Abstract
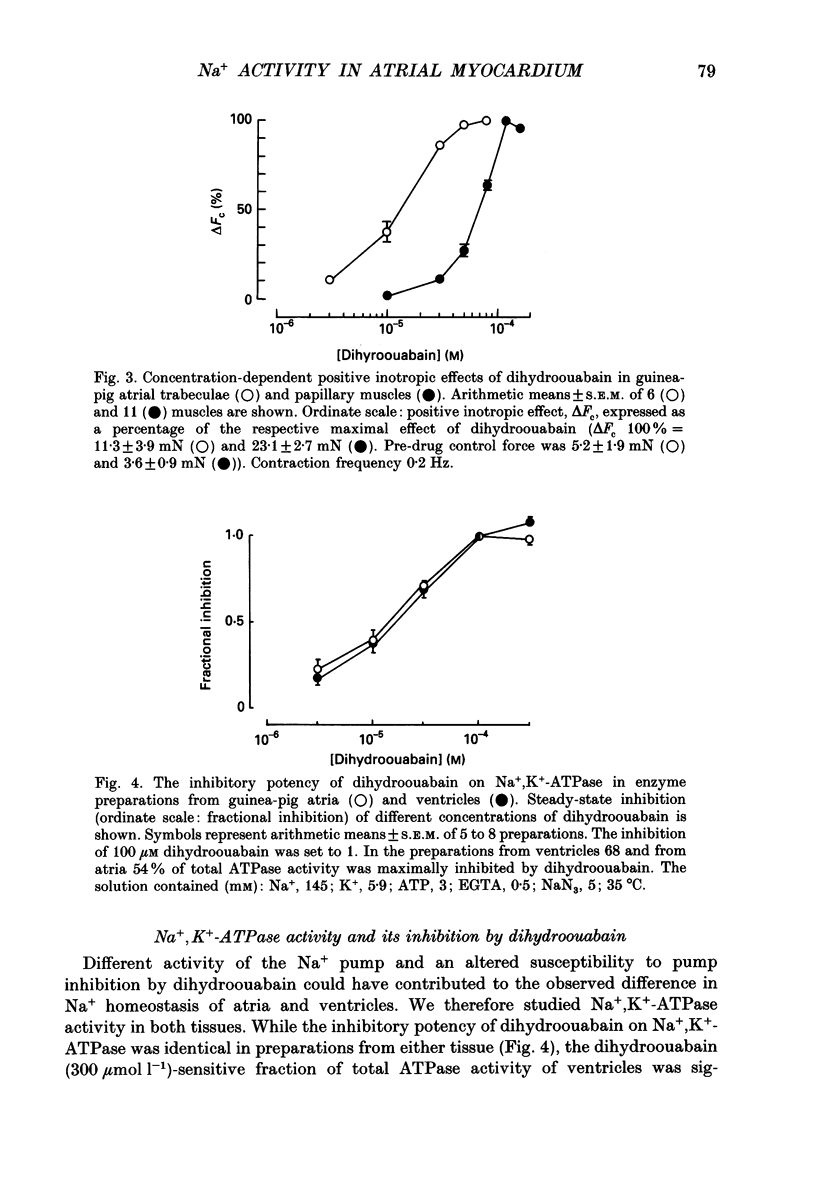
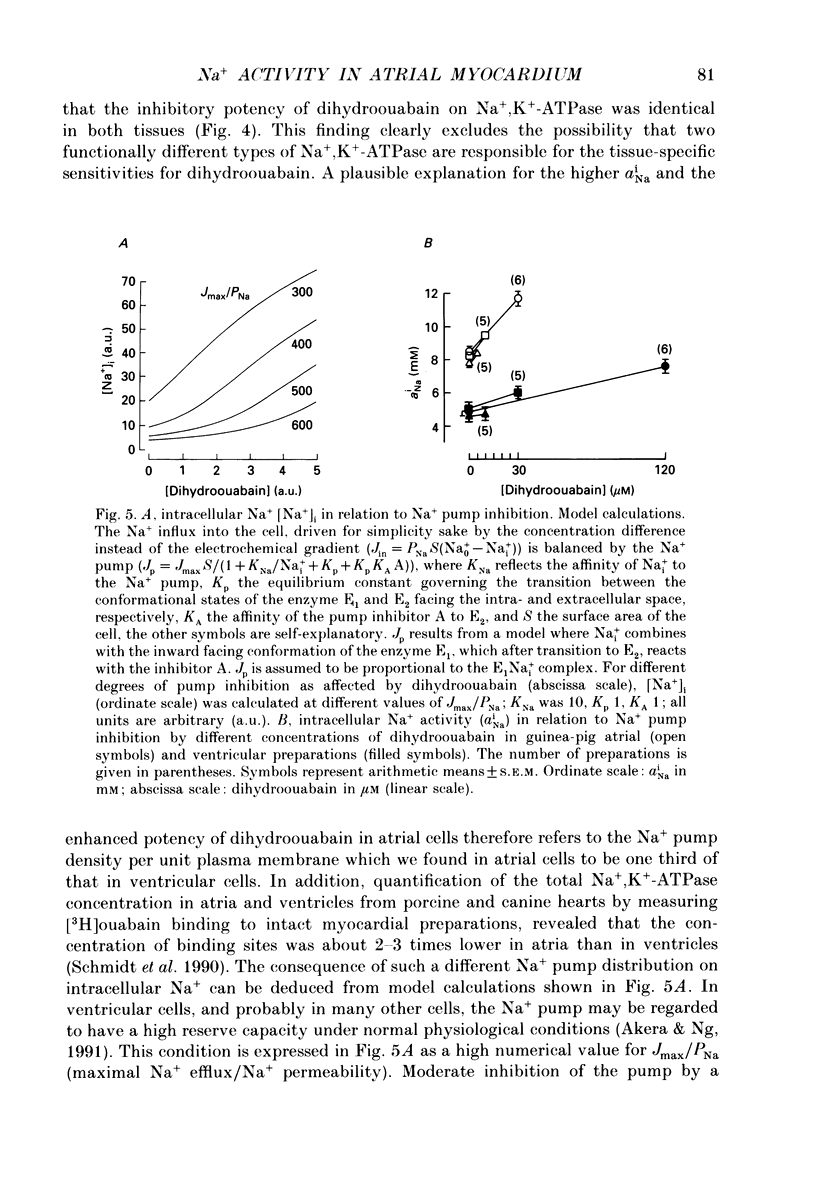
1. Intracellular Na+ activity (aNai) and membrane resting potential were studied in quiescent guinea-pig atrial and papillary muscles by means of Na(+)-sensitive and conventional microelectrodes. The effects of the cardioactive steroid dihydroouabain (DHO) on aiNa, force of contraction and sarcolemmal Na+, K(+)-ATPase activity were also investigated. 2. In thirty atria and twenty-two papillary muscles, aNai amounted to 8.0 +/- 0.2 and 4.7 +/- 0.3 mM, respectively (mean +/- S.E.M.). When both tissues were from the same animal, with the same ion-sensitive microelectrode mean aNai values of 7.9 +/- 0.2 and 5.1 +/- 0.5 mM (P < 0.01) were obtained from eight atrial and eight papillary muscles, respectively. 3. Membrane resting potentials (Em) were significantly (P < 0.001) more negative in the papillary muscles (-83.5 +/- 0.7 mV; n = 8) than in the atrium (-78.1 +/- 0.5 mV; n = 8). Deviation of Em from EK (determined by K(+)-sensitive microelectrodes) was 3.0 +/- 0.2 mV in ventricular (P < 0.05) and 6.1 +/- 0.3 mV in atrial preparations (P < 0.05). 