Abstract
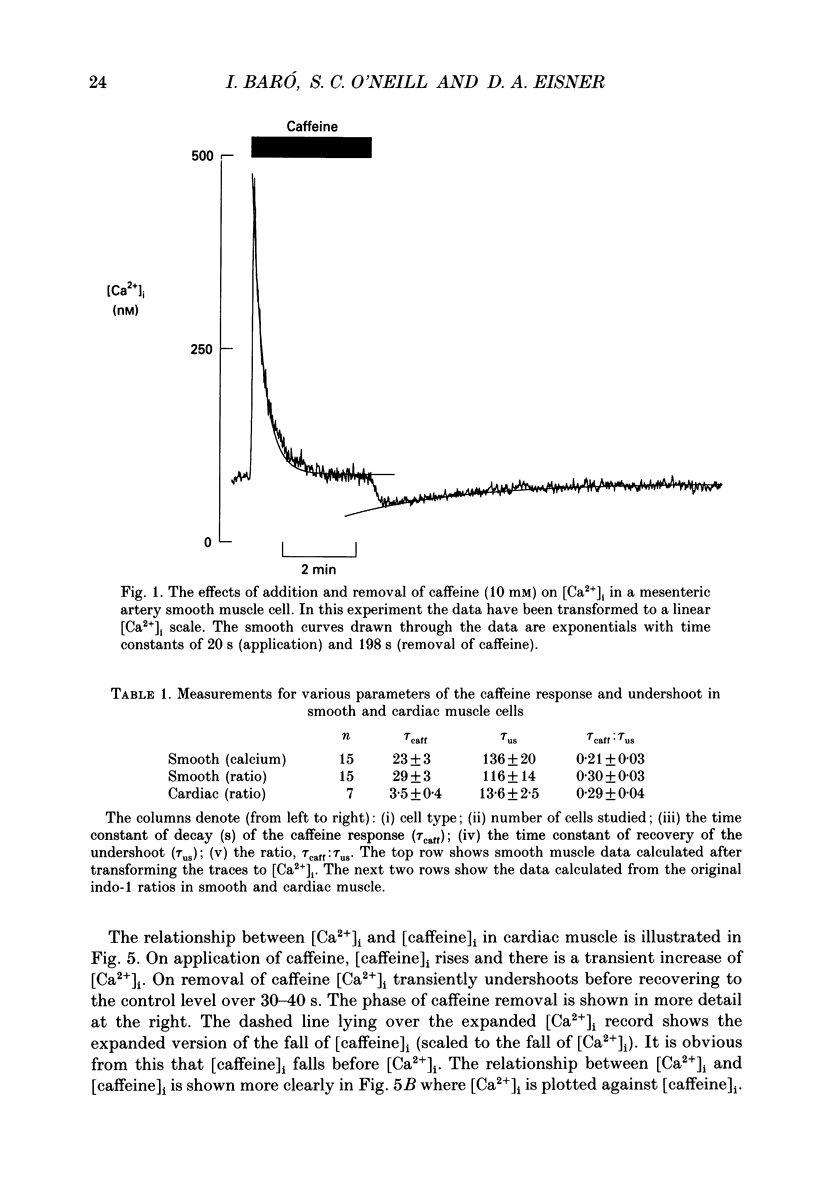
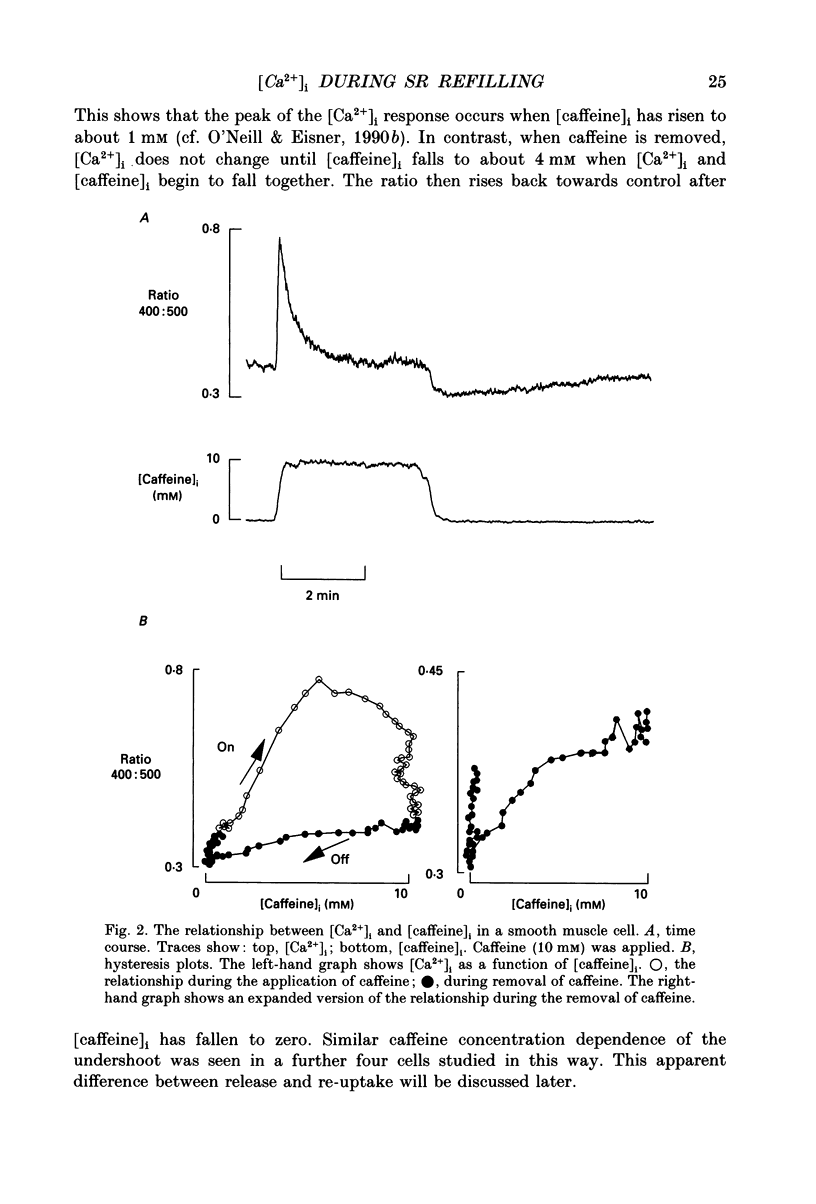
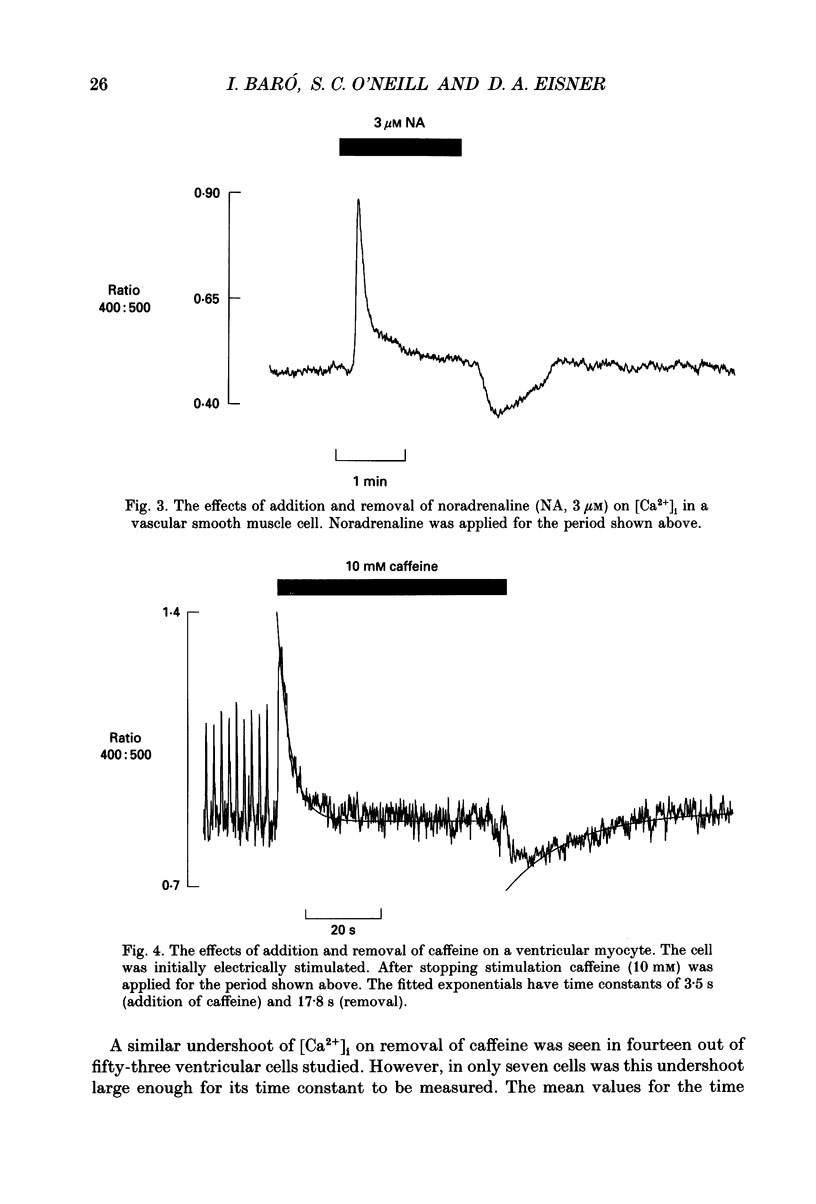
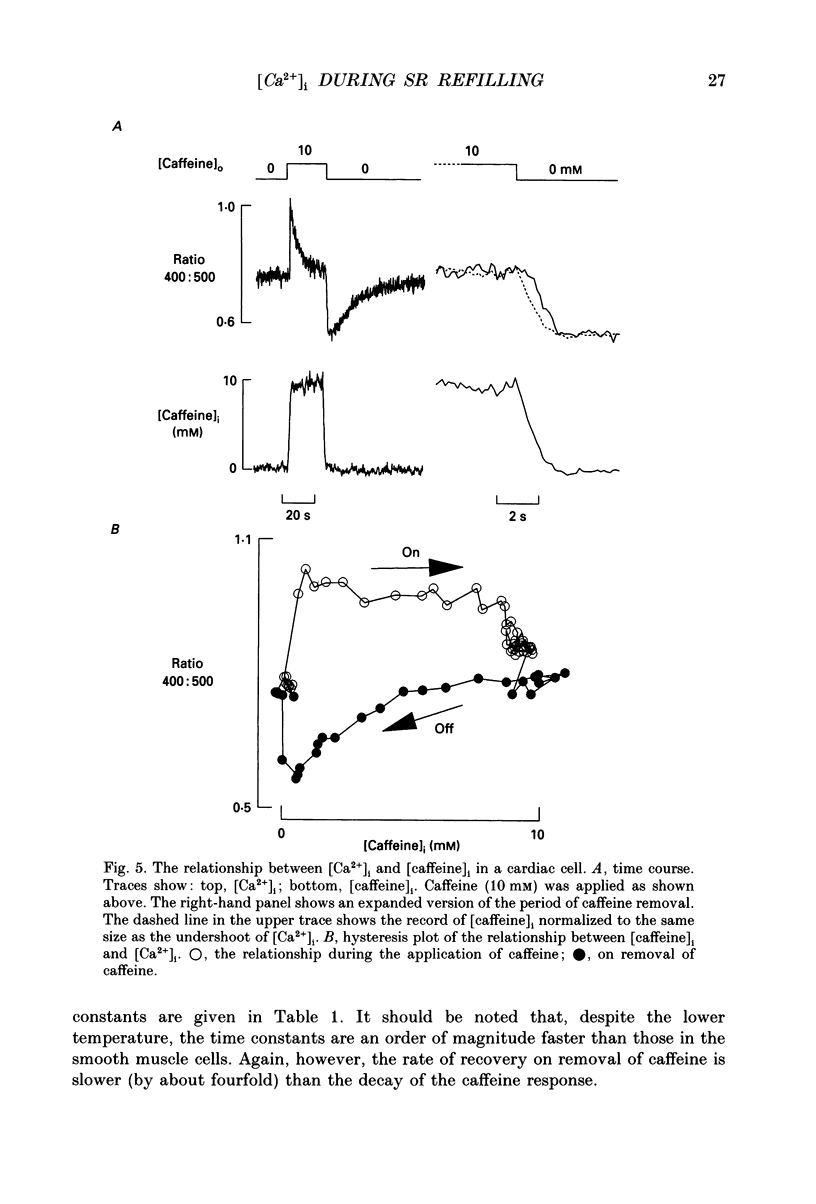
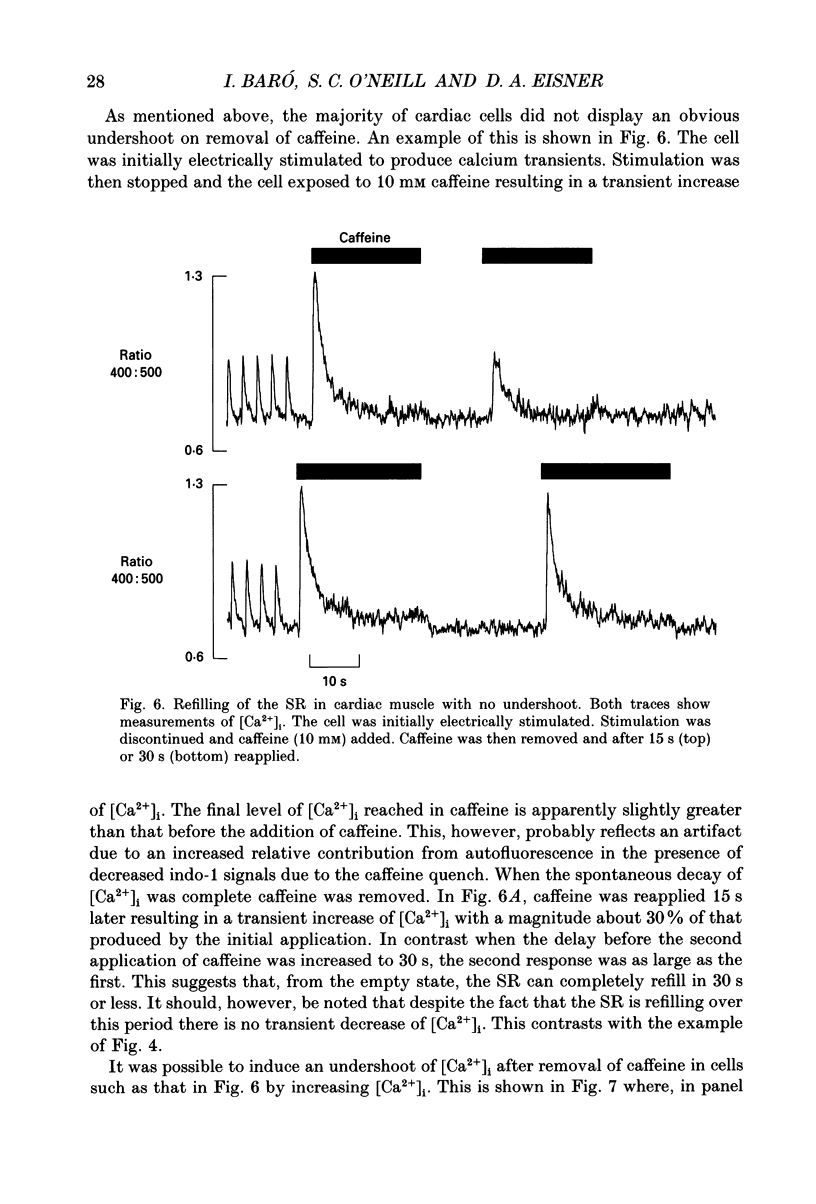
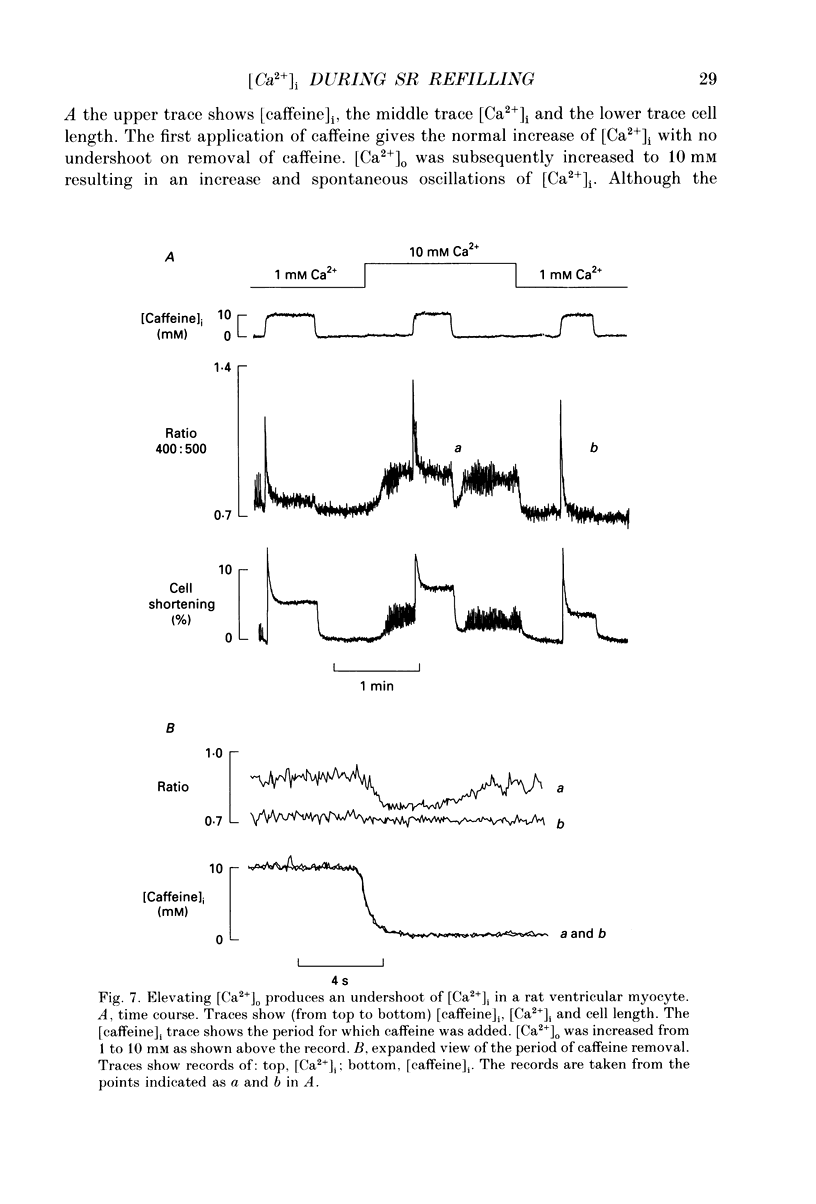
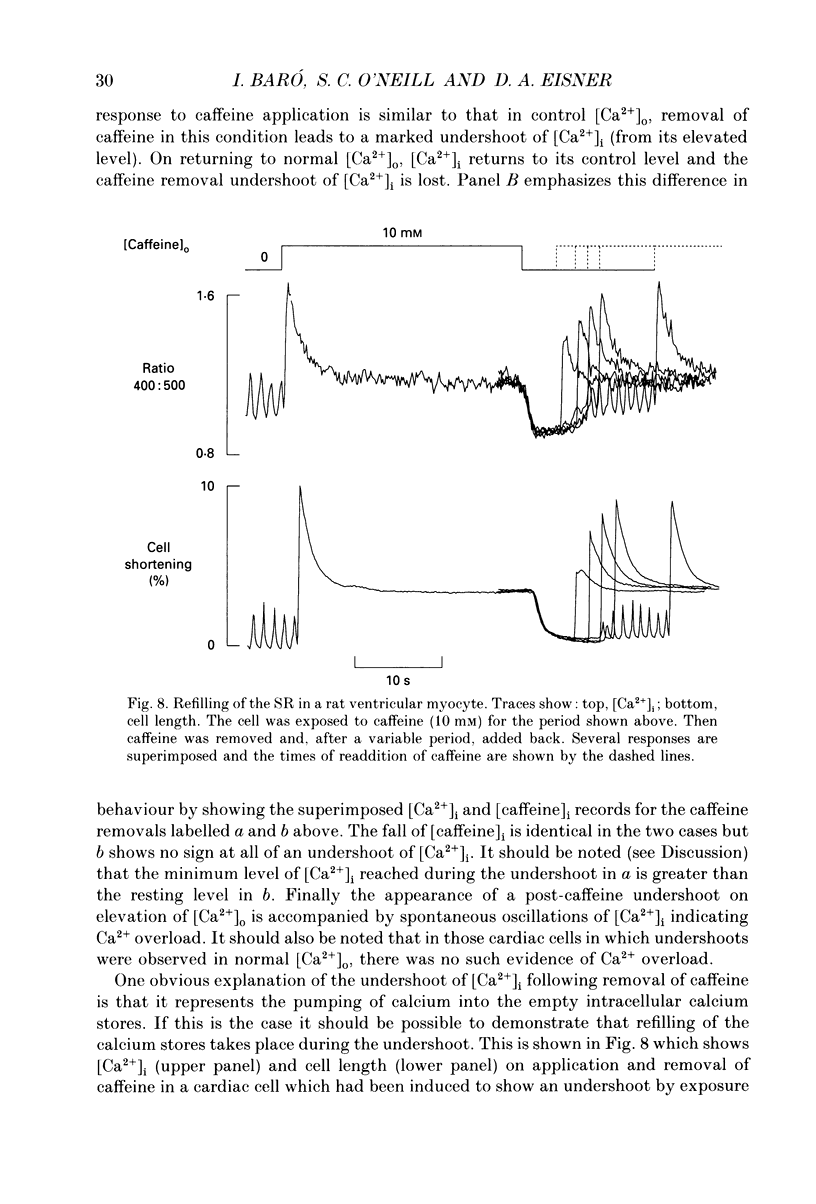
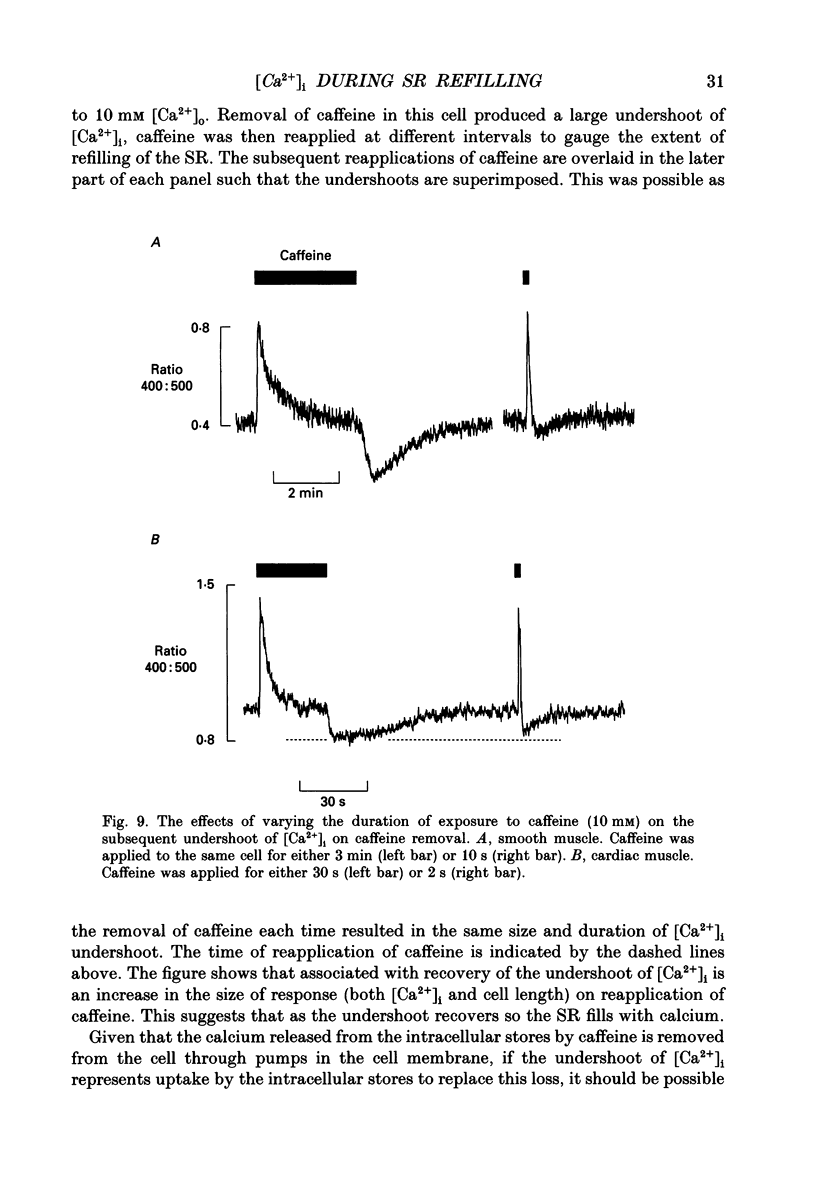
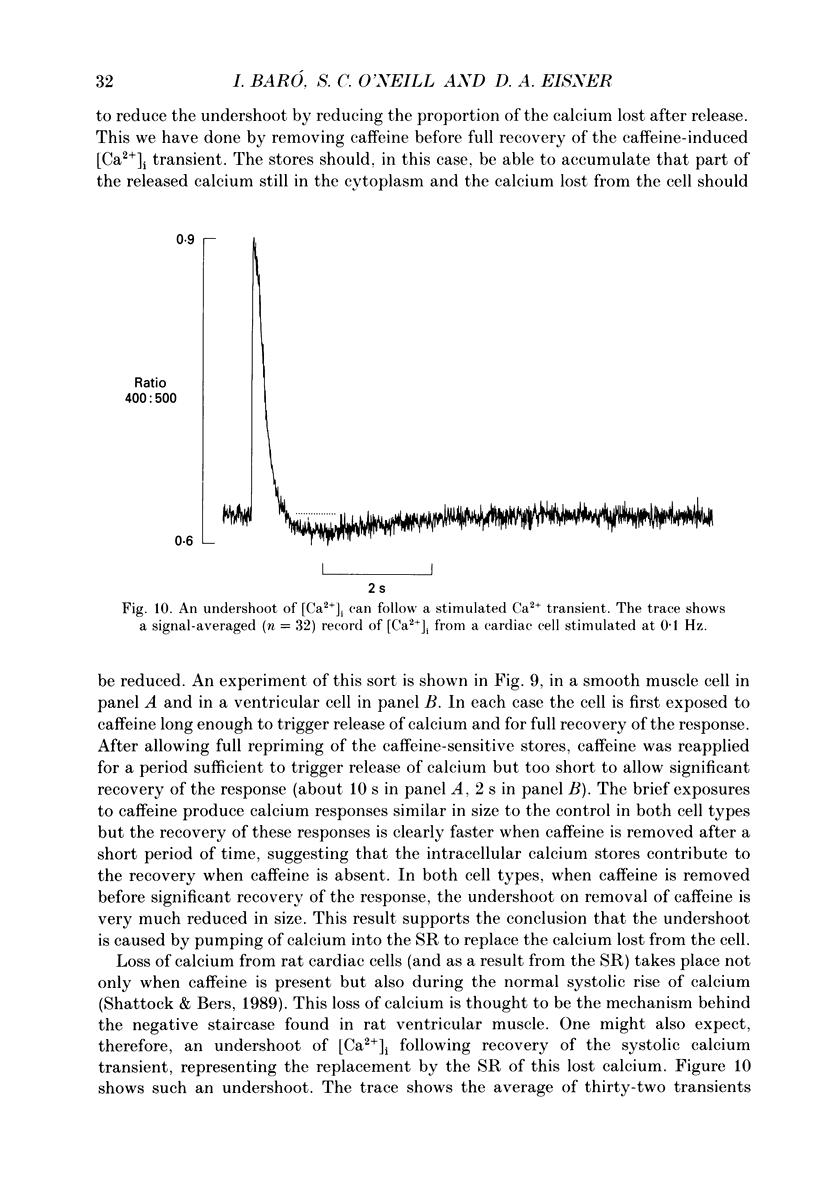
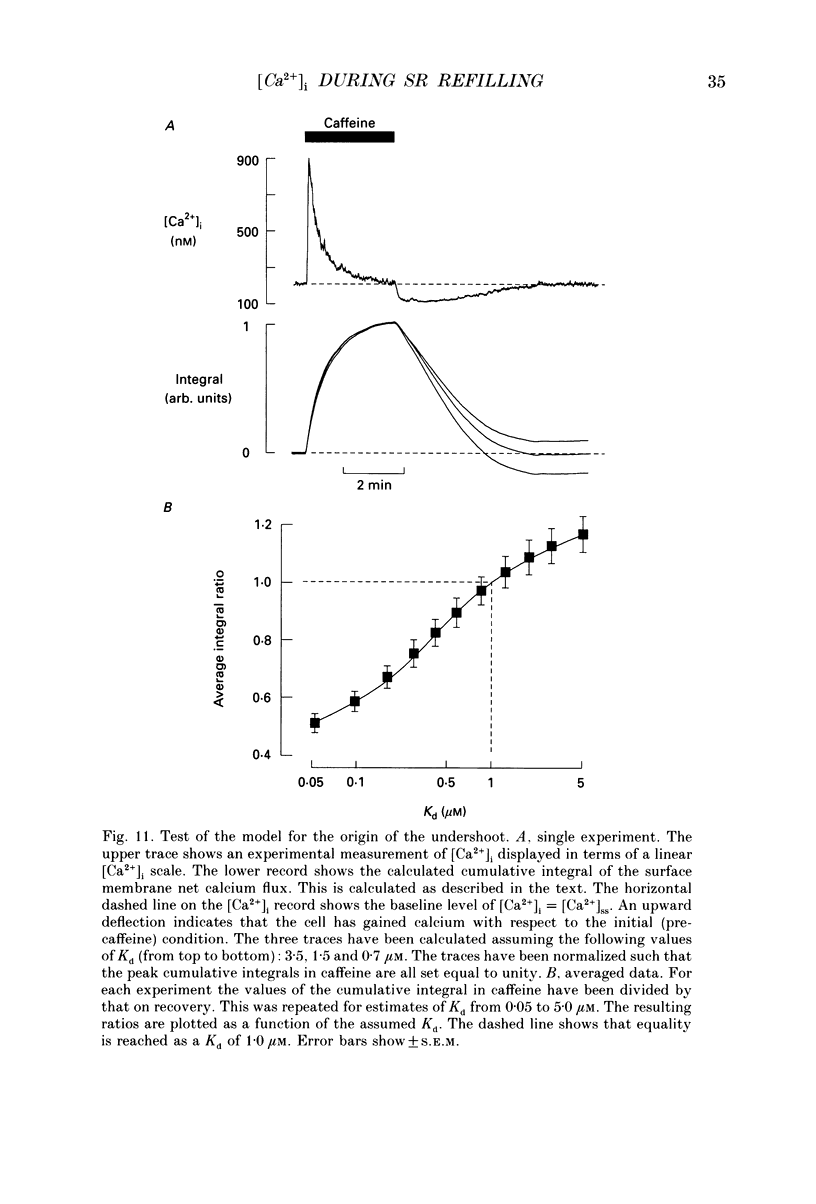
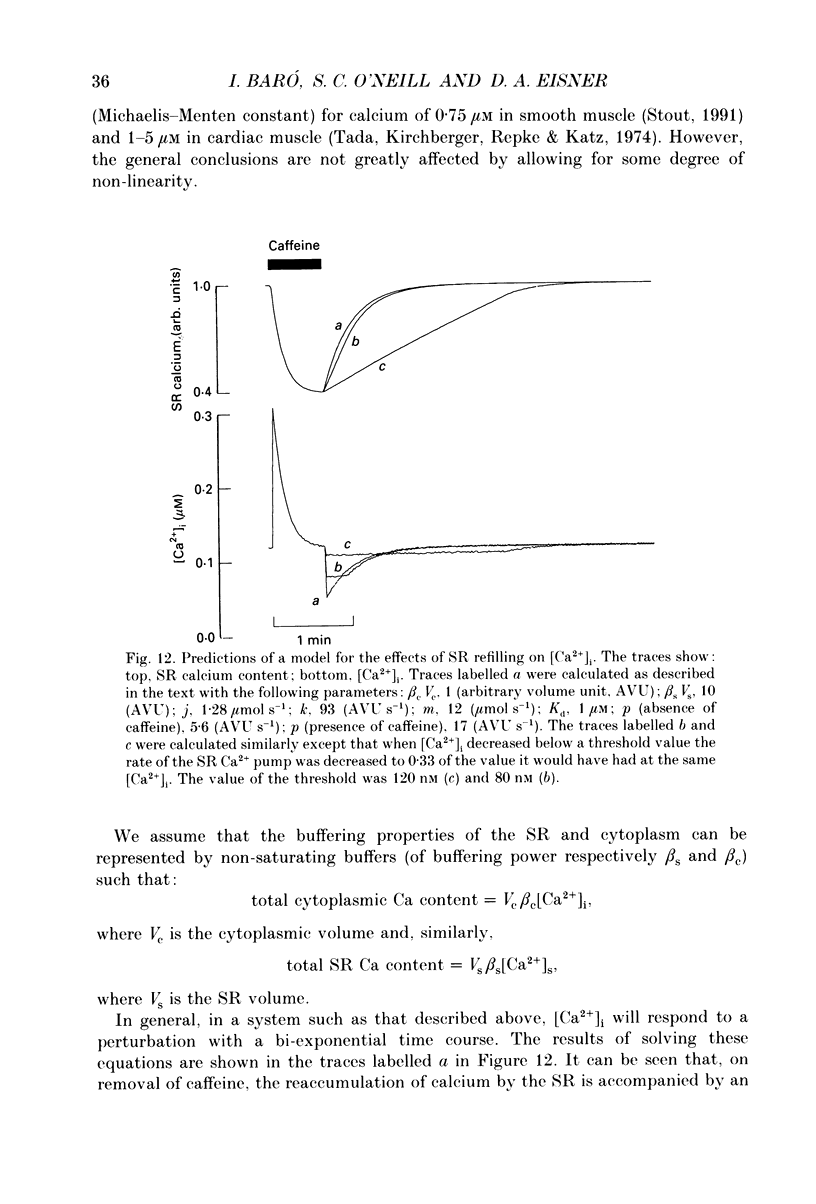
