Abstract
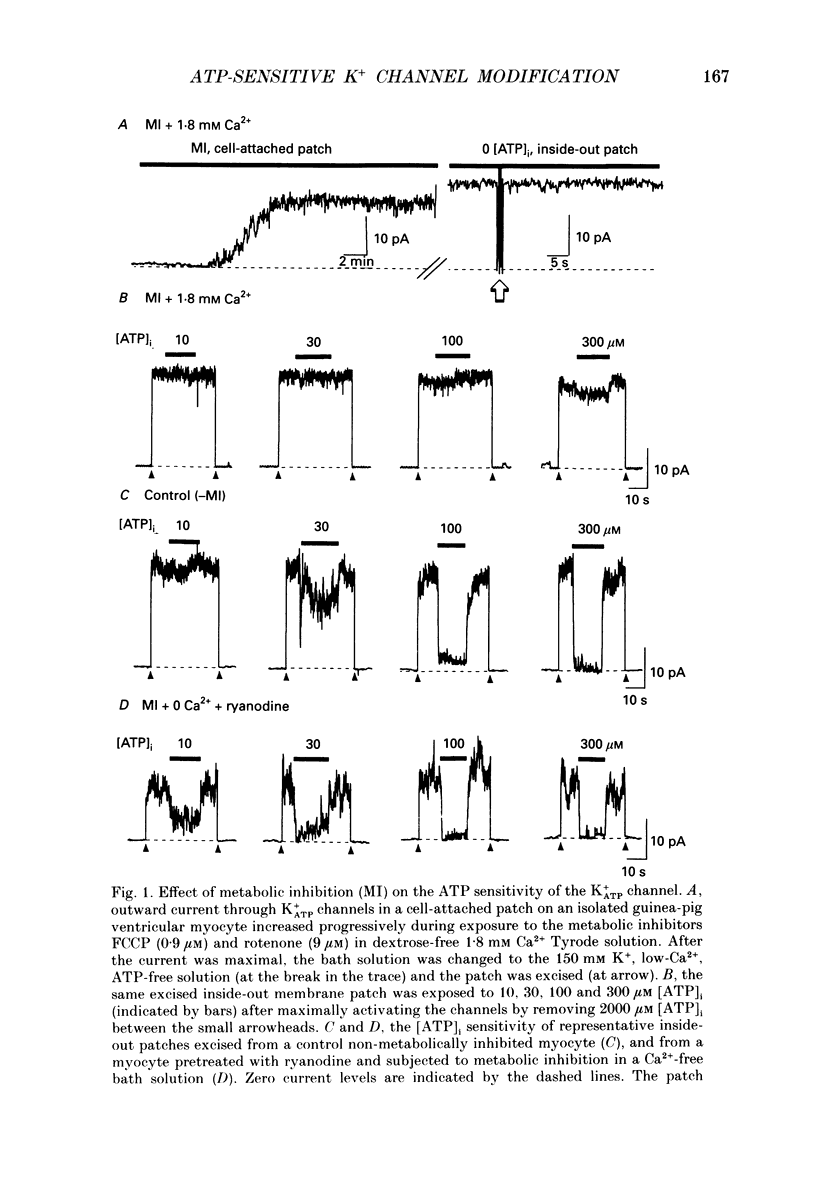
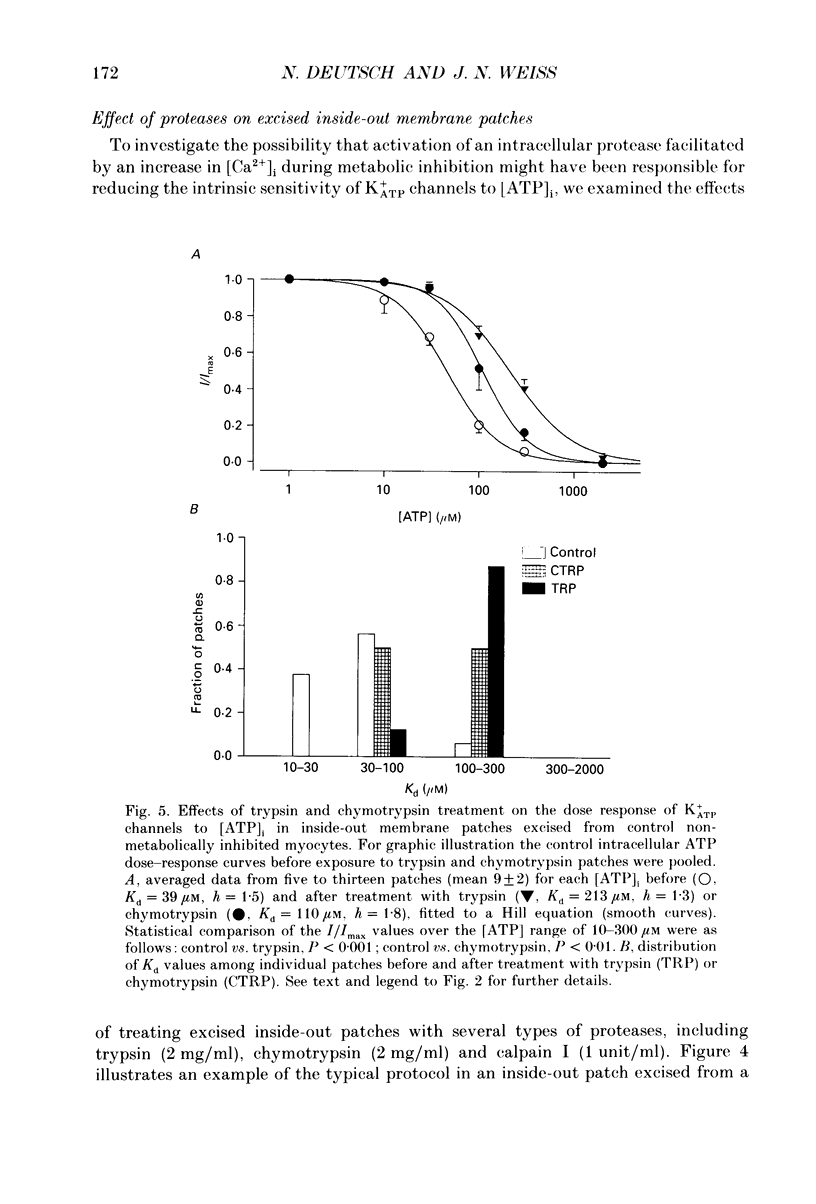
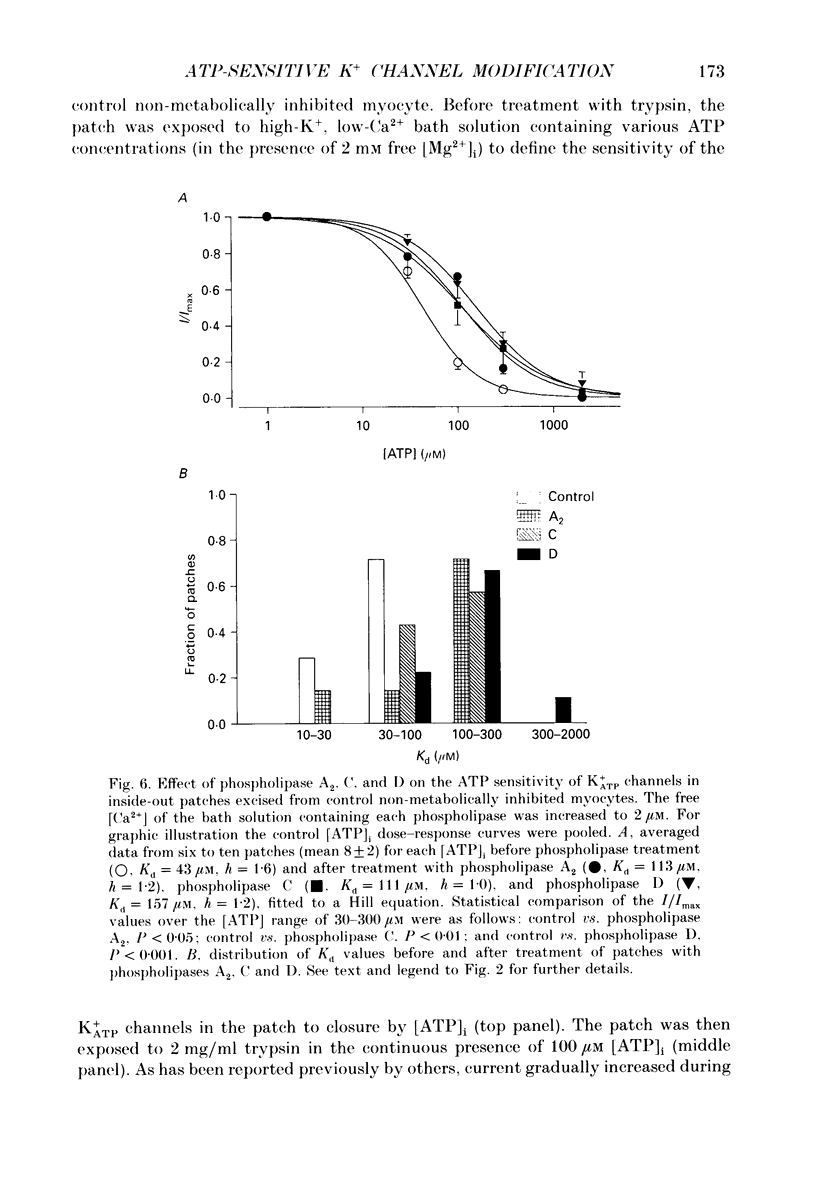
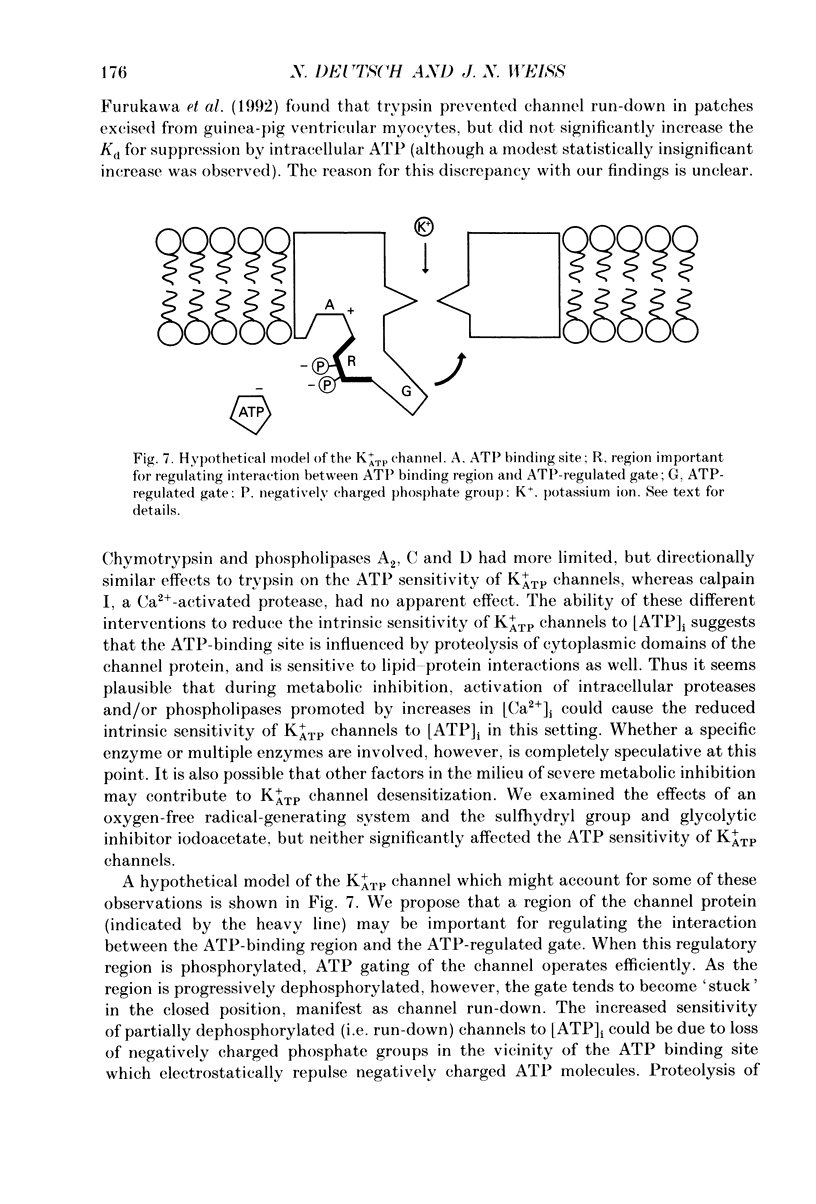
1. ATP-sensitive K+ (K+ATP) channels are believed to make an important contribution to the increased cellular K+ efflux and shortening of the action potential duration (APD) during metabolic inhibition, hypoxia, and ischaemia in the heart. The mechanisms by which the activity of the K+ATP channel is regulated during conditions of metabolic