Abstract
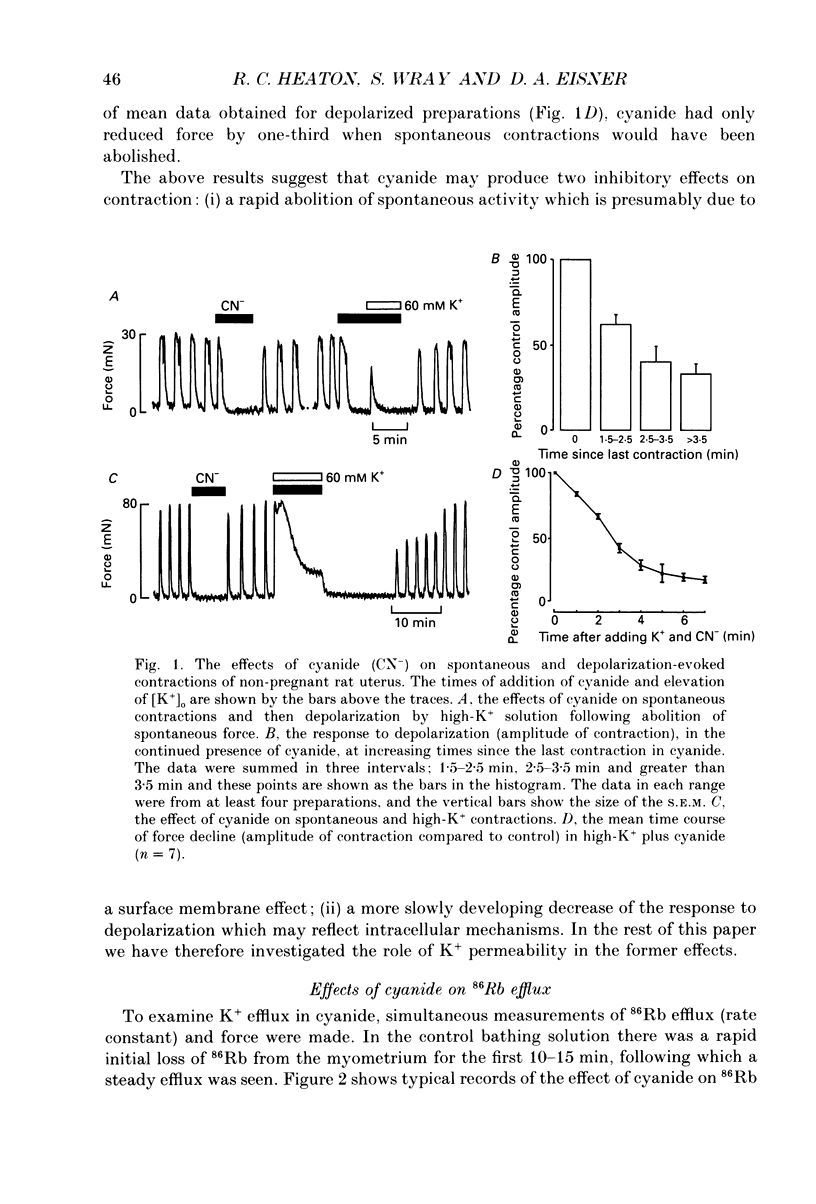
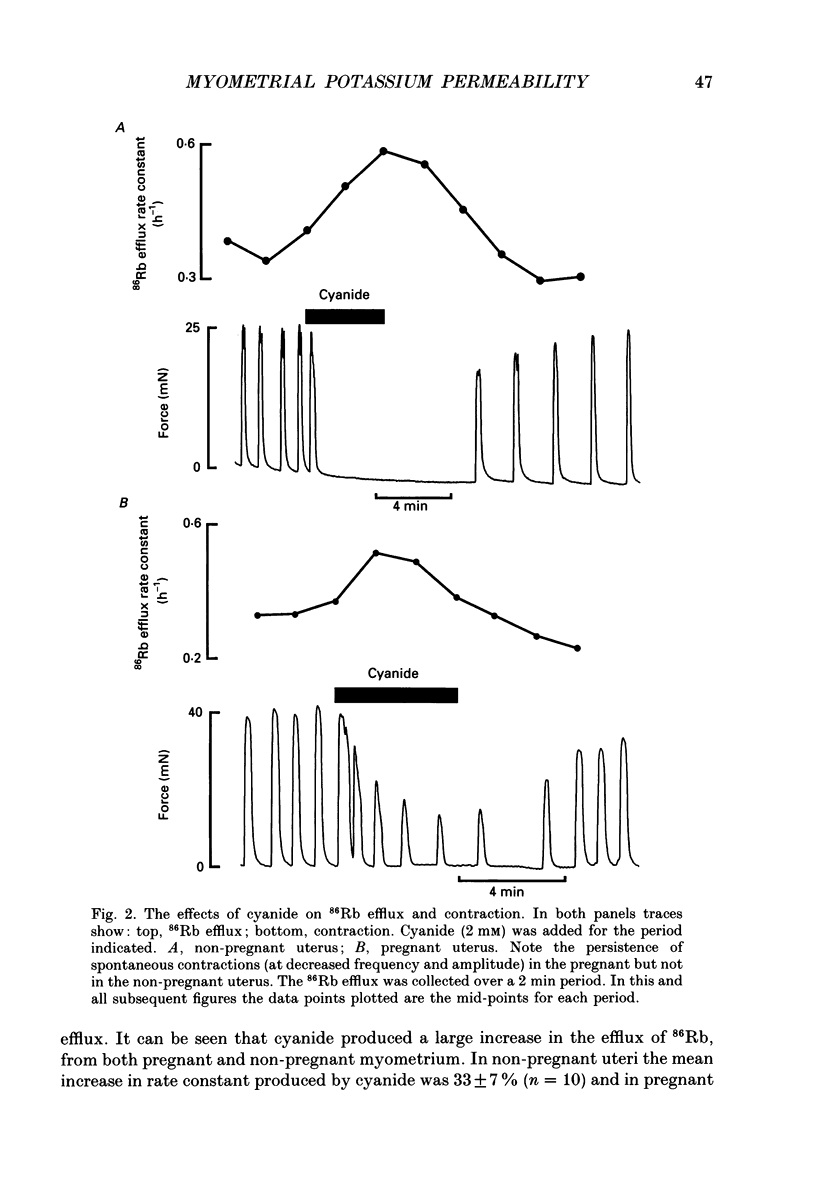
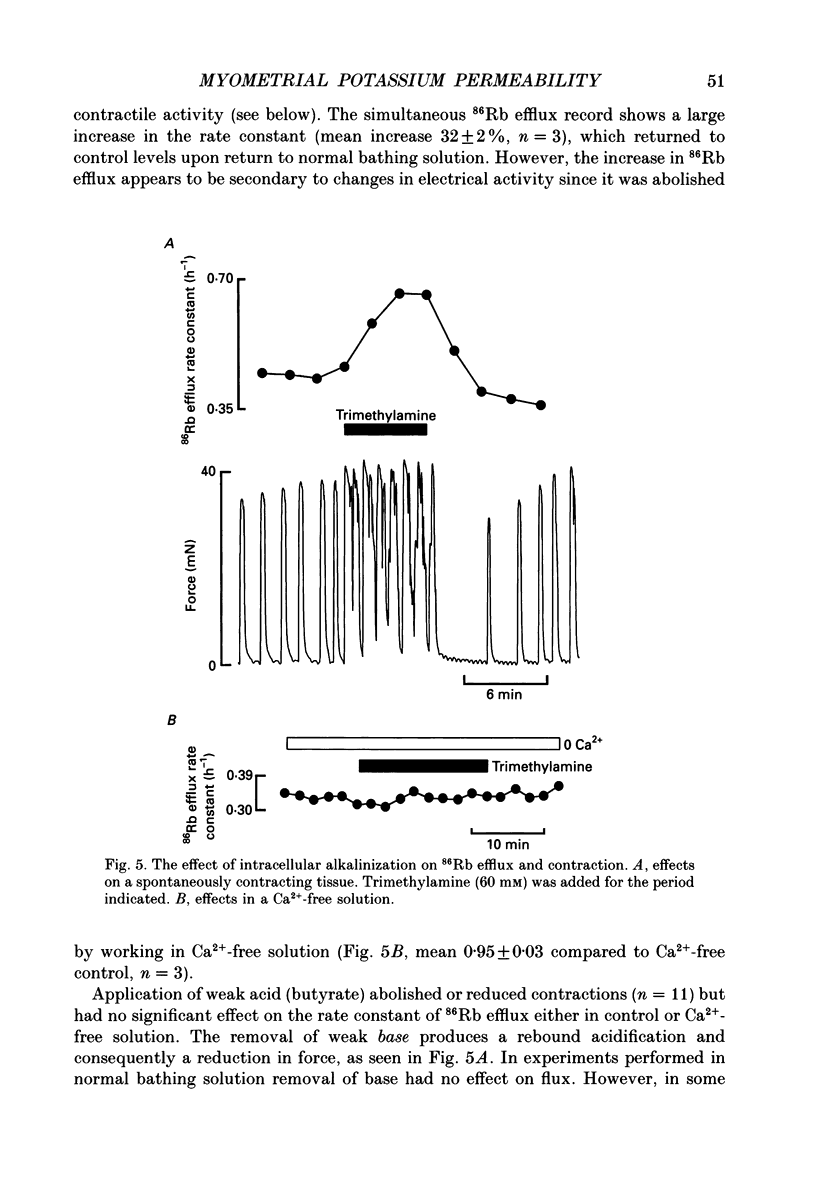
1. We have investigated the role of changes of potassium efflux in the inhibition of uterine force produced by cyanide. K+ efflux (86Rb) was measured from pregnant and non-pregnant rat myometrial strips during metabolic inhibition with cyanide and following manoeuvres to displace intracellular pH (pHi). 2. Cyanide greatly reduced or abolished spontaneous contractions. If the membrane was depolarized directly at this stage (by elevating external K+) then contraction redeveloped. This suggests that the initial depression of force is due to a failure of membrane excitation. 