4. Inhibition of the Na+ pump by DHO increased aNai of the atrium within 10 min by 0.6 +/- 0.1 (n = 7), 1.3 +/- 0.1 (n = 5) and 3.2 +/- 0.2 mM (n = 5) at 5, 10 and 30 microM, respectively. In the papillary muscle, 10 microM DHO was without effect while aNai rose by 1.0 +/- 0.1 (n = 5) and 2.9 +/- 0.2 mM (n = 6) at 30 and 120 microM DHO. 5. Consistent with the aNai measurements, the potency of DHO to increase force of the isometric contraction was three times higher in atrium than in papillary muscle (stimulation frequency 0.2 Hz). 6. Hydrolytic activity of sarcolemmal Na+,K(+)-ATPase isolated from atria amounted to only one third of that detected in ventricles (0.07 +/- 0.01, n = 6, versus 0.2 +/- 0.01 mumol phosphate released min-1 (g tissue)-1, n = 5). The inhibitory potencies of DHO on sarcolemmal Na+,K(+)-ATPase preparations were found to be identical in the enzymes from either tissue. 7. It is concluded that a lower Na+ pump density is responsible for the higher aNai and for the lower resting membrane potential in atrial as compared to ventricular cells. The regulation of cellular Na+ homeostasis in atrial muscle appears to be closer to the limits of its capacity than in ventricle, explaining the higher sensitivity of the atrium to interventions which impede Na+ pump activity.
