Abstract
Background
Catheter-related bloodstream infection (CR-BSI) stands as one of the leading causes of hospital-acquired infections, often resulting in high healthcare expenditure and mortality rates. Despite efforts, reducing the incidence of CR-BSI remains a significant challenge.
Objective
This study aimed to assess the impact of a multidisciplinary organizational intervention on reducing intravenous CR-BSI.
Methods
A quality improvement team was established to implement various interventions, utilizing the FOCUS-PDCA continuous quality improvement model and fishbone diagram for analysis and improvement.
Results
After the interventions, operational indicators for catheter insertion, maintenance, and removal improved from 82.50% ± 1.15%, 83.60% ± 1.60%, and 81.60% ± 1.80–95.30% ± 1.00%, 96.20% ± 1.62%, and 97.25% ± 0.50%, respectively. Additionally, catheter dwell time decreased from 7.50 ± 0.85 days to 3.50 ± 0.75 days, and the quarterly infection rate was reduced from 2.328% ± 1.85–0.305% ± 0.95% following the implementation of the intervention.
Discussion
Despite the available evidence, there remains a noticeable gap between the ideal evidence-based practices and their practical implementation. We aim to eradicate CR-BSIs within the surgical intensive care units (ICUs) of hospitals. To achieve this goal, we have introduced a comprehensive quality improvement framework designed not only to benefit our own ICU but also to serve as a model for implementation in other similar healthcare settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-024-05002-7.
Keywords: General surgical nursing departments, Nosocomial infection, Central venous line, Continuous quality improvement, Organizational creativity
Background
Central venous catheters (CVCs) play a pivotal role in the treatment and care of surgical inpatients. However, the prevalence of catheter-related bloodstream infections (CR-BSIs) remains a significant concern in healthcare settings [1]. Annually, hospitals in the United States witness approximately 80,000 CR-BSI cases, tragically resulting in nearly 30,000 fatalities [2]. CR-BSIs impose a cost of over $2 billion annually on the healthcare system of the United States, with an average expenditure of $45,000 per patient [3]. Within the vast patient population in China, there exists a significant incidence of CR-BSIs, accompanied by substantial medical expenses [4]. This underscores the critical need for effective interventions to mitigate the impact of CR-BSIs and improve patient outcomes.
Recent reports have emphasized the effectiveness of implementing multidisciplinary teams, continuously monitoring insertion and maintenance practices, and adopting antimicrobial catheters to overcome barriers in reducing CR-BSI rates and enhancing patient safety [3, 5].
We have been steadfastly confronting the challenge of CR-BSIs for many years. The substantial body of research in this area affirms the effectiveness of CR-BSI prevention, with numerous studies consistently demonstrating the potential for significant reductions in their incidence, accompanied by remarkable improvements in morbidity, mortality rate, and the associated economic burden [6–9]. Additionally, several organizations, including the Centers for Disease Control and Prevention (CDC), the Society of Critical Care Medicine, and the Association for Professionals in Infection Prevention and Epidemiology, have diligently crafted guidelines aimed at preventing CR-BSIs [10]. Evidence-based strategies have been validated for their efficacy, including rigorous hand hygiene, the use of antiseptic skin preparations, adherence to strict aseptic principles during CVC insertion, a preference for the subclavian vein catheterization, and an unwavering commitment to the maintenance of stringent aseptic practices during catheter placement [11].