impairment are not completely clear. Extrinsic factors such as increased [ADP]i, acidosis, and stimulation of adenosine receptors appear to decrease the K+ATP channel's sensitivity to closure by [ATP]i. The purpose of this study was to determine whether the K+ATP channel itself is intrinsically altered by the processes associated with metabolic impairment. 2. Isolated guinea-pig ventricular myocytes were metabolically inhibited in glucose-free 1.8 mM Ca2+ Tyrode solution containing 9 microM rotenone and 0.9 microM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) while recording unitary currents through K+ATP channels in cell-attached patches. When K+ATP channel activity became maximal, the patch was excised (inside-out) into 150 mM K+ bath solution containing different ATP concentrations. The Kd for suppression by [ATP]i ([ATP]i causing half-maximal suppression of current through K+ATP channels) was markedly increased to 305 microM (n = 9) compared to patches excised from control myocytes not exposed to metabolic inhibitors (Kd = 46 microM, n = 28). 3. A [Ca2+]i-dependent process was involved in K+ATP channel modification during metabolic inhibition. Removal of extracellular Ca2+ during metabolic inhibition led to an intermediate decrease in the ATP sensitivity of the K+ATP channels (Kd = 120 microM, n = 6). In myocytes that were pretreated with 10 microM ryanodine in addition to removing extracellular Ca2+, the reduction in ATP sensitivity was completely prevented (Kd = 23 microM, n = 6). 