Abstract
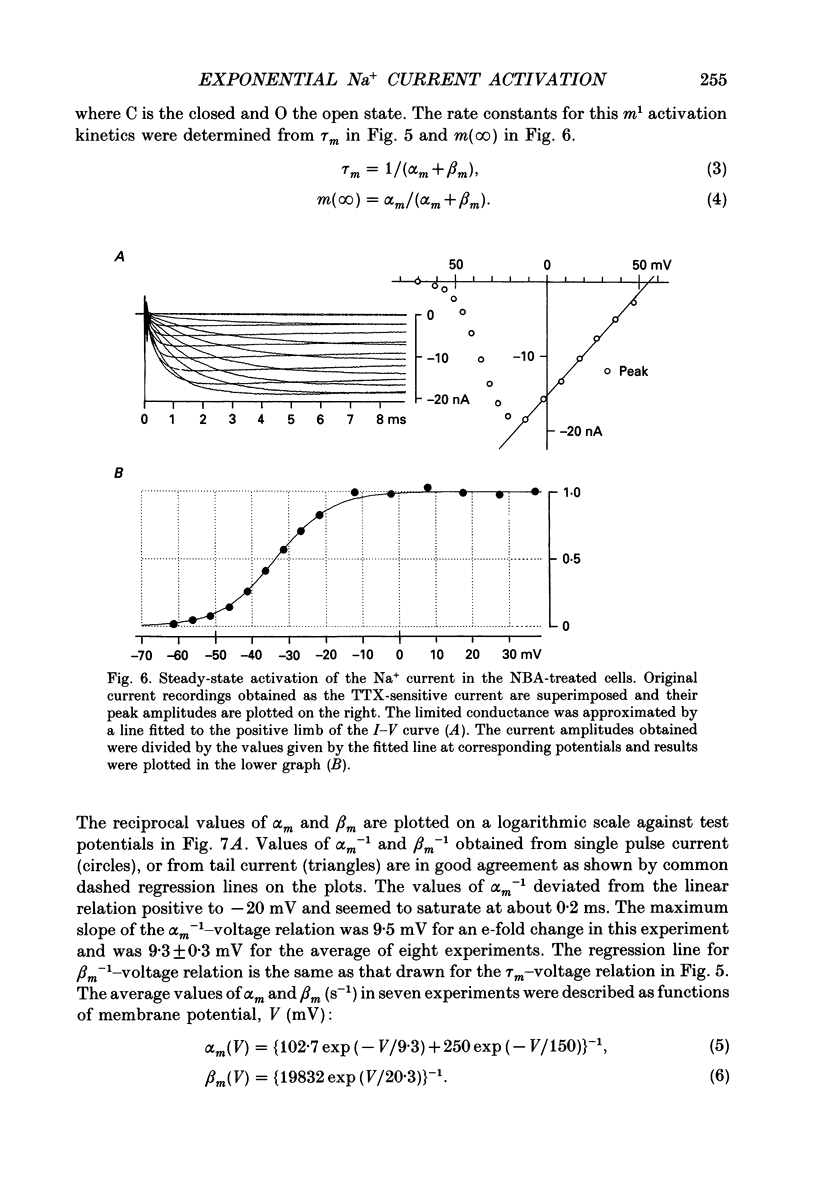
1. The activation kinetics of the Na+ current was investigated in single ventricular cells of the guinea-pig heart using an improved oil-gap voltage clamp method. The inactivation of the current was removed by an intracellular application of N-bromoacetamide (NBA) for less than 1 min. Although the NBA treatment slightly decreased the peak amplitudes (81.7 +/- 13.4% of control, n = 15), the Na+ current remained stable after the removal of inactivation. 2. On depolarization, the activation of Na+ current took an exponential time course after the capacitive current decreased to 5% of its peak amplitude (40-100 microseconds after the pulse onset). The time course of deactivation, recorded on repolarization from 1.2 ms depolarization, was also a single exponential. 3. The time constants of activation and deactivation were almost identical when compared at a given test potential within a range of -50 to -30 mV. These findings indicate that the cardiac Na+ current activation is determined by m1 kinetics, or one rate-limiting step. 4. At potentials negative to -60 mV, the deactivation was complete, and its time constant decreased e-fold per 20.3 +/- 1.8 mV hyperpolarization (n = 7). 5. The degree of steady-state activation (m(infinity)) was fitted to a Boltzmann equation with a slope factor of 7.4 +/- 0.3 mV and a half-maximum potential of -33.3 +/- 0.8 mV (n = 8). 6. Rate constants for the rate-limiting activation step between a closed state and an open state (alpha m, beta m), were determined from m(infinity) and tau m over a potential range between -100 and +50 mV. On a logarithmic scale, beta m-1 was a linear function of the membrane potential over the range -100 and -30 mV. 7. Fitting the newly determined activation kinetics to the rising phase of the action potential indicated that the activation kinetics in the present study is relevant to the physiological action potential. The density of the Na+ channels thus obtained was 1075 +/- 186 pF-1 (n = 6). 8. The measurements in the NBA-treated Na+ current were compared with those obtained without treatment.