Methods
Study Design
Interdisciplinary approach
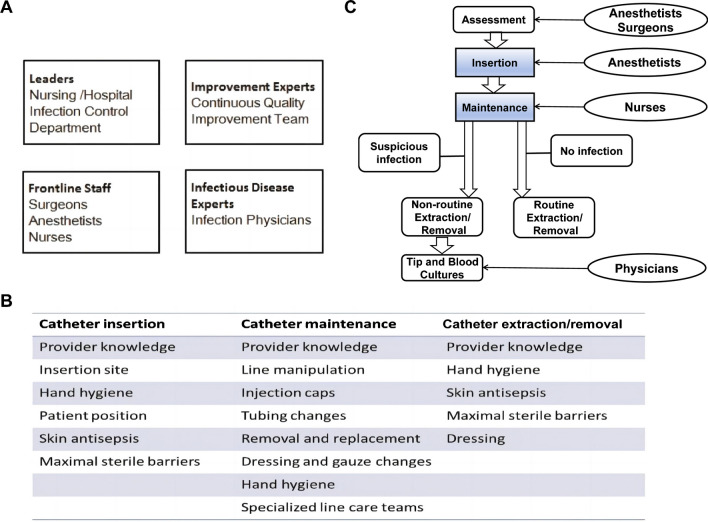
We assembled an interdisciplinary team comprising a lead general surgeon, an infectious disease specialist, an anesthetist, a continuous quality improvement (CQI) expert, and all frontline staff (Fig. 1A). The team aimed to develop a systematic flowchart and checklist for measuring catheter utilization and understanding how this data could guide ongoing improvement efforts to minimize CR-BSIs. The process of catheter utilization encompassed assessment of indications, insertion, maintenance, and removal. Based on published literature on CR-BSIs, the team compiled a list of potential risk factors, categorizing them based on whether they are associated with insertion, routine maintenance, or removal (Fig. 1B). In our general surgery ward, junior staff were responsible for catheter insertion, while nurses of various levels handled maintenance. Recognizing catheter utilization as an area where effective improvements could be measured, the team focused on enhancing the process of insertion, maintenance, and removal.
Fig. 1.
Strategies for CQI. A. Formation of an interdisciplinary team. The interdisciplinary team consists of a general surgery leader, an infectious disease specialist, an anesthesiologist, frontline staff, and a CQI expert B. Identification of risk factors for catheter insertion, routine maintenance, and removal. The team compiled a list of risk factors associated with catheter usage by reviewing relevant literature on CR-BSI C. System process map for catheter use. We developed a comprehensive process map to measure real-time catheter use and CQI. Routine Extraction/Removal: This is performed when treatment is no longer needed or when the catheter has expired. Non-routine Extraction/Removal: This is performed due to suspected infection, catheter-related complications, or catheter damage
Formation of the insertion team
A dedicated CVC insertion team was established, comprising senior anesthetists, junior anesthetists, and interns. Catheter insertions were performed only when hemodynamic changes were deemed necessary, such as in cases of surgery, severe trauma, shock, and acute circulatory failure, or when required for high nutritional therapy or intravenous antibiotic treatment. The expertise of senior anesthetists, their understanding of infection prevention, and their strict adherence to standardized checklists significantly bolstered the team’s capabilities. It has been documented that inexperienced healthcare providers elevate the risk of infection during catheter insertion [10]. Therefore, anesthetists conducted catheter insertions in the operating room or vascular access room, following aseptic procedures and under appropriate supervision. In addition to developing the innovative checklist, senior anesthetists also provided the scientific rationale underpinning the recommended actions.
The effectiveness of CQI in various healthcare settings inspired us to adopt a similar approach. CQI focuses on addressing iterative challenges faced by frontline staff, guided by real-time process measurements to make informed decisions. Through systematic measurement of crucial procedures impacting clinical outcomes, CQI empowers teams to optimize enhancement endeavors and maintain progress [12–14]. Infection control practitioners closely monitored both the quality of catheter insertions (inputs) and the occurrences of CR-BSI (outputs). Before implementing CQI, we developed a systematic flowchart and checklist to monitor the utilization of catheters in real time (Fig. 1C and Supplementary file 1).
We chose the FOCUS-PDCA model for CQI to analyze and enhance our procedures. This model, developed by Hospital Corporation of America (HCA), has proven to be an effective strategy for performance improvement [15]. It has been successfully employed in various medical specialties, including nursing, laboratory science, trauma care, and surgical procedures. The acronym “FOCUS-PDCA” represents a methodology centered on identifying a process for improvement, assembling a knowledgeable team, clarifying the existing understanding of the process, understanding the reasons for change, and selecting a strategy to initiate the PDCA cycle. The PDCA cycle, also known as the Deming cycle, signifies an iterative quality improvement process that includes four stages: plan and make improvements, evaluate the outcomes, take action, and sustain the gains [15, 16].