3. Cyanide reversibly increased 86Rb efflux (30-35%) in both pregnant and nonpregnant uteri and contraction was reduced. The increase in 86Rb efflux with cyanide was not secondary to changes of membrane potential as it also occurred in both high-K+ and Ca(2+)-free solutions. 4. Glibenclamide (20 microM), an antagonist of K+ATP channels, reduced the cyanide-evoked increase of 86Rb efflux by about 50%. The glibenclamide-insensitive component of efflux persisted in a Ca(2+)-free solution. Despite its action on 86Rb efflux, glibenclamide did not restore contraction. 5. Intracellular pH falls during metabolic inhibition. We therefore investigated whether reducing pHi (in the absence of cyanide) had an effect on 86Rb efflux. Application of the weak acid butyrate (60 mM, at constant external pH, 7.4) had no significant effect on 86Rb efflux. Thus it is unlikely that the acidification in hypoxia contributes to the increased K+ efflux. 6. Intracellular alkalinization produced by the weak base trimethylamine (60 mM) increased the frequency of uterine contraction and the 86Rb efflux. However, there was no effect on the 86Rb efflux in a Ca(2+)-free solution. The increased efflux is therefore presumably a consequence of the increased frequency. 7. It is concluded that metabolic inhibition produced by cyanide, produces an increase in K+ efflux from the myometrium. Part of this efflux is glibenclamide sensitive. This increased K+ efflux will lead to hyperpolarization of the myometrial membrane and thus decrease excitation. Thus reduced surface membrane excitability will contribute to the fall of force in hypoxia; specifically it may cause the initial loss of spontaneous contractions in the uterus.