Full text
PDF



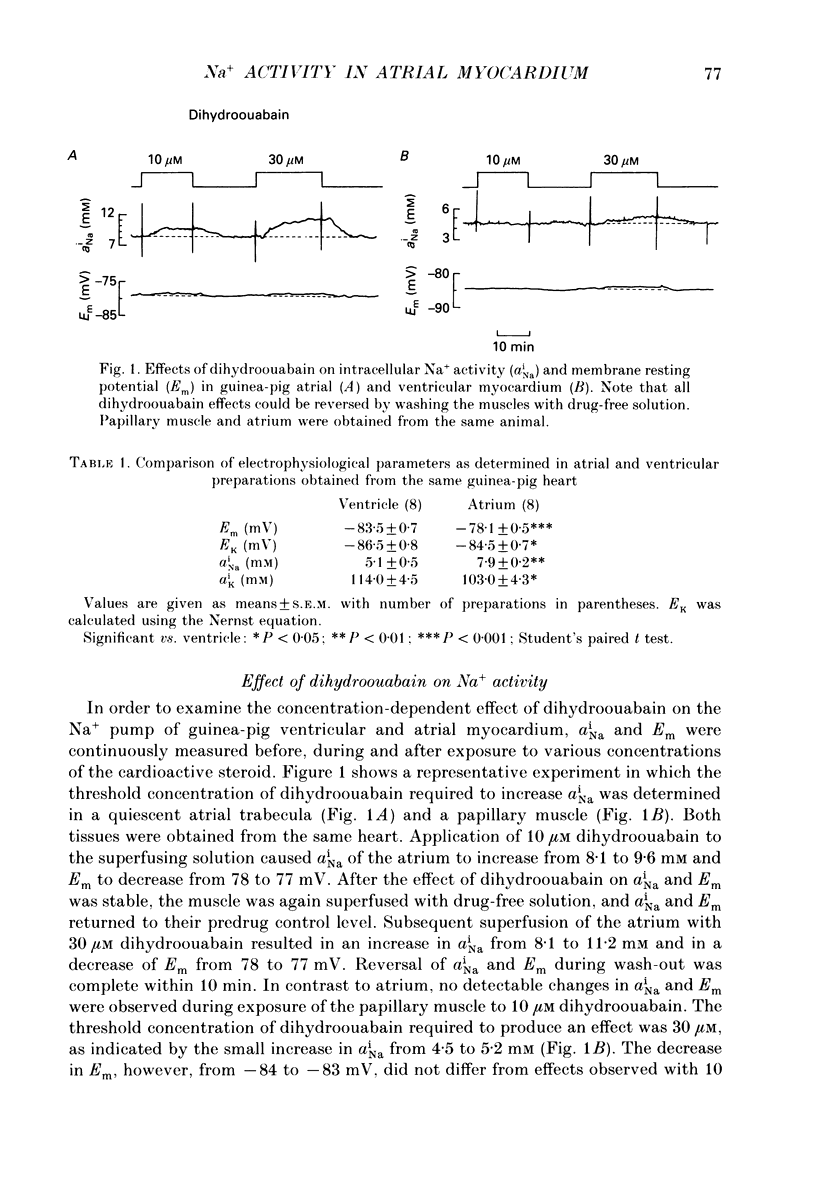
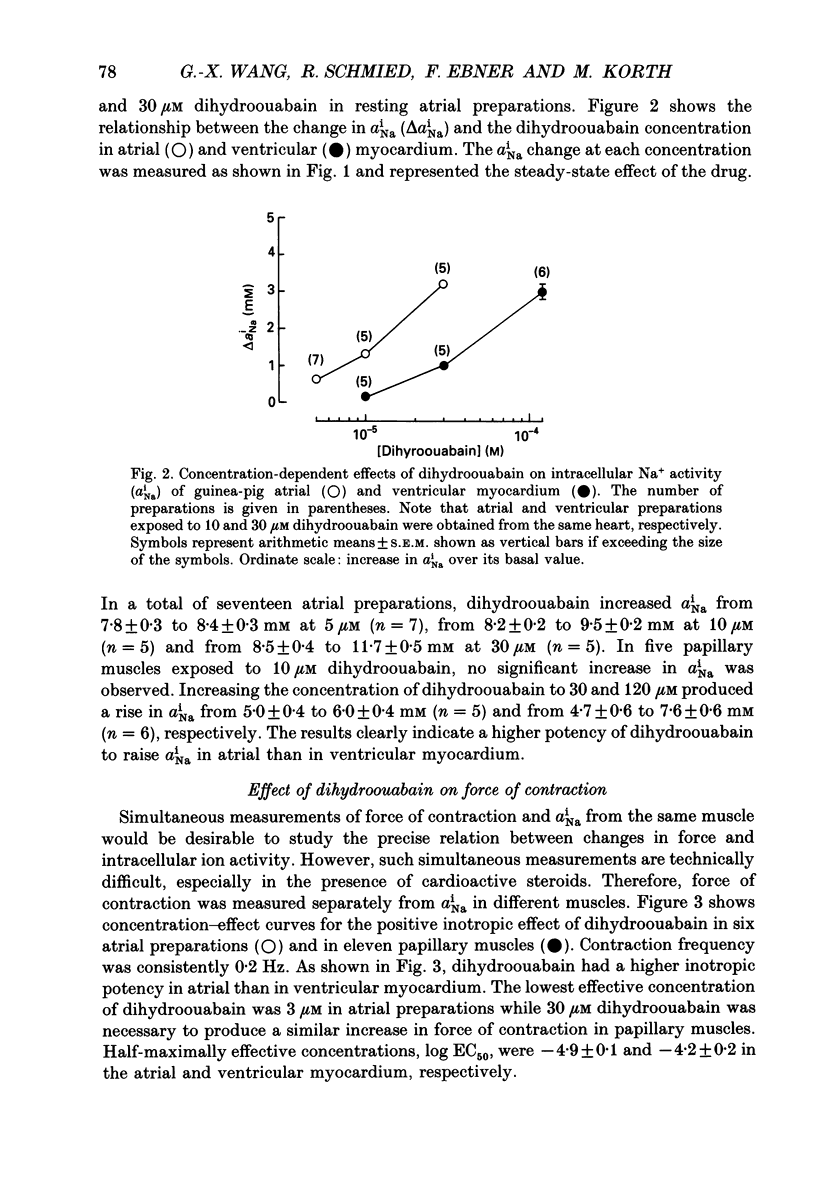




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Ng Y. C. Digitalis sensitivity of Na+,K(+)-ATPase, myocytes and the heart. Life Sci. 1991;48(2):97–106. doi: 10.1016/0024-3205(91)90402-w. [DOI] [PubMed] [Google Scholar]
- Bals S., Bechem M., Paffhausen W., Pott L. Spontaneous and experimentally evoked [Ca2+]i-transients in cardiac myocytes measured by means of a fast Fura-2 technique. Cell Calcium. 1990 Jun-Jul;11(6):385–396. doi: 10.1016/0143-4160(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Singer D. H., Fozzard H. A. Intra- and extracellular potassium activities, acetylcholine and resting potential in guinea pig atria. Circ Res. 1984 Jan;54(1):65–73. doi: 10.1161/01.res.54.1.65. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill D. M., Wasserstrom J. A. Intracellular sodium and the positive inotropic effect of veratridine and cardiac glycoside in sheep Purkinje fibers. Circ Res. 1986 Jan;58(1):109–119. doi: 10.1161/01.res.58.1.109. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Dagostino M., Lee C. O. Neutral carrier Na+- and Ca2+-selective microelectrodes for intracellular application. Biophys J. 1982 Dec;40(3):199–207. doi: 10.1016/S0006-3495(82)84475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F. Factors influencing the onset of ouabain inhibition of Na,K-ATPase from guinea-pig myocardium. Br J Pharmacol. 1990 Oct;101(2):337–343. doi: 10.1111/j.1476-5381.1990.tb12711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y. Difference in calcium content of atrial and ventricular muscle. Jpn J Physiol. 1975;25(4):467–479. doi: 10.2170/jjphysiol.25.467. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Grupp I., Im W. B., Lee C. O., Lee S. W., Pecker M. S., Schwartz A. Relation of sodium pump inhibition to positive inotropy at low concentrations of ouabain in rat heart muscle. J Physiol. 