Abstract
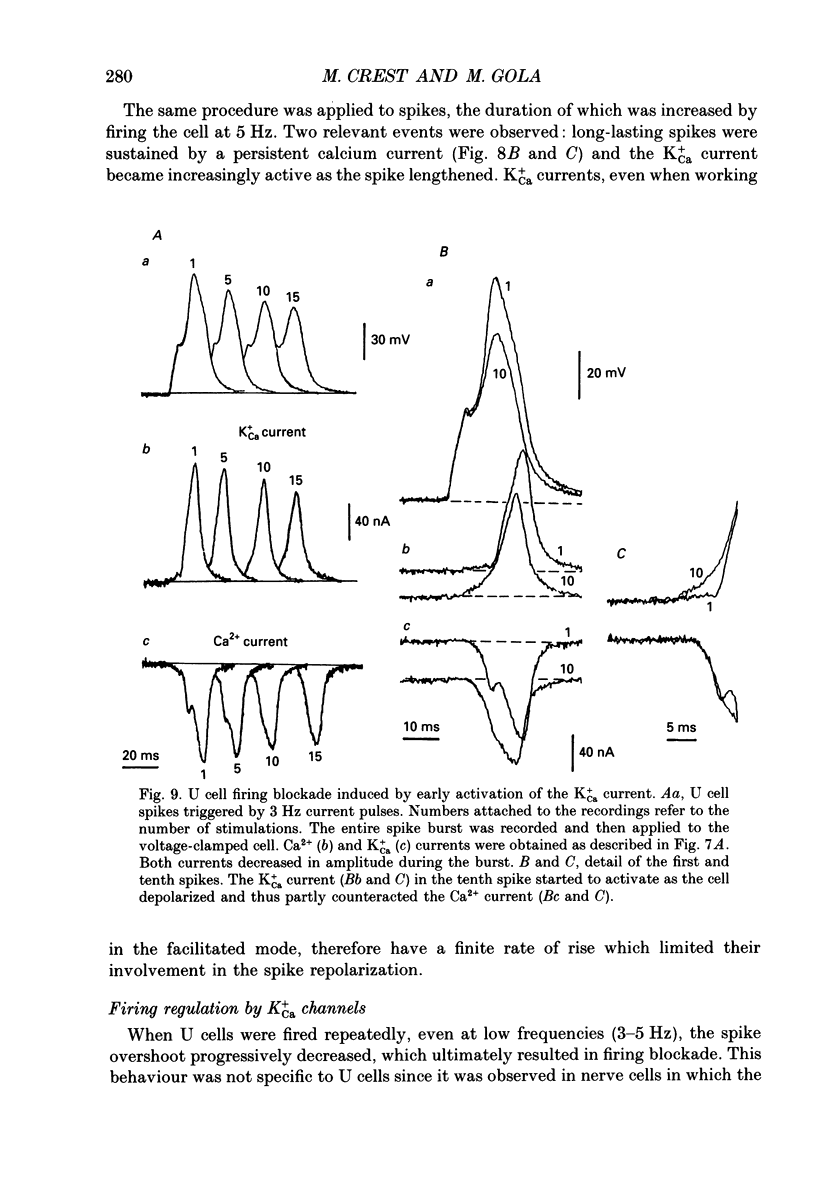
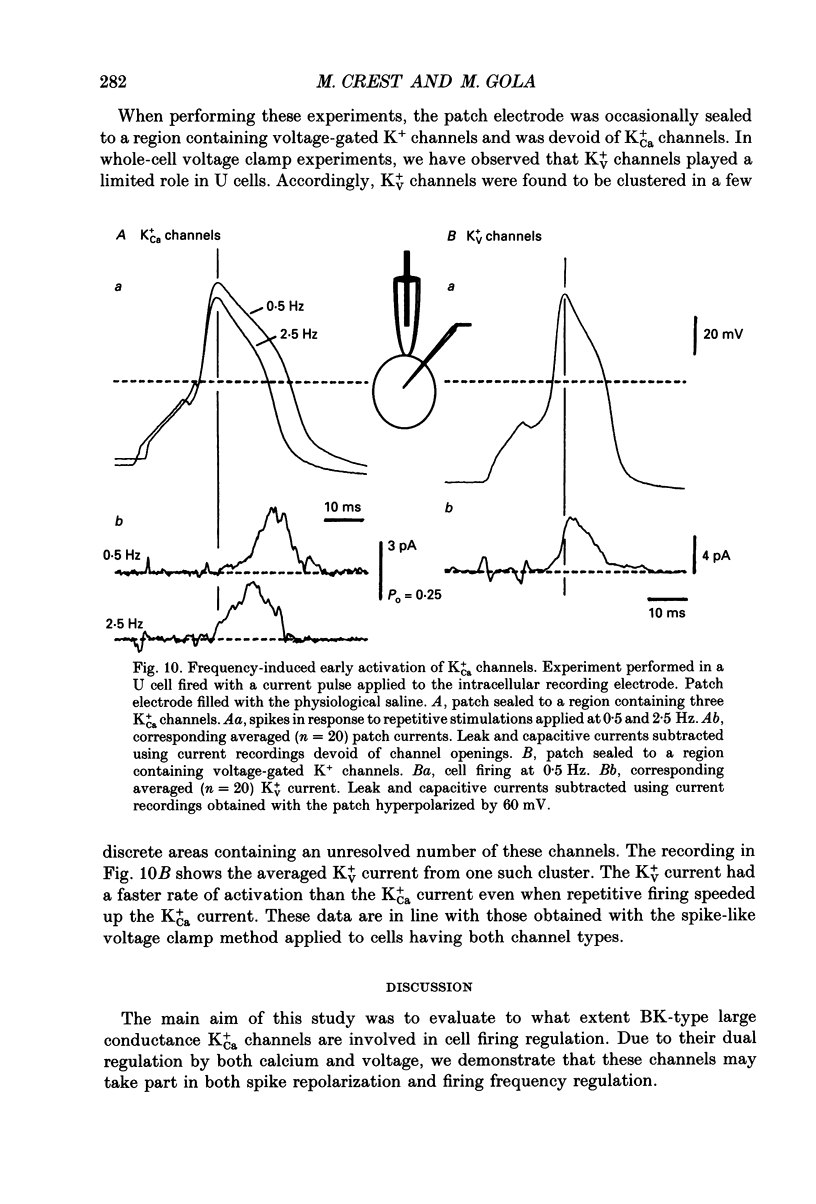
1. The role of BK-type calcium-dependent K+ channels (K+Ca) in cell firing regulation was evaluated by performing whole-cell voltage clamp and patch clamp experiments on the U cell neurones in the snail Helix pomatia. These cells were selected because most of the repolarizing K+ current flowed through K+Ca channels. 2. U cells generated overshooting Ca(2+)-dependent spikes in Na(+)-free saline. In response to prolonged depolarizing current, they fired a limited number of spikes of decreasing amplitude, and behaved like fast-adapting or phasic neurones. 3. Under voltage clamp conditions, the K+Ca current had a slow onset at voltages that induced small Ca2+ entries. By manipulating the Ca2+ entry (either with appropriate voltage programmes or by changing the Ca2+ content of the bath), the K+Ca channel opening was found to be rate limited by the Ca2+ binding step and not by the voltage-dependent conformational change to the open state. 