Full text
PDF




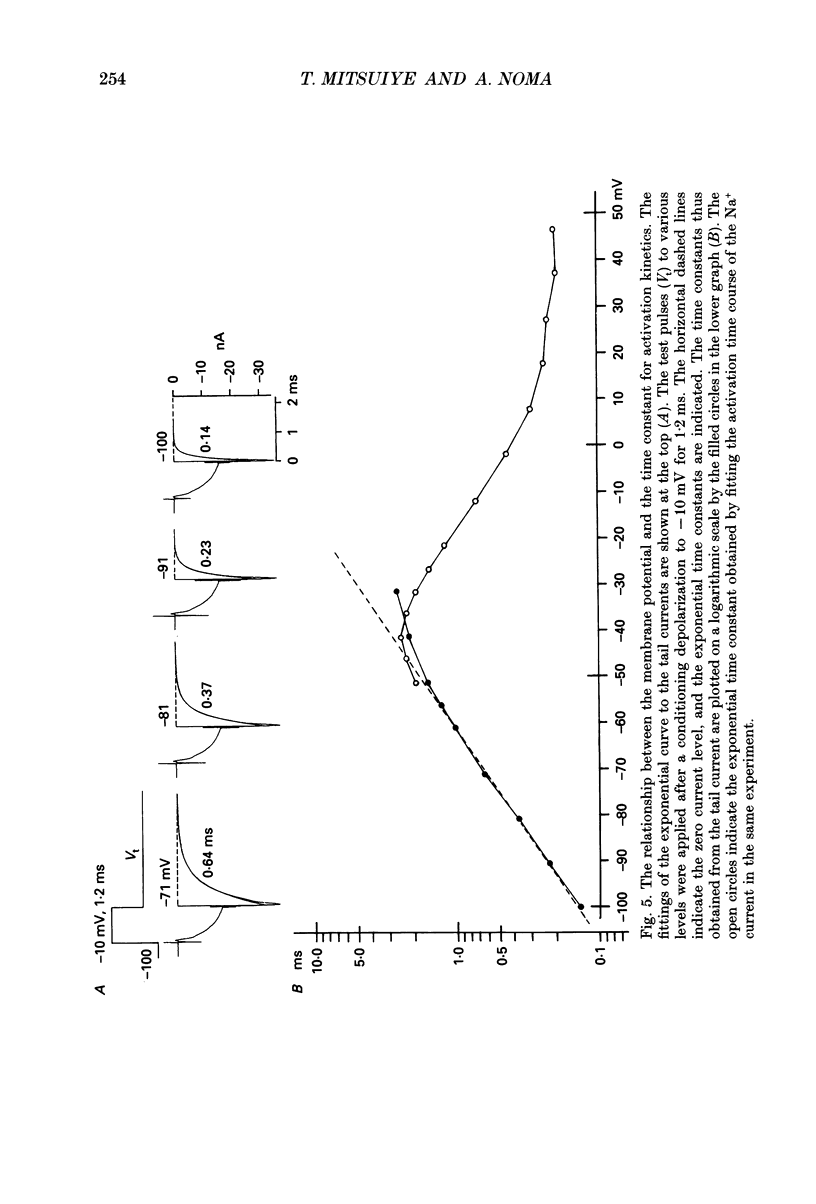






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
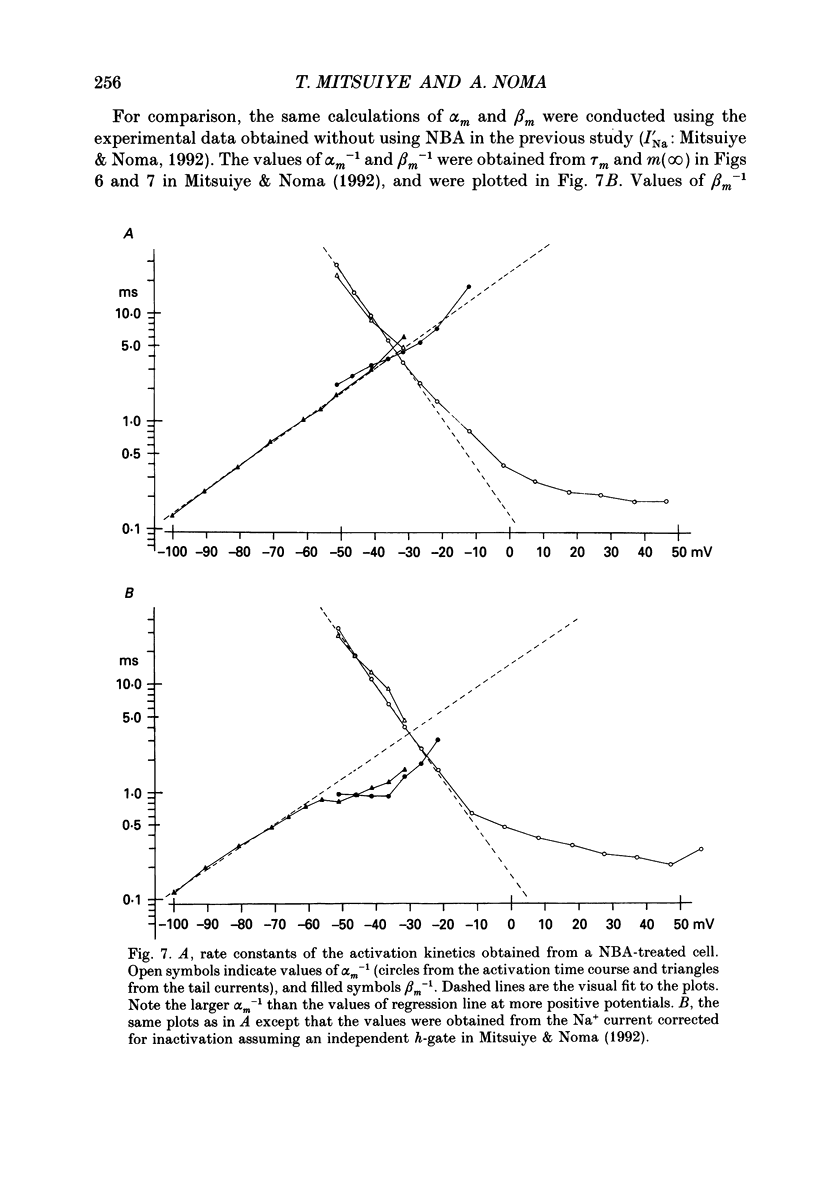
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
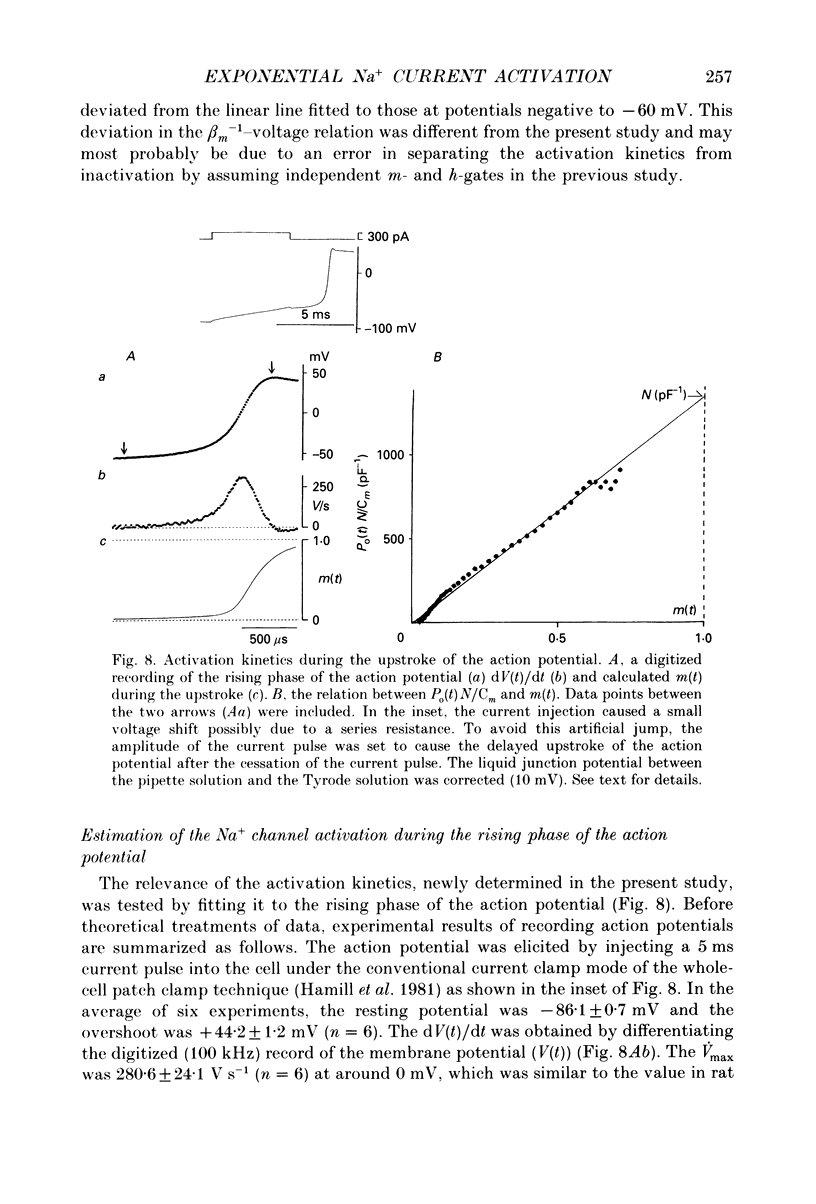
- Berman M. F., Camardo J. S., Robinson R. B., Siegelbaum S. A. Single sodium channels from canine ventricular myocytes: voltage dependence and relative rates of activation and inactivation. J Physiol. 1989 Aug;415:503–531. doi: 10.1113/jphysiol.1989.sp017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F. Mechanisms of closure of cardiac sodium channels in rabbit ventricular myocytes: single-channel analysis. Circ Res. 1987 Jun;60(6):897–913. doi: 