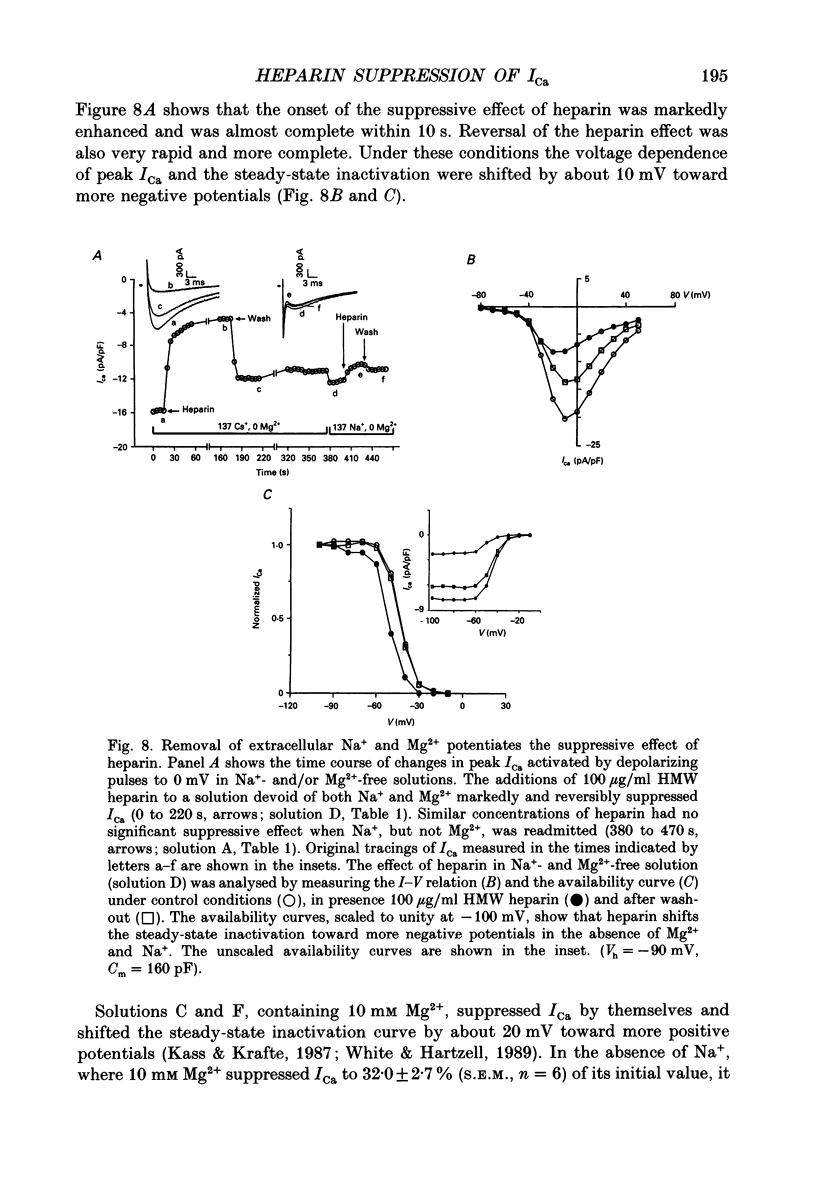
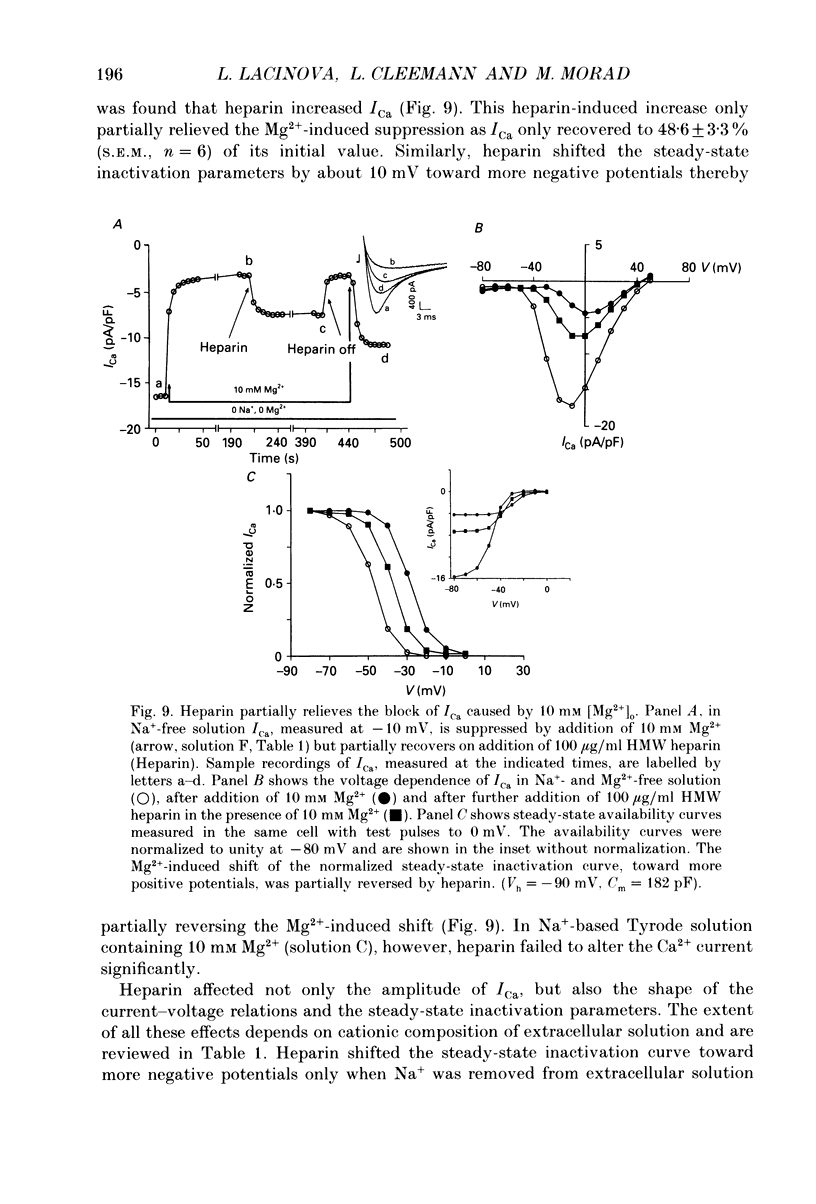
Abstract
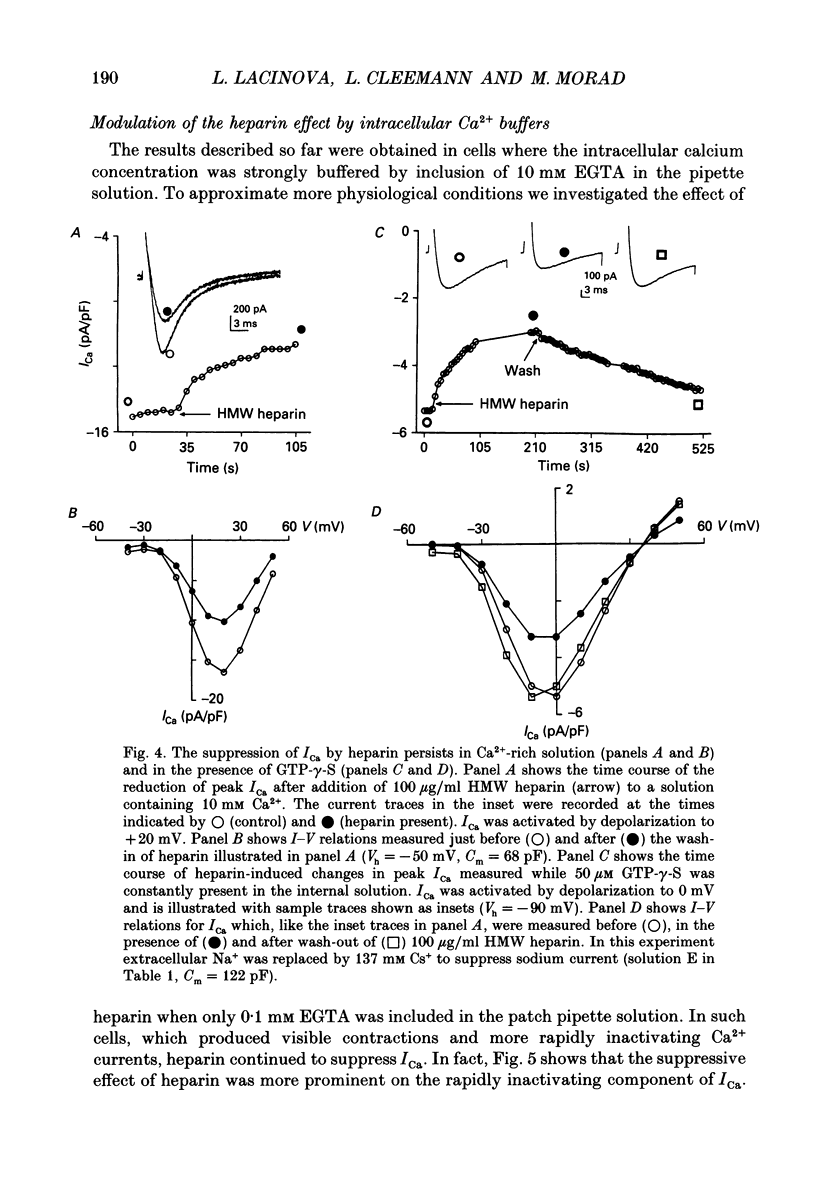
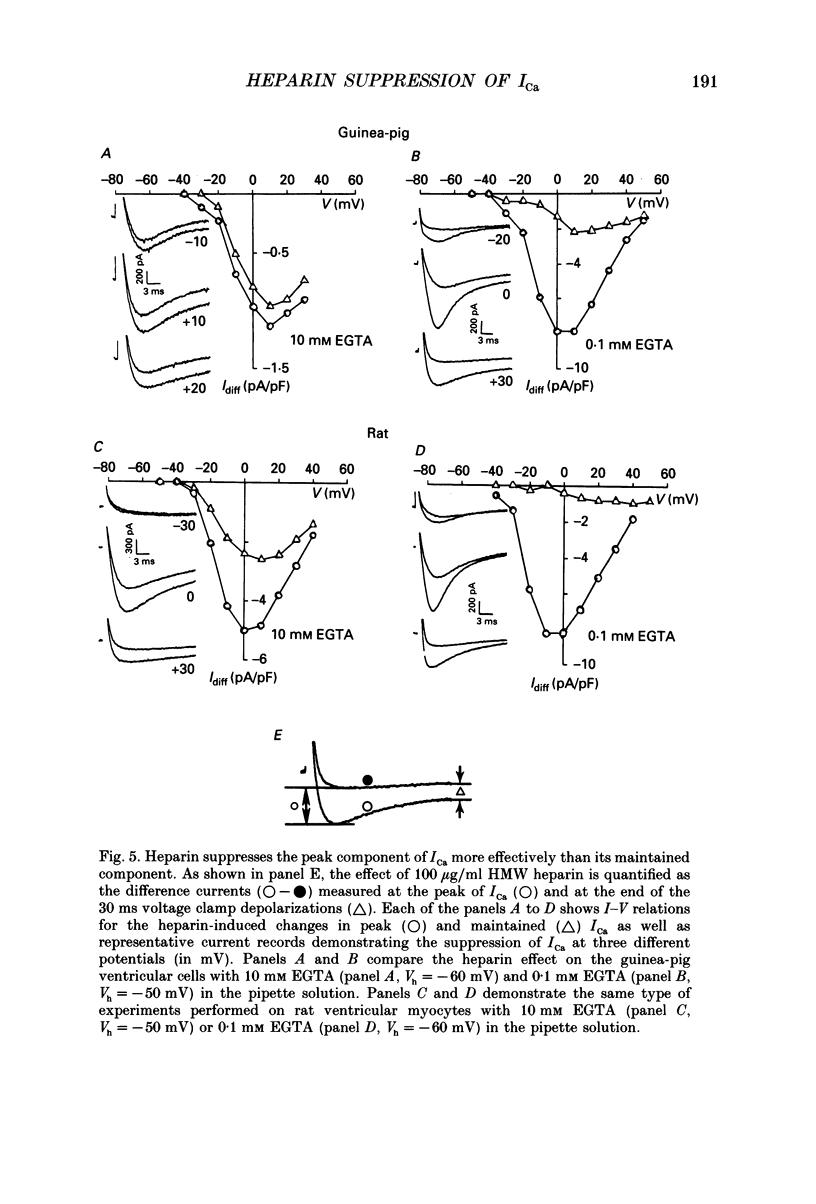
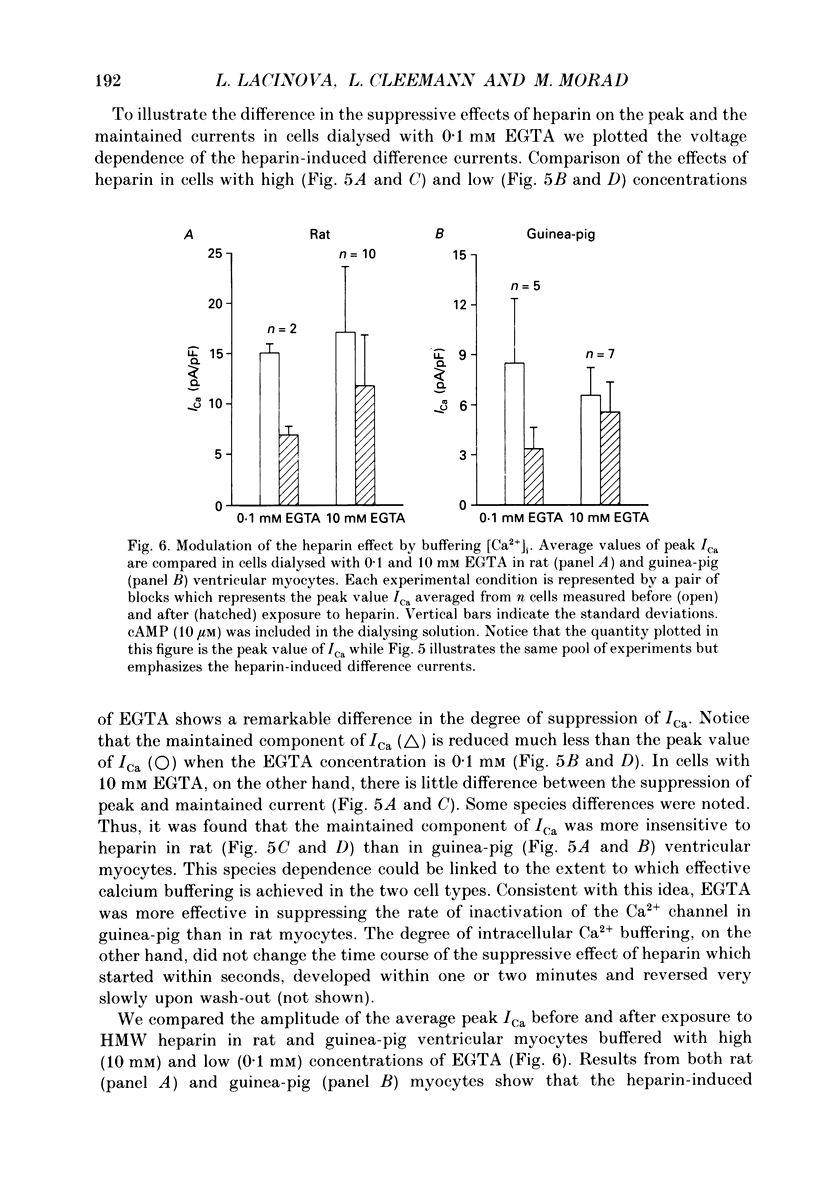
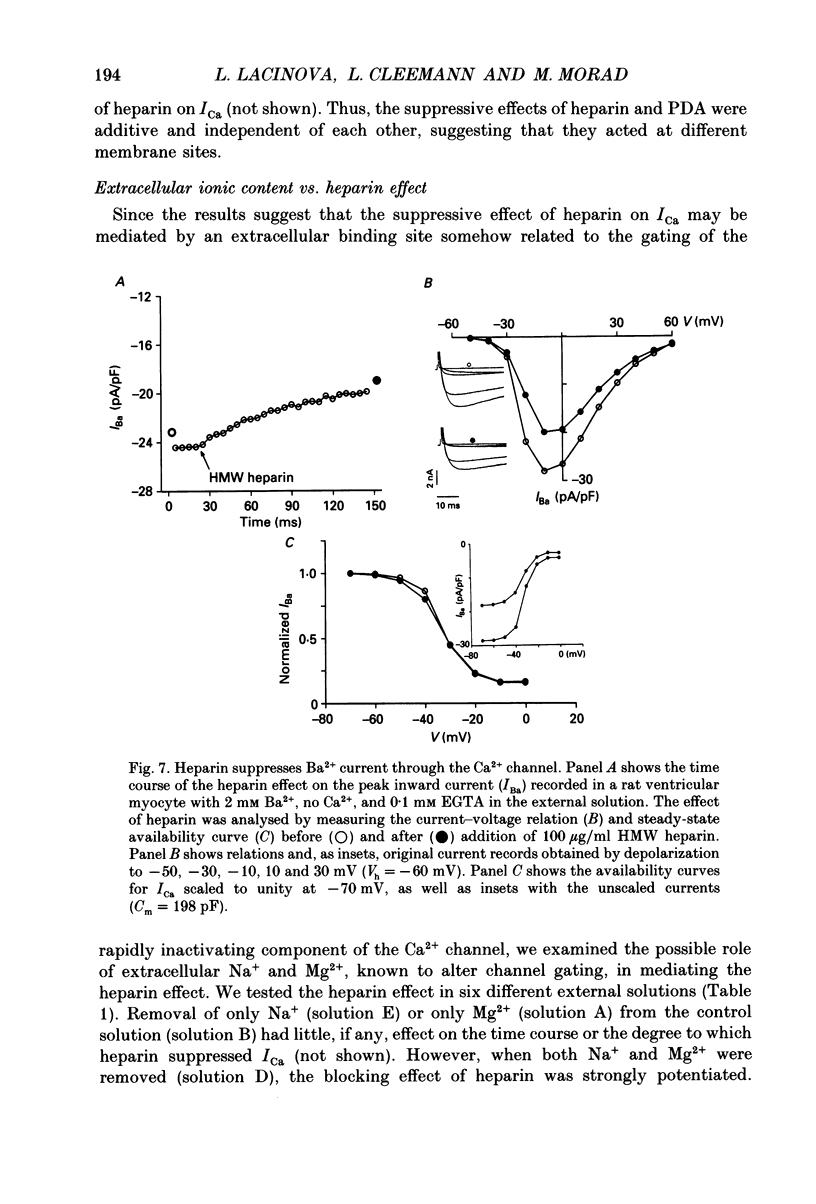
1. The effect of heparin on L-type Ca2+ channels in rabbit, rat and guinea-pig cardiac myocytes was studied using the whole-cell patch clamp method. 2. Sodium salts of heparin uniformly suppressed the Ca2+ current, ICa, independent of their molecular weight, in the rat and guinea-pig ventricular and rabbit atrial myocytes. The suppression of ICa by heparin was dose dependent and reached its maximum, about 30%, around 10 microM. Heparin did not alter the voltage-dependence or the steady-state inactivation properties of ICa. These effects were specific to heparin as another polysaccharide, dextran, failed to have any effect on ICa. 3. The suppressive effect of heparin was not diminished when [Ca2+]o was increased to 10 mM, or when Ba2+ was the charge carrier through the Ca2+ channel. 