Full text
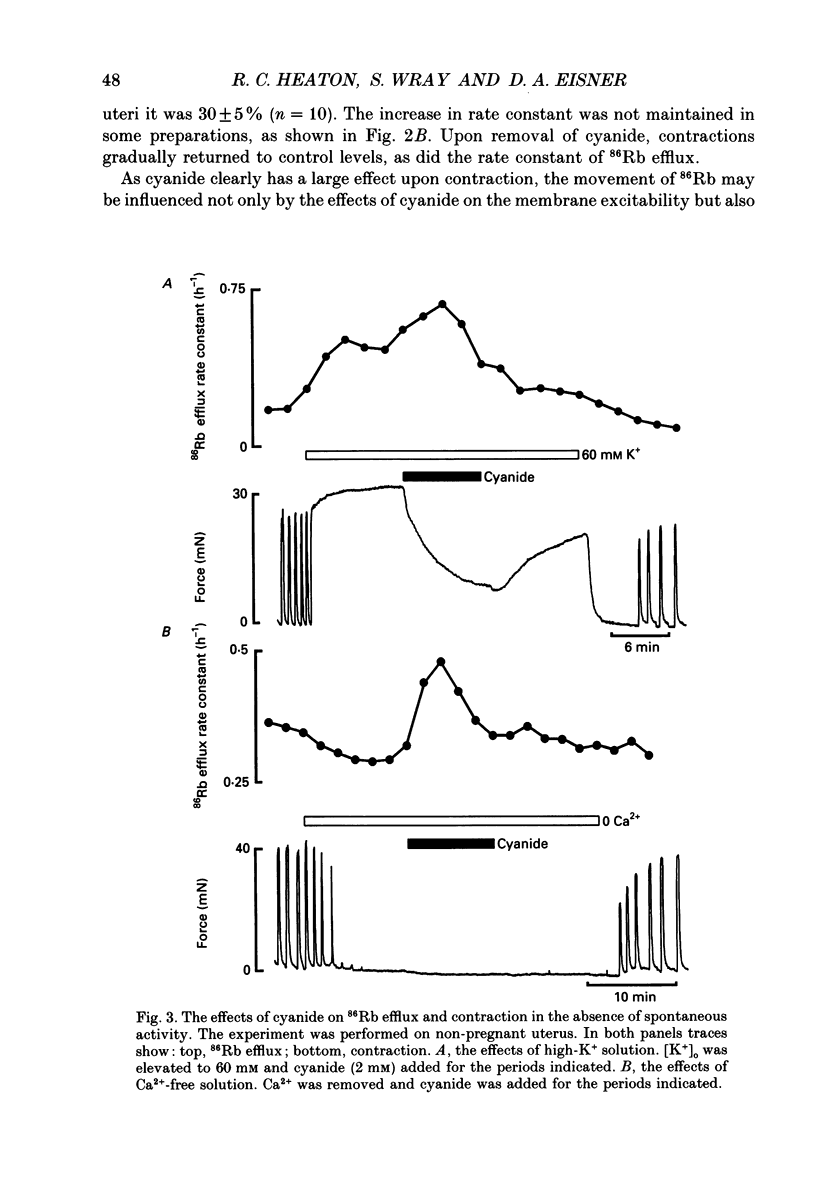
PDF



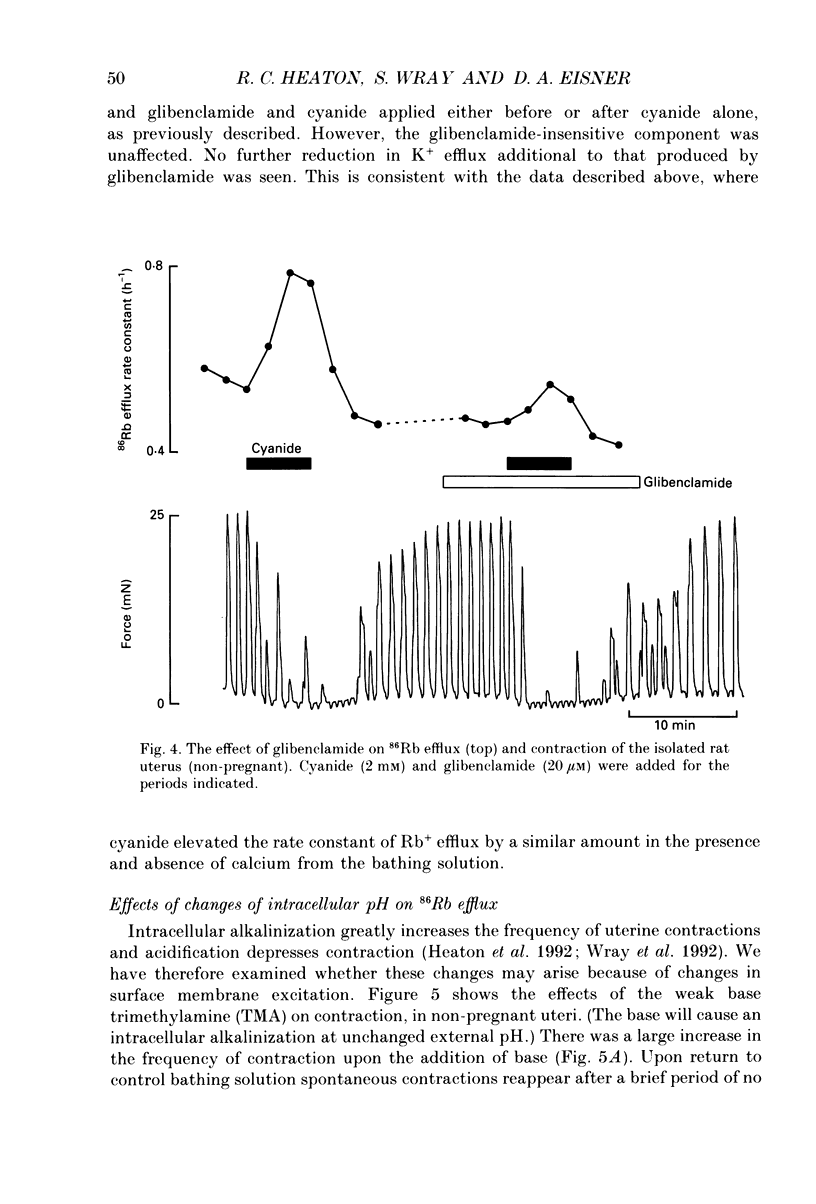





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Foster C. D., Brading A. F., Parekh A. B. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990 Apr;99(4):779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiss F. C., Jr Effect of labor on uterine blood flow. Observations on gravid ewes. Am J Obstet Gynecol. 1965 Dec 1;93(7):917–923. doi: 10.1016/0002-9378(65)90150-x. [DOI] [PubMed] [Google Scholar]
- Heaton R. C., Taggart M. J., Wray S. The effects of intracellular and extracellular alkalinization on contractions of the isolated rat uterus. Pflugers Arch. 1992 Oct;422(1):24–30. doi: 10.1007/BF00381509. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M., Amédée T., Edwards D., Mironneau J., Savineau J. P., Small R. C., Weston A. H. The relaxant action of BRL 34915 in rat uterus. Br J Pharmacol. 1987 Aug;91(4):803–813. doi: 10.1111/j.1476-5381.1987.tb11279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol. 1981 Jul;316:33–46. doi: 10.1113/jphysiol.1981.sp013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984 May;16(5):389–394. doi: 10.1016/s0022-2828(84)80610-0. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G., Smith G. L. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989 Jun;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov S. L., Lux H. D. Cytoplasmic alkalinization increases high-threshold calcium current in chick dorsal root ganglion neurones. Pflugers Arch. 1991 Sep;419(2):138–143. doi: 10.1007/BF00372999. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- O'Driscoll K., Foley M., MacDonald D. Active management of labor as an alternative to cesarean section for dystocia. Obstet Gynecol. 1984 Apr;63(4):485–490. [PubMed] [Google Scholar]