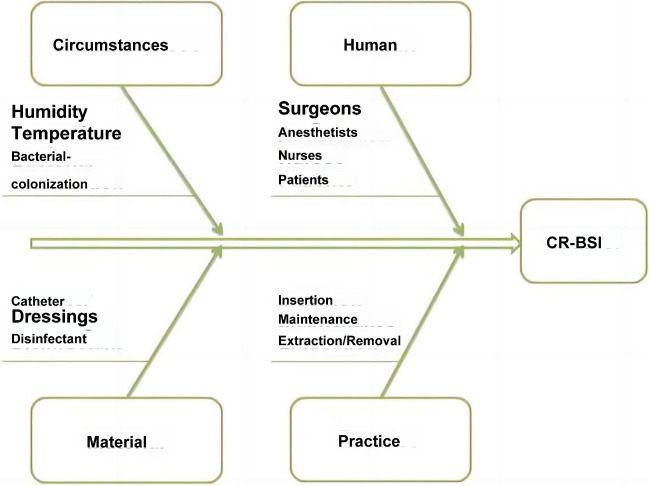
The fishbone diagram, originally a component of quality assurance plans in the manufacturing sector, has found extensive application as a healthcare safety tool for conducting root cause analysis of system errors in medical facilities and other healthcare settings [17, 18]. This diagram aids in categorizing complex medical errors into distinct groups, with the visual representation of interconnected categories in the fishbone structure revealing potential areas for improvement (Fig. 2).
Fig. 2.
Fishbone Diagram of the Comprehensive Evaluation Framework for CR-BSI. The fishbone diagram represents the potential causes of CR-BSI, encompassing the categories of environment, materials, personnel, and/or practice protocols
We delineate the process of improving catheter utilization, which involves assembling a multidisciplinary team, gaining insights from existing literature on catheter utilization, identifying infection causes using the fishbone diagram, choosing a process management strategy, planning and making improvements based on the checklist, conducting measurement checks, and taking action to maintain and sustain the improvements.
Intervention measure 1: Formation of the maintenance team
We established a CVC maintenance team, which included nursing management, nursing personnel, and infection control staff. Nursing leadership played a pivotal role in strengthening the team by adhering to a comprehensive checklist, leveraging their expertise in preventing bloodstream infections, and facilitating initial team discussions. Regular meetings were held by the maintenance team to strategize and implement effective measures. All members of the nursing team acknowledged the challenge of reducing infection rates among general surgery patients. A group of experienced nurses collaborated closely with the team to incorporate catheter maintenance into health education programs, with the goal of minimizing variations in nursing practices and strategies. To ensure consistency and adherence to best practices, a dedicated surgical nurse documented all action items and took responsibility for the daily catheter maintenance tasks. Strict guidelines were followed for catheter removal in cases of occlusion, phlebitis, local/bloodstream infection, uncontrollable bleeding, or discontinuation of specific medications.
Intervention measure 2: Catheter insertion practices
Inserters are required to strictly adhere to standard insertion site protocols and rigorously follow all infection-reduction procedures, even in life-threatening situations, within the emergency care unit, intensive care unit (ICU), or operating room.
Intervention measure 3: Hand Hygiene and sterile gloves
Ensuring proper hand hygiene and employing sterile gloves before catheter insertion contribute to the reduction of CR-BSIs. Acknowledging the efficacy of hand sanitizers for hand disinfection compared to soap and water, we endorse the practice of proper hand hygiene and the use of sterile gloves.
Intervention measure 4: Chlorhexidine skin preparation
Effective skin disinfection at the puncture site plays a pivotal role in infection reduction. Notably, chlorhexidine skin preparation has demonstrated superior efficacy in reducing CR-BSIs compared to povidone-iodine and/or alcohol. Even in cases where alternative disinfectants are employed, we recognize the significance of considering chlorhexidine.
Intervention measure 5: Maximum sterile barriers
Numerous studies emphasize the critical role of maximum sterile barriers (gloves, gowns, masks, and patient drapes) in preventing infections during catheter insertion, maintenance, and removal [19, 20]. Our checklist records individual barriers used or neglected throughout the intervention. In our context, “maximum” denotes the comprehensive utilization of all four protective barriers.
Intervention measure 6: Dressings
Transparent dressings are scheduled for replacement every 7 days, whereas gauze dressings are changed every 3 days. Dressings should be promptly replaced if they become soiled, wet, loose, or bloodstained, if the date of last change is unknown, or if any other signs indicating susceptibility to infection are observed. Additionally, it is recommended to perform chlorhexidine skin preparation when changing dressings.
Intervention measure 7: Standardized practices