1985 Mar;360:149–160. doi: 10.1113/jphysiol.1985.sp015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S., Mohr K. Sodium load and high affinity ouabain binding in rat and guinea-pig cardiac tissue. Br J Pharmacol. 1985 Mar;84(3):685–688. doi: 10.1111/j.1476-5381.1985.tb16150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. O. 200 years of digitalis: the emerging central role of the sodium ion in the control of cardiac force. Am J Physiol. 1985 Nov;249(5 Pt 1):C367–C378. doi: 10.1152/ajpcell.1985.249.5.C367. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y. C., Akera T. Relative abundance of two molecular forms of Na+,K+-ATPase in the ferret heart: developmental changes and associated alterations of digitalis sensitivity. Mol Pharmacol. 1987 Aug;32(1):201–205. [PubMed] [Google Scholar]
- Ng Y. C., Akera T. Two classes of ouabain binding sites in ferret heart and two forms of Na+-K+-ATPase. Am J Physiol. 1987 May;252(5 Pt 2):H1016–H1022. doi: 10.1152/ajpheart.1987.252.5.H1016. [DOI] [PubMed] [Google Scholar]
- Ng Y. C., Yao A. Z., Akera T. Tissue-specific isoform regulation of Na+-K+-ATPase by thyroid hormone in ferrets. Am J Physiol. 1989 Aug;257(2 Pt 2):H534–H539. doi: 10.1152/ajpheart.1989.257.2.H534. [DOI] [PubMed] [Google Scholar]
- Orlowski J., Lingrel J. B. Thyroid and glucocorticoid hormones regulate the expression of multiple Na,K-ATPase genes in cultured neonatal rat cardiac myocytes. J Biol Chem. 1990 Feb 25;265(6):3462–3470. [PubMed] [Google Scholar]
- Poole-Wilson P. A., Cameron I. R. ECS, intracellular pH, and electrolytes of cardiac and skeletal muscle. Am J Physiol. 1975 Nov;229(5):1299–1304. doi: 10.1152/ajplegacy.1975.229.5.1299. [DOI] [PubMed] [Google Scholar]
- Reiter M. Die Wertbestimmung inotrop wirkender Arzneimittel am isolierten Papillarmuskel. Arzneimittelforschung. 1967 Oct;17(10):1249–1253. [PubMed] [Google Scholar]