10.1161/01.res.60.6.897. [DOI] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F., Strauss H. C. Unitary sodium channels in isolated cardiac myocytes of rabbit. Circ Res. 1983 Dec;53(6):823–829. doi: 10.1161/01.res.53.6.823. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanck D. A., Sheets M. F., Fozzard H. A. Gating currents associated with Na channels in canine cardiac Purkinje cells. J Gen Physiol. 1990 Mar;95(3):439–457. doi: 10.1085/jgp.95.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985 Nov;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimitsuki T., Mitsuiye T., Noma A. Negative shift of cardiac Na+ channel kinetics in cell-attached patch recordings. Am J Physiol. 1990 Jan;258(1 Pt 2):H247–H254. doi: 10.1152/ajpheart.1990.258.1.H247. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuiye T., Noma A. A new oil-gap method for internal perfusion and voltage clamp of single cardiac cells. Pflugers Arch. 1987 Sep;410(1-2):7–14. doi: 10.1007/BF00581889. [DOI] [PubMed] [Google Scholar]
- Mitsuiye T., Noma A. Exponential activation of the cardiac Na+ current in single guinea-pig ventricular cells. J Physiol. 1992;453:261–277. doi: 10.1113/jphysiol.1992.sp019228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanley B. E., Hanck D. A., Chay T., Fozzard H. A. Kinetic analysis of single sodium channels from canine cardiac Purkinje cells. J Gen Physiol. 1990 Mar;95(3):411–437. doi: 10.1085/jgp.95.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]