4. Despite the slow activation rate observed in voltage-clamped cells, 25-30% of the available K+Ca current was found to be active during isolated spikes. These data were based on patch clamp, spike-like voltage clamp and hybrid current clamp-voltage clamp experiments. 5. The fact that spikes led the slowly rising K+Ca current to shift into a fast activating mode was accounted for by the large surge of Ca2+ current concomitant with spike upstroke. The early calcium surge resulted in local increases in cytosolic calcium, which speeded up the binding of calcium ions to the closed K+Ca channels. From changes in the null Ca2+ current voltage, it was calculated that the submembrane [Ca2+]i increase to 50-80 microM during the spike. 6. Due to their fast voltage dependence, K+Ca channels appeared to play no role in shaping the interspike trajectory. 7. Even in the fast activating mode, the K+Ca current had a finite rate of rise and was not involved in repolarizing short duration Na(+-dependent action potentials. The current became more and more active, however, when voltage-gated K+ channels were progressively inactivated during firing. 8. The fast adaptation exhibited by U cells upon sustained depolarization was not paralleled by a recruitment of K+Ca channels because of the cumulative Ca2+ entries. During a spike burst, the K+Ca current progressively overlapped the depolarizing Ca2+ current, which ultimately stopped the firing. The early opening of K+Ca channels was ascribed to residual Ca2+ accumulation that kept part of the channels in the Ca(2+)-bound state ready to be opened quickly by cell depolarization.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
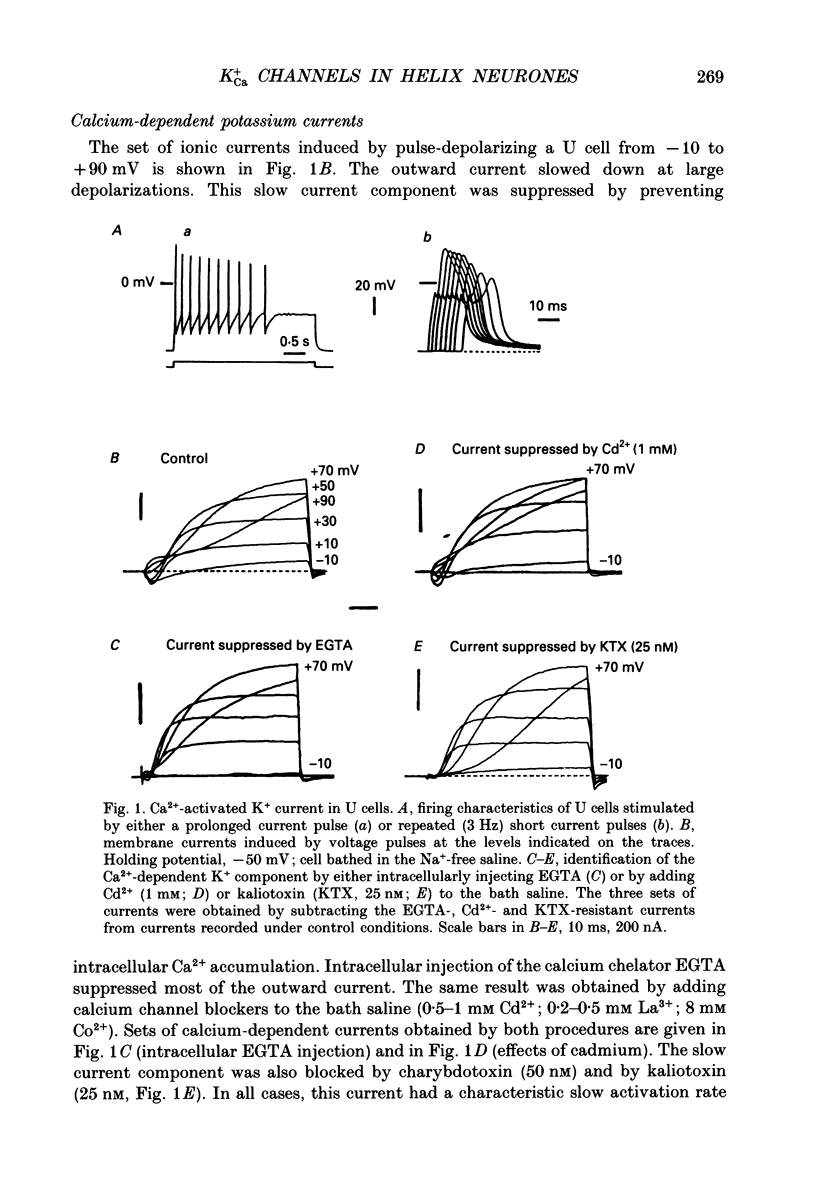
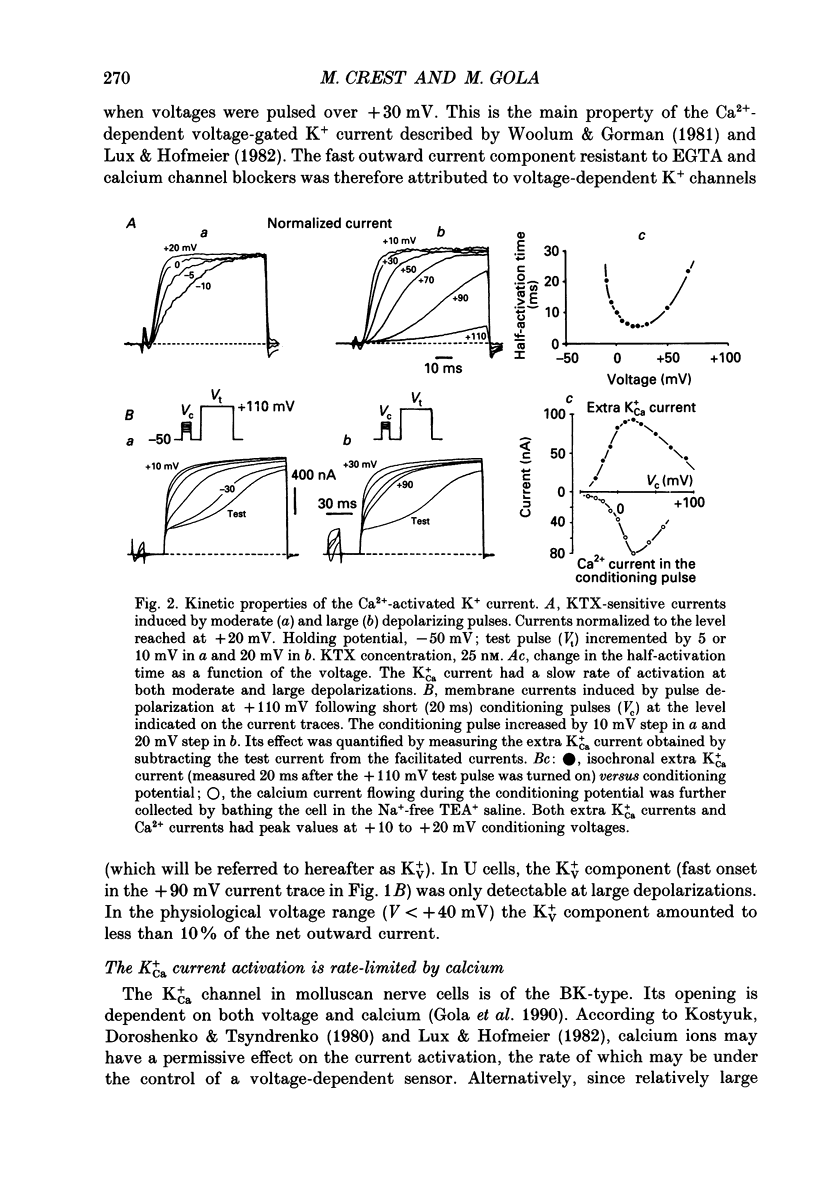
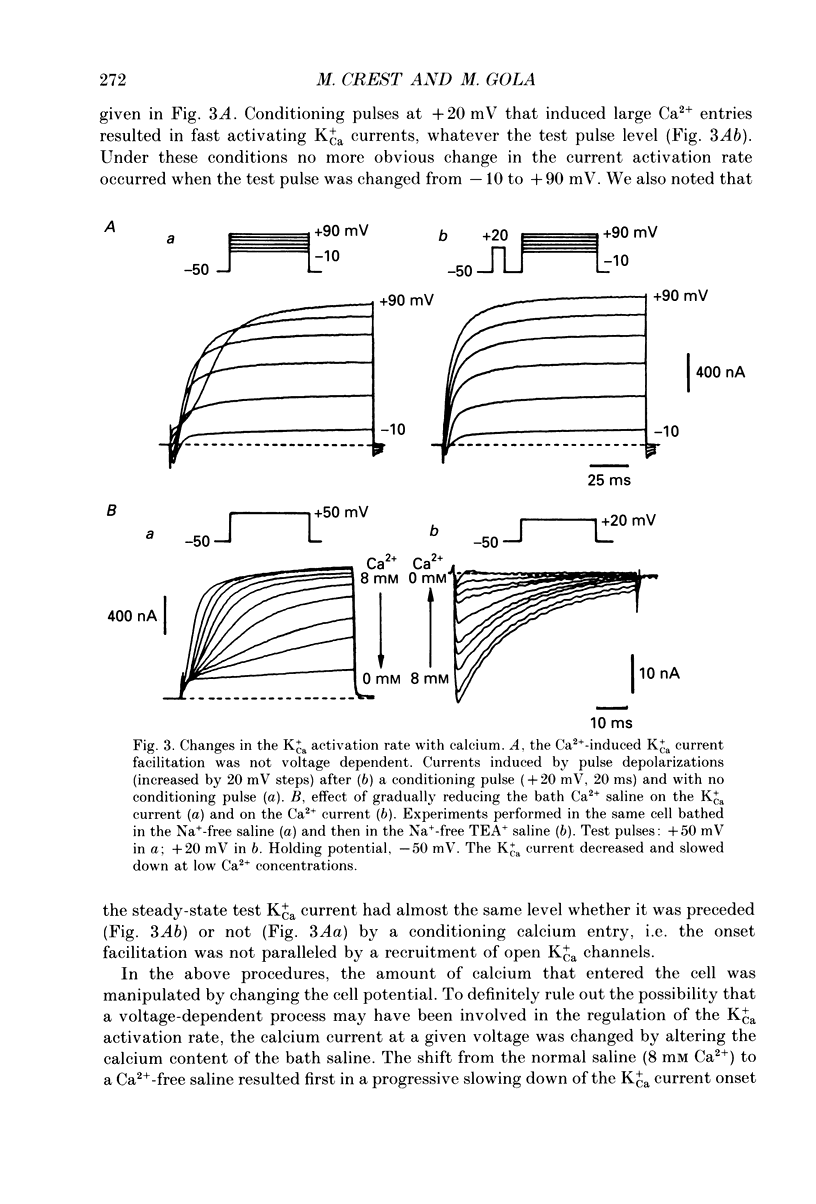
PDF





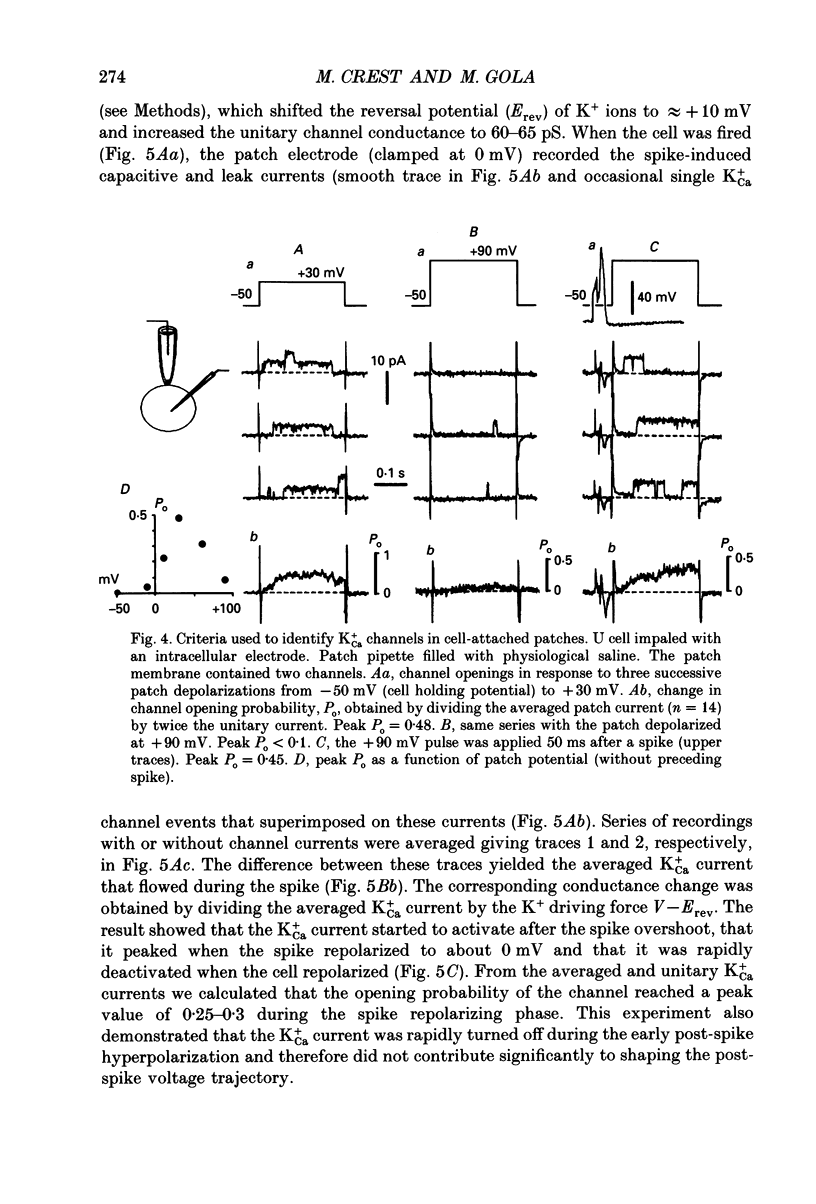
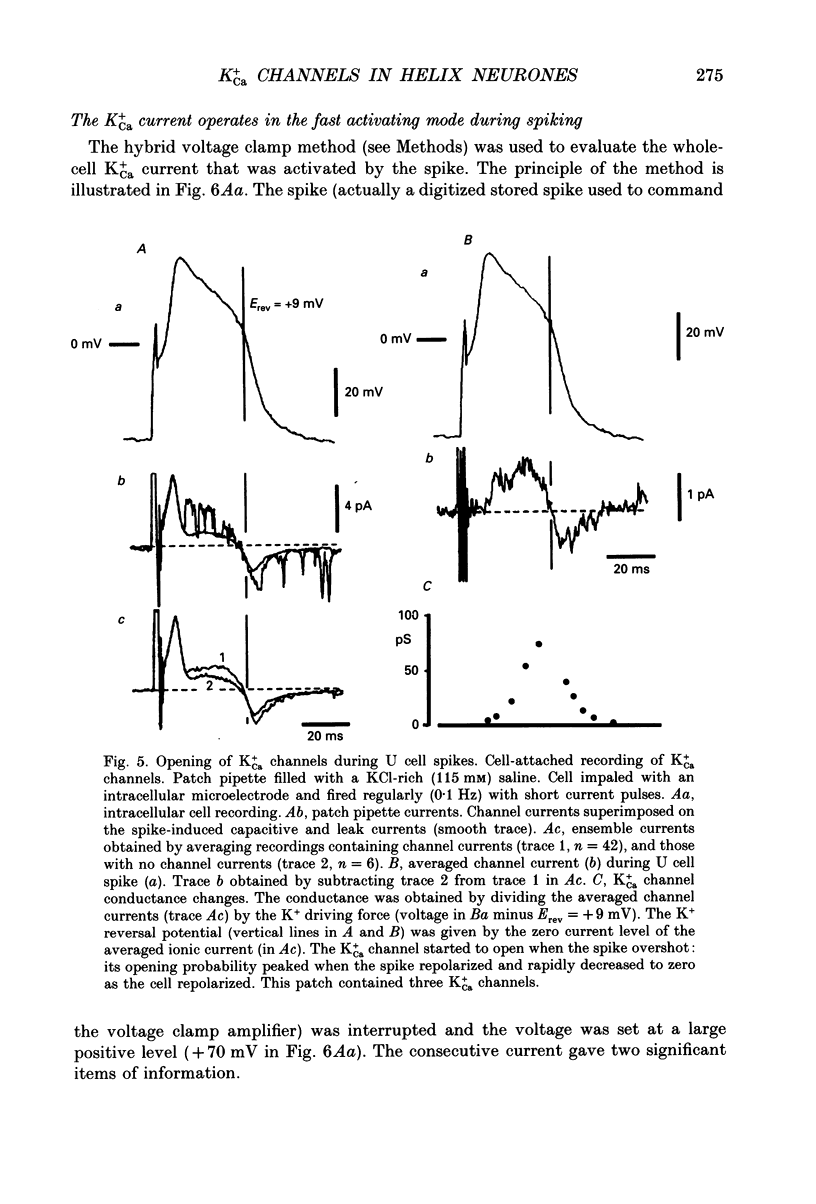
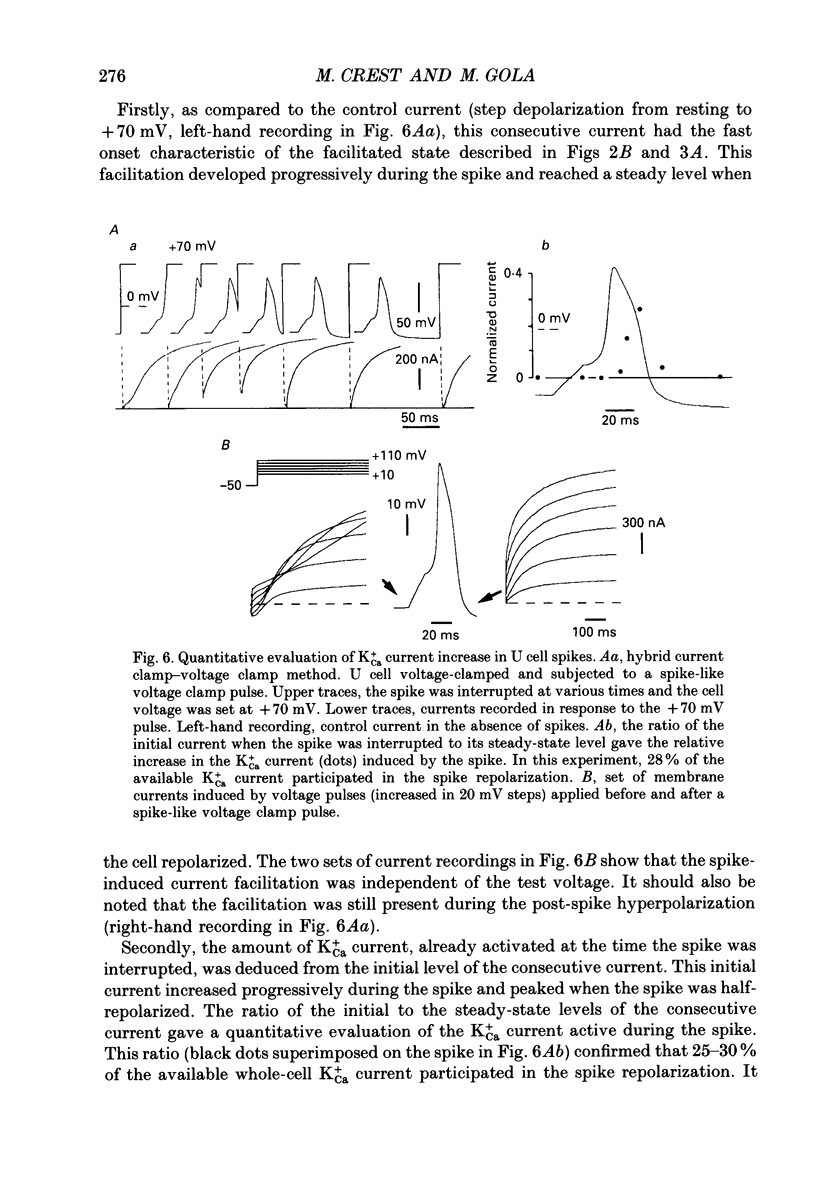