4. Spectrophotometric assays showed that heparin-induced changes in [Ca2+]o generally were too small to alter ICa significantly. 5. In myocytes buffered with 0.1 mM EGTA, the suppressive effect of heparin was more prominent on the inactivating than on the maintained component of ICa. 6. When extracellular Na+ was replaced by Cs+, the heparin suppressive effect was accompanied by a 10 mV shift of both the voltage dependence of activation and the steady-state inactivation parameters toward more negative potentials. 7. When both Mg2+ and Na+ were omitted from the bathing solutions, the suppressive effect of heparin was significantly enhanced such that almost 80% of the current was blocked. 8. In Cs(+)-based solutions 10 mM [Mg2+]o suppressed ICa by about 70% and heparin partially relieved this block. Heparin, however, did not counteract the Mg(2+)-induced suppression of ICa in Na(+)-based solution. 9. Extracellularly applied heparin did not alter the isoprenaline-induced enhancement of ICa or interfere with the blocking effect of phorbol esters on ICa. 10. Heparin thus appears to interfere with the permeation of Ca2+ through the channel by a mechanism regulating the Ca(2+)-induced inactivation of the Ca2+ channel. Na+ and Mg2+ appear to alter the kinetics and the magnitude of the suppressive effect of heparin on the Ca2+ channel, suggesting an interaction of these cations with either the Ca2+ or the heparin-binding sites of the channel.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choay J. Structure and activity of heparin and its fragments: an overview. Semin Thromb Hemost. 1989 Oct;15(4):359–364. doi: 10.1055/s-2007-1002730. [DOI] [PubMed] [Google Scholar]
- Cutler L. S., Christian C. P. Inhibition of rat salivary gland adenylate cyclase by glycosaminoglycans and high molecular weight polyanions. Arch Oral Biol. 1984;29(8):629–633. doi: 10.1016/0003-9969(84)90133-x. [DOI] [PubMed] [Google Scholar]
- Grant D., Long W. F., Williamson F. B. The dependence on counter-cation of the degree of hydration of heparin. Biochem Soc Trans. 1990 Dec;18(6):1283–1284. doi: 10.1042/bst0181283. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Takikawa R., Iguchi M., Hamada E., Sugimoto T., Kurachi Y. Heparin uncouples the muscarinic receptors from GK protein in the atrial cell membrane of the guinea-pig heart. Pflugers Arch. 1990 Sep;417(1):126–128. doi: 10.1007/BF00370783. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Krafte D. S. Negative surface charge density near heart calcium channels. Relevance to block by dihydropyridines. J Gen Physiol. 1987 Apr;89(4):629–644. doi: 10.1085/jgp.89.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzka D. A., Morad M. Properties of calcium channels in guinea-pig gastric myocytes. J Physiol. 1989 Jun;413:175–197. doi: 10.1113/jphysiol.1989.sp017648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Lefkowitz R. J., Caron M. G., Benovic J. L. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1989 May;86(9):3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattai J., Kwak J. C. Quantitative similarity of zinc and calcium binding to heparin in excess salt solution. Biophys Chem. 1988 Sep;31(3):295–299. doi: 10.1016/0301-4622(88)80035-8. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. Characterization of the murexide method: dual-wavelength spectrophotometry of cations under physiological conditions. Anal Biochem. 1978 Mar;85(1):165–179. doi: 10.1016/0003-2697(78)90287-7. [DOI] [PubMed] [Google Scholar]
- Reches A., Eldor A., Salomon Y. The effects of dextran sulfate, heparin and PGE1 on adenylate cyclase activity and aggregation of human platelets. Thromb Res. 1979;16(1-2):107–116. doi: 10.1016/0049-3848(79)90274-3. [DOI] [PubMed] [Google Scholar]
- Richard S., Tiaho F., Charnet P., Nargeot J., Nerbonne J. M. Two pathways for Ca2+ channel gating differentially modulated by physiological stimuli. Am J Physiol. 1990 Jun;258(6 Pt 2):H1872–H1881. doi: 10.1152/ajpheart.1990.258.6.H1872. [DOI] [PubMed] [Google Scholar]
- Silva M. E., Dietrich C. P. Structure of heparin. Characterization of the products formed from heparin by the action of a heparinase and a heparitinase from Flavobacterium heparinum. J Biol Chem. 1975 Sep 10;250(17):6841–6846. [PubMed] [Google Scholar]
- Thurn A. L., Underhill C. B. Heparin-induced aggregation of lymphoid cells. J Cell Physiol. 1986 Mar;126(3):352–358. doi: 10.1002/jcp.1041260305. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Magnesium ions in cardiac function. Regulator of ion channels and second messengers. Biochem Pharmacol. 1989 Mar 15;38(6):859–867. doi: 10.1016/0006-2952(89)90272-4. [DOI] [PubMed] [Google Scholar]
- Willuweit B., Aktories K. Heparin uncouples alpha 2-adrenoceptors from the Gi-protein in membranes of human platelets. Biochem J. 1988 Feb 1;249(3):857–863. doi: 10.1042/bj2490857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. C., Kirchberger M. A. Modulation by polyelectrolytes of canine cardiac microsomal calcium uptake and the possible relationship to phospholamban. J Biol Chem. 1989 Oct 5;264(28):16644–16651. [PubMed] [Google Scholar]
- Zaragoza R., Battle-Tracy K. M., Owen N. E. Heparin inhibits Na(+)-H+ exchange in vascular smooth muscle cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C46–C53. doi: 10.1152/ajpcell.1990.258.1.C46. [DOI] [PubMed] [Google Scholar]