- Piper I., Minshall E., Downing S. J., Hollingsworth M., Sadraei H. Effects of several potassium channel openers and glibenclamide on the uterus of the rat. Br J Pharmacol. 1990 Dec;101(4):901–907. doi: 10.1111/j.1476-5381.1990.tb14178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve H. L., Vaughan P. F., Peers C. Glibenclamide inhibits a voltage-gated K+ current in the human neuroblastoma cell line SH-SY5Y. Neurosci Lett. 1992 Jan 20;135(1):37–40. doi: 10.1016/0304-3940(92)90130-y. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Toro L., Stefani E., Erulkar S. Hormonal regulation of potassium currents in single myometrial cells. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2892–2895. doi: 10.1073/pnas.87.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh N., Lamp S. T., Weiss J. N. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ Res. 1991 Sep;69(3):623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Venkatesh N., Lamp S. T. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992 Feb;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Duggins K., Iles R., Nyman L., Osman V. The effects of metabolic inhibition and acidification on force production in the rat uterus. Exp Physiol. 1992 Mar;77(2):307–319. doi: 10.1113/expphysiol.1992.sp003590. [DOI] [PubMed] [Google Scholar]
- Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. J Physiol. 1990 Apr;423:411–423. doi: 10.1113/jphysiol.1990.sp018030. [DOI] [PMC free article] [PubMed] [Google Scholar]