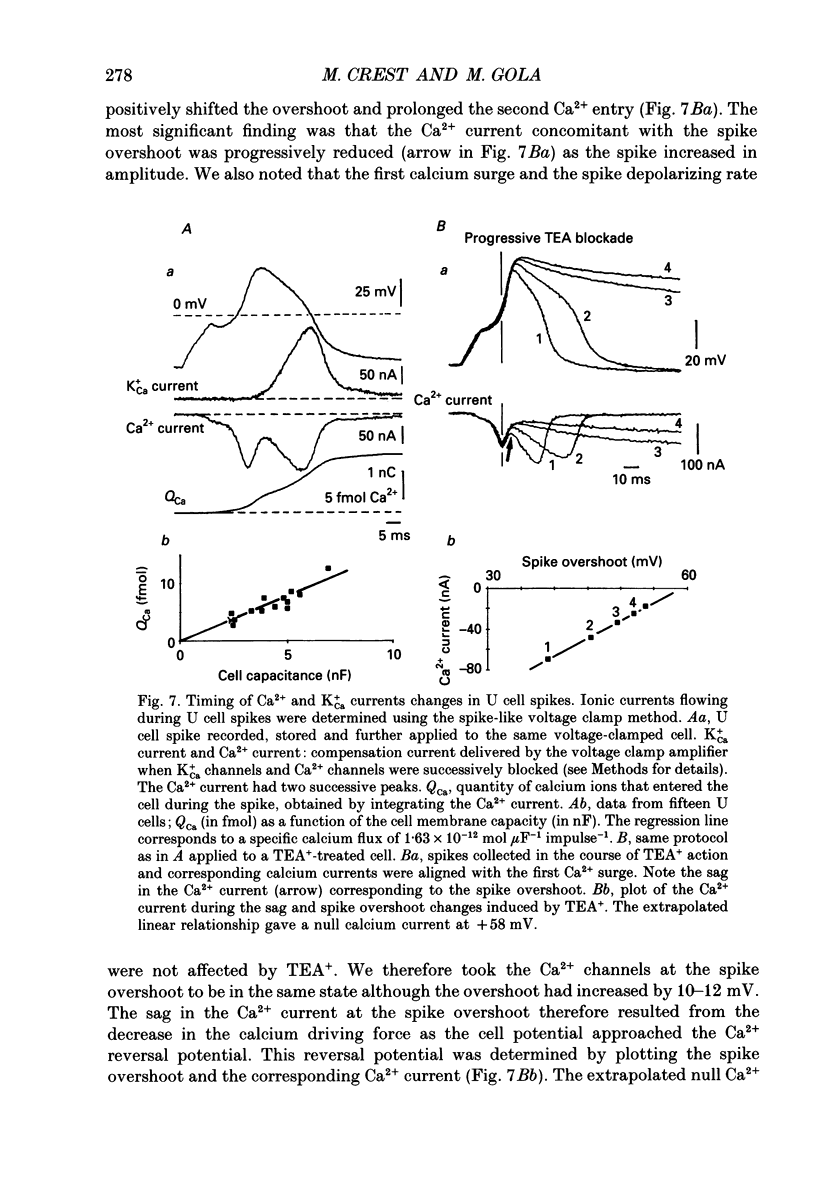
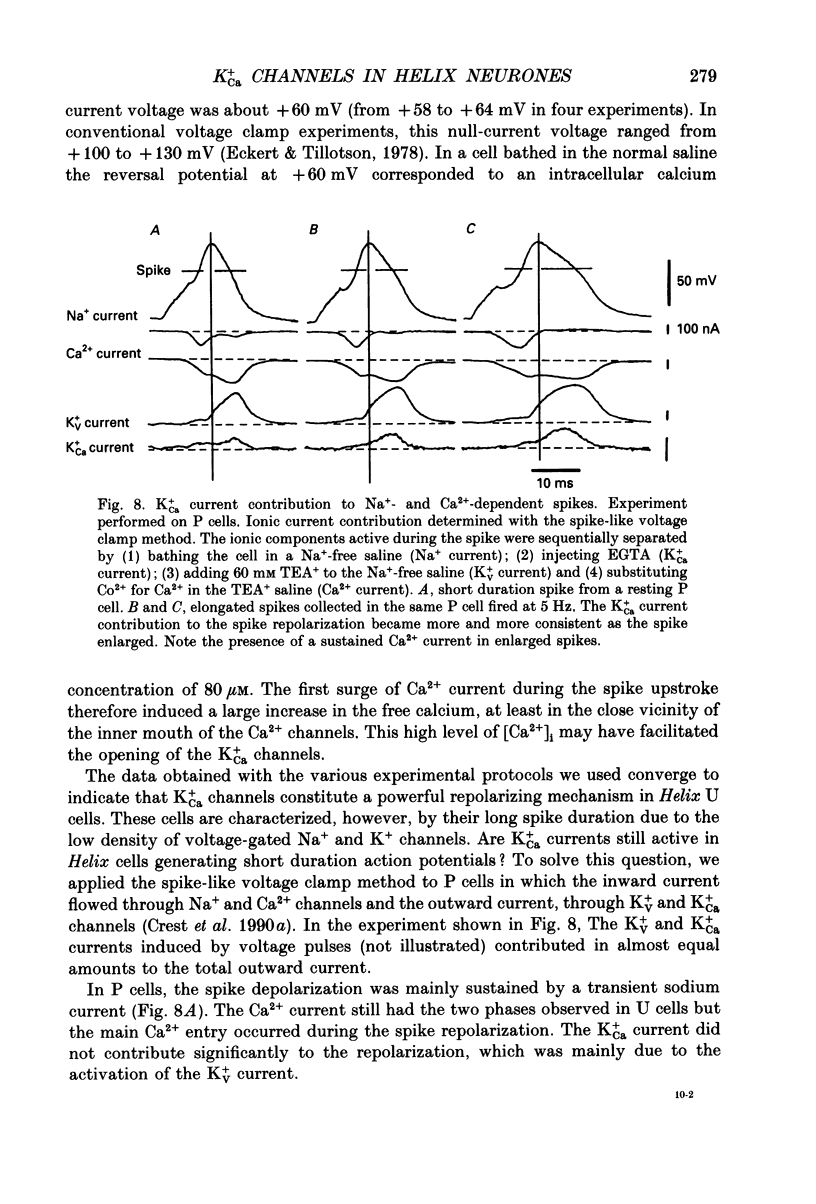






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Williamson A. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol. 1988 May;399:191–205. doi: 10.1113/jphysiol.1988.sp017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Schacher S., Siegelbaum S. A. Action potentials, macroscopic and single channel currents recorded from growth cones of Aplysia neurones in culture. J Physiol. 1986 May;374:289–313. doi: 10.1113/jphysiol.1986.sp016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crest M., Jacquet G., Gola M., Zerrouk H., Benslimane A., Rochat H., Mansuelle P., Martin-Eauclaire M. F. Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca(2+)-activated K+ channels characterized from Androctonus mauretanicus mauretanicus venom. J Biol Chem. 1992 Jan 25;267(3):1640–1647. [PubMed] [Google Scholar]
