: 10.1113/expphysiol.1989.sp003259. [DOI] [PubMed] [Google Scholar]
- HO C., STURTEVANT J. M. THE KINETICS OF THE HYDRATION OF CARBON DIOXIDE AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3499–3501. [PubMed] [Google Scholar]
- Landò L., Zucker R. S. "Caged calcium" in Aplysia pacemaker neurons. Characterization of calcium-activated potassium and nonspecific cation currents. J Gen Physiol. 1989 Jun;93(6):1017–1060. doi: 10.1085/jgp.93.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Joyner R. W., Nicholson C. Equilibrium potential for the postsynaptic response in the squid giant synapse. J Gen Physiol. 1974 Nov;64(5):519–535. doi: 10.1085/jgp.64.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglion of the squid. J Physiol. 1967 Sep;192(2):379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter action in the giant synapse of the squid. Nature. 1969 Sep 20;223(5212):1284–1286. doi: 10.1038/2231284a0. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Capiod T., Walker J. W., Trentham D. R. Kinetics of the conductance evoked by noradrenaline, inositol trisphosphate or Ca2+ in guinea-pig isolated hepatocytes. J Physiol. 1990 Mar;422:585–602. doi: 10.1113/jphysiol.1990.sp018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Reid G. P., Trentham D. R. Synthesis and properties of caged nucleotides. Methods Enzymol. 1989;172:288–301. doi: 10.1016/s0076-6879(89)72019-x. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Bishop S. H., Himoe A. Detection of carbamate as a product of the carbamate kinase-catalyzed reaction by stopped flow spectrophotometry. J Biol Chem. 1972 Jul 25;247(14):4437–4440. [PubMed] [Google Scholar]
- Young J. Z. The giant fibre synapse of Loligo. Brain Res. 1973 Jul 27;57(2):457–460. doi: 10.1016/0006-8993(73)90149-2. [DOI] [PubMed] [Google Scholar]
- de Santis A., Eusebi F., Miledi R. Kainic acid and synaptic transmission in the stellate ganglion of the squid. Proc R Soc Lond B Biol Sci. 1978 Sep 29;202(1149):527–532. doi: 10.1098/rspb.1978.0084. [DOI] [PubMed] [Google Scholar]