4. In inside-out membrane patches excised from control non-metabolically inhibited myocytes, elevated free [Ca2+]i (2 microM) did not alter the sensitivity of the K+ATP channel to closure by [ATP]i, suggesting that in metabolically inhibited myocytes elevated [Ca2+]i acted indirectly. K+ATP channel run-down was found to increase the sensitivity of K+ATP channels to closure to [ATP]i (Kd = 16 microM, n = 13). 5. Inside-out membrane patches excised from control non-metabolically inhibited myocytes were also exposed to various proteases, phospholipases and other reagents that may be activated during metabolic inhibition. Trypsin and chymotrypsin treatment increased the Kd from 39 to 213 microM (n = 8) and 110 microM (n = 5), respectively. Calpain I had no apparent effect on the Kd.(ABSTRACT TRUNCATED AT 400 WORDS)
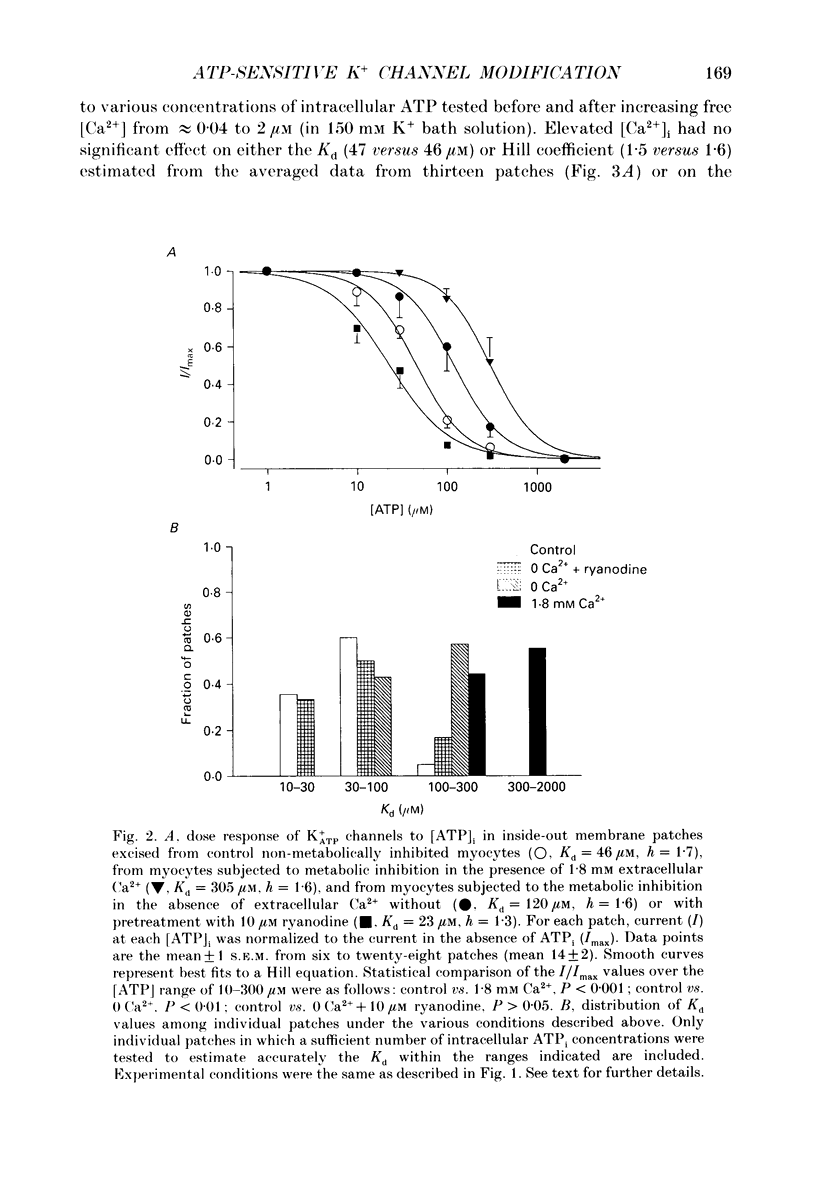
Full text
PDF




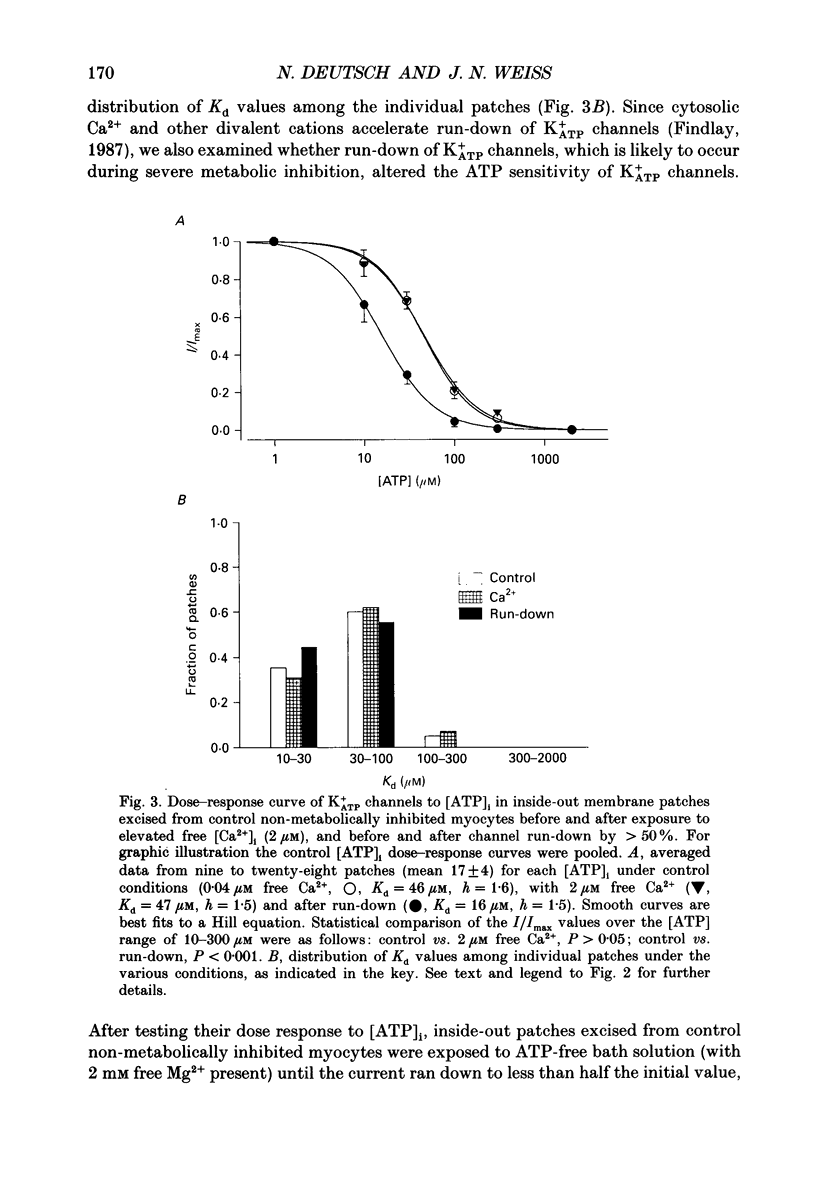





Selected References
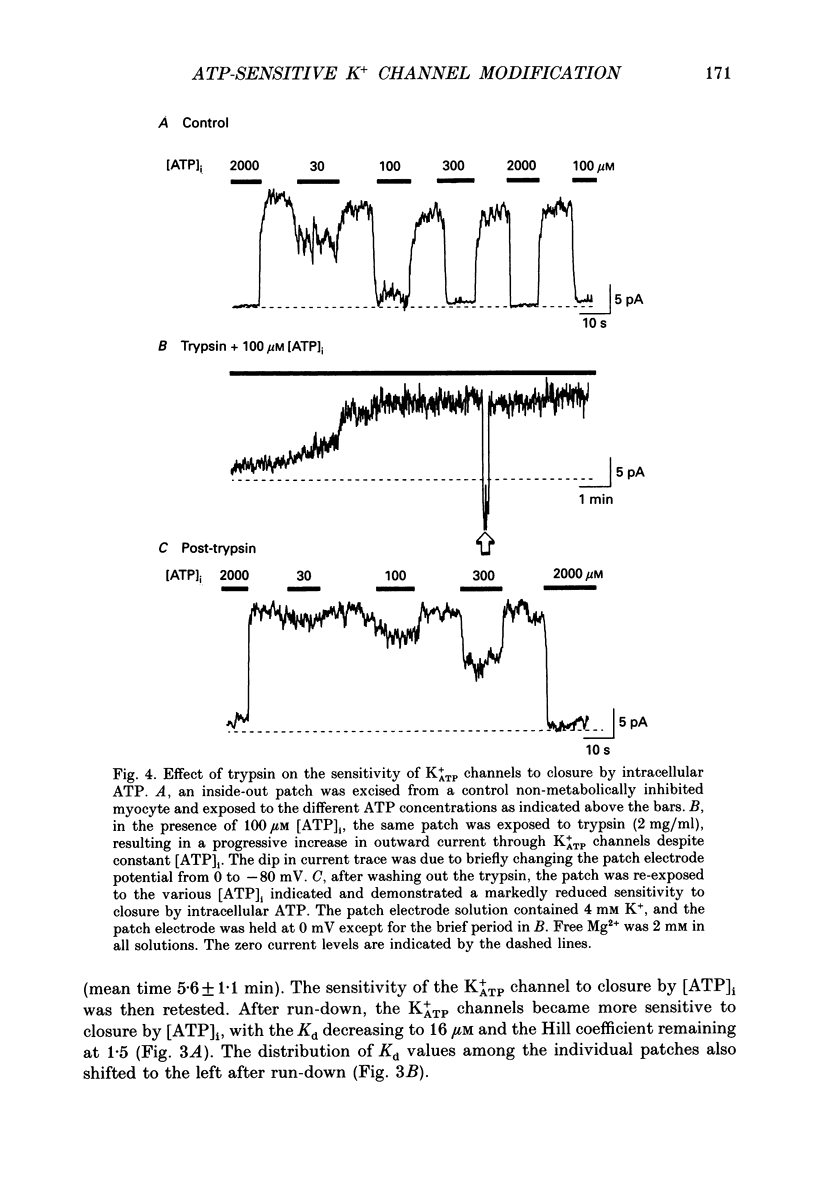
These references are in PubMed. This may not be the complete list of references from this article.