1. Intracellular calcium concentration ([Ca2+]i) was measured in single myocytes isolated from either the cardiac ventricle or the mesenteric artery of the rat. 2. In both cardiac and smooth muscle, the application of caffeine produced an increase of [Ca2+]i which spontaneously decayed back to resting levels. In vascular smooth muscle cells, removal of caffeine produced a transient fall of [Ca2+]i to below the resting level. [Ca2+]i then returned to control levels. A transient undershoot of [Ca2+]i on removal of caffeine was also sometimes seen in cardiac cells. When the undershoot was absent in cardiac cells it could be induced by elevating [Ca2+]o. 3. In vascular smooth muscle cells noradrenaline increased [Ca2+]i and an undershoot of [Ca2+]i could be produced by its removal. In cardiac cells a small undershoot could sometimes be seen following the systolic Ca2+ transient produced by electrical stimulation. 4. In both cardiac and vascular cells the time constant of decay of the caffeine response (tau caff) was less than that of the recovery from the undershoot (tau us). On average the ratio tau us:tau caff was about 5 in smooth muscle. In cardiac cells the recovery of the undershoot was also considerably slower than that of the caffeine response. 5. If caffeine was removed before the rise of [Ca2+]i had fully decayed spontaneously then the magnitude of the undershoot was reduced. 6. It is suggested that the undershoot of [Ca2+]i on removal of caffeine results from refilling of the