- Schmidt T. A., Svendsen J. H., Haunsø S., Kjeldsen K. Quantification of the total Na,K-ATPase concentration in atria and ventricles from mammalian species by measuring 3H-ouabain binding to intact myocardial samples. Stability to short term ischemia reperfusion. Basic Res Cardiol. 1990 Jul-Aug;85(4):411–427. doi: 10.1007/BF01907133. [DOI] [PubMed] [Google Scholar]
- Schmied R., Wang G. X., Korth M. Intracellular Na+ activity and positive inotropic effect of sulmazole in guinea pig ventricular myocardium. Comparison with a cardioactive steroid. Circ Res. 1991 Feb;68(2):597–604. doi: 10.1161/01.res.68.2.597. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Lobaugh L. A., Liu S., Shigeto N., Lieberman M. Intracellular sodium affects ouabain interaction with the Na/K pump in cultured chick cardiac myocytes. J Gen Physiol. 1990 Jan;95(1):77–95. doi: 10.1085/jgp.95.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J., Farshi S. K. Rat cardiac ventricle has two Na+,K+-ATPases with different affinities for ouabain: developmental changes in immunologically different catalytic subunits. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8404–8407. doi: 10.1073/pnas.84.23.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J., Farshi S. K. Two forms of the Na,K-ATPase catalytic subunit detected by their immunological and electrophoretic differences. Alpha and alpha+ in mammalian heart. Prog Clin Biol Res. 1988;268B:149–156. [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., DUDEL J. Zum Mechanismus der Membranwirkung des Acetylcholin an der Herzmuskelfaser. Pflugers Arch. 1958;266(3):324–334. doi: 10.1007/BF00416781. [DOI] [PubMed] [Google Scholar]
- Wang D. Y., Chae S. W., Gong Q. Y., Lee C. O. Role of aiNa in positive force-frequency staircase in guinea pig papillary muscle. Am J Physiol. 1988 Dec;255(6 Pt 1):C798–C807. doi: 10.1152/ajpcell.1988.255.6.C798. [DOI] [PubMed] [Google Scholar]
- Young R. M., Lingrel J. B. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem Biophys Res Commun. 1987 May 29;145(1):52–58. doi: 10.1016/0006-291x(87)91286-1. [DOI] [PubMed] [Google Scholar]