- Barry W. H., Peeters G. A., Rasmussen C. A., Jr, Cunningham M. J. Role of changes in [Ca2+]i in energy deprivation contracture. Circ Res. 1987 Nov;61(5):726–734. doi: 10.1161/01.res.61.5.726. [DOI] [PubMed] [Google Scholar]
- Benndorf K., Friedrich M., Hirche H. Alterations of ionic currents after reoxygenation in isolated cardiocytes of guinea-pigs. Pflugers Arch. 1991 Apr;418(3):238–247. doi: 10.1007/BF00370522. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Cuevas J., Bassett A. L., Cameron J. S., Furukawa T., Myerburg R. J., Kimura S. Effect of H+ on ATP-regulated K+ channels in feline ventricular myocytes. Am J Physiol. 1991 Sep;261(3 Pt 2):H755–H761. doi: 10.1152/ajpheart.1991.261.3.H755. [DOI] [PubMed] [Google Scholar]
- Deutsch N., Klitzner T. S., Lamp S. T., Weiss J. N. Activation of cardiac ATP-sensitive K+ current during hypoxia: correlation with tissue ATP levels. Am J Physiol. 1991 Sep;261(3 Pt 2):H671–H676. doi: 10.1152/ajpheart.1991.261.3.H671. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre J. F., Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim Biophys Acta. 1990 Nov 2;1029(1):167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I., Faivre J. F. ATP-sensitive K channels in heart muscle. Spare channels. FEBS Lett. 1991 Feb 11;279(1):95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Cook F., Hashimotso B., Stone P., Muller J., Loscalzo A. Evidence that hospital care for acute myocardial infarction has not contributed to the decline in coronary mortality between 1973-1974 and 1978-1979. Circulation. 1982 May;65(5):936–942. doi: 10.1161/01.cir.65.5.936. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Nicolaus A., Goknur A. B., Berkoff H. A. Synchronous depletion of ATP in isolated adult rat heart cells. J Mol Cell Cardiol. 1988 Sep;20(9):837–846. doi: 10.1016/s0022-2828(88)80008-7. [DOI] [PubMed] [Google Scholar]
- Hill J. L., Gettes L. S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980 Apr;61(4):768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Kantor P. F., Coetzee W. A., Carmeliet E. E., Dennis S. C., Opie L. H. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res. 1990 Feb;66(2):478–485. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- Keung E. C., Li Q. Lactate activates ATP-sensitive potassium channels in guinea pig ventricular myocytes. J Clin Invest. 1991 Nov;88(5):1772–1777. doi: 10.1172/JCI115497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Hohl C. M., Altschuld R. A., Stokes B. T. Energy depletion-repletion and calcium transients in single cardiomyocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C427–C434. doi: 10.1152/ajpcell.1989.257.3.C427. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Mohabir R., Lee H. C., Kurz R. W., Clusin W. T. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991 Dec;69(6):1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990 Apr;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol Pharmacol. 1989 Dec;36(6):897–902. [PubMed] [Google Scholar]
- Trube G., Hescheler J., Schröter K. Regulation and function of ATP-dependent K+ channels in pancreatic B cells. Soc Gen Physiol Ser. 1989;44:83–95. [PubMed] [Google Scholar]
- Venkatesh N., Lamp S. T., Weiss J. N. Sulfonylureas, ATP-sensitive K+ channels, and cellular K+ loss during hypoxia, ischemia, and metabolic inhibition in mammalian ventricle. Circ Res. 1991 Sep;69(3):623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Venkatesh N., Lamp S. T. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992 Feb;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. Extracellular K+ accumulation during myocardial ischemia in isolated rabbit heart. Am J Physiol. 1982 Apr;242(4):H619–H628. doi: 10.1152/ajpheart.1982.242.4.H619. [DOI] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. [K+]o accumulation and electrophysiological alterations during early myocardial ischemia. Am J Physiol. 1982 Aug;243(2):H318–H327. doi: 10.1152/ajpheart.1982.243.2.H318. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Escande D., Schumacher C. A., Thuringer D., Mestre M., Fiolet J. W., Janse M. J. Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res. 1990 Oct;67(4):835–843. doi: 10.1161/01.res.67.4.835. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Kléber A. G. The combined effects of hypoxia, high K+, and acidosis on the intracellular sodium activity and resting potential in guinea pig papillary muscle. Circ Res. 1986 Feb;58(2):249–256. doi: 10.1161/01.res.58.2.249. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Müller M., Lazdunski M. Activation and inhibition of ATP-sensitive K+ channels by fluorescein derivatives. J Biol Chem. 1992 Mar 5;267(7):4557–4563. [PubMed] [Google Scholar]