- Deitmer J. W., Eckert R. Two components of Ca-dependent potassium current in identified neurons of Aplysia californica. Pflugers Arch. 1985 Apr;403(4):353–359. doi: 10.1007/BF00589246. [DOI] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Dourado M. M., Wisgirda M. E. Characteristics of multiple Ca(2+)-activated K+ channels in acutely dissociated chick ciliary-ganglion neurones. J Physiol. 1991 Nov;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. Potassium activation associated with intraneuronal free calcium. Science. 1978 Apr 28;200(4340):437–439. doi: 10.1126/science.644308. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Ducreux C., Chagneux H. Ca2(+)-activated K+ current involvement in neuronal function revealed by in situ single-channel analysis in Helix neurones. J Physiol. 1990 Jan;420:73–109. doi: 10.1113/jphysiol.1990.sp017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Hussy N., Crest M., Ducreux C. Time course of Ca and Ca-dependent K currents during molluscan nerve cell action potentials. Neurosci Lett. 1986 Oct 20;70(3):354–359. doi: 10.1016/0304-3940(86)90578-1. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Intracellular calcium and the control of neuronal pacemaker activity. Fed Proc. 1981 Jun;40(8):2233–2239. [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Potassium conductance and internal calcium accumulation in a molluscan neurone. J Physiol. 1980 Nov;308:287–313. doi: 10.1113/jphysiol.1980.sp013472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988 Apr;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Y., Ono K., Yoshida A., Akaike N. Delayed activation of large-conductance Ca2+-activated K channels in hippocampal neurons of the rat. Biophys J. 1989 Jul;56(1):207–212. doi: 10.1016/S0006-3495(89)82665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J., Yang J., Kleinhaus A. L. Voltage-clamp analysis of the ionic conductances in a leech neuron with a purely calcium-dependent action potential. J Neurophysiol. 1987 Dec;58(6):1468–1484. doi: 10.1152/jn.1987.58.6.1468. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Doroshenko P. A., Tsyndrenko A. Y. Calcium-dependent potassium conductance studied on internally dialysed nerve cells. Neuroscience. 1980;5(12):2187–2192. doi: 10.1016/0306-4522(80)90135-9. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A., Perkel D. J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991 Jan;11(1):23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch. 1987 Dec;410(6):614–622. doi: 10.1007/BF00581321. [DOI] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2(+)-activated K+ channels in a pituitary cell line. J Physiol. 1990 Jun;425:117–132. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Hofmeier G. Activation characteristics of the calcium-dependent outward potassium current in Helix. Pflugers Arch. 1982 Jul;394(1):70–77. doi: 10.1007/BF01108310. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr. 1991 Aug;23(4):537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- Mosfeldt Laursen A., Rekling J. C. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience. 1989;30(3):619–637. doi: 10.1016/0306-4522(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Swandulla D., Lux H. D. Activation of three types of membrane currents by various divalent cations in identified molluscan pacemaker neurons. J Gen Physiol. 1989 Dec;94(6):997–1014. doi: 10.1085/jgp.94.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Levitan I. B. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989 Jan;2(1):1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K. Two distinct calcium-activated potassium currents in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:591–609. doi: 10.1113/jphysiol.1987.sp016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987 Dec 1;435(1-2):387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- Woolum J. C., Gorman A. L. Time dependence of the calcium-activated potassium current. Biophys J. 1981 Oct;36(1):297–302. doi: 10.1016/S0006-3495(81)84729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