SR decreasing [Ca2+]i. The data from vascular cells can be fitted by this model if the dissociation constant, Kd, of the surface membrane Ca2+ pump for [Ca2+]i is about 1 microM. 7. Using the model, it is concluded from the ratio of the time constants shown above that the caffeine releasable content of the sarcoplasmic reticulum constitutes about 80% of total cellular calcium in both cardiac and smooth muscle.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Baró I., Eisner D. A. The effects of thapsigargin on [Ca2+]i in isolated rat mesenteric artery vascular smooth muscle cells. Pflugers Arch. 1992 Jan;420(1):115–117. doi: 10.1007/BF00378652. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Caffeine-induced release and reuptake of Ca2+ by Ca2+ stores in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:99–117. doi: 10.1113/jphysiol.1992.sp019408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Highsmith S., Bloebaum P., Snowdowne K. W. Sarcoplasmic reticulum interacts with the Ca(2+) indicator precursor fura-2-am. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1153–1162. doi: 10.1016/s0006-291x(86)80403-x. [DOI] [PubMed] [Google Scholar]
- O'Neill S. C., Donoso P., Eisner D. A. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am J Physiol. 1989 Feb;256(2 Pt 2):H328–H333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- Shattock M. J., Bers D. M. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989 Apr;256(4 Pt 1):C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Kort A. A., Bhatnagar G. M., Lakatta E. G. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J Gen Physiol. 1983 Jul;82(1):119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout M. A. Calcium transport by sarcoplasmic reticulum of vascular smooth muscle: I. MgATP-dependent and MgATP-independent calcium uptake. J Cell Physiol. 1991 Dec;149(3):383–395. doi: 10.1002/jcp.1041490305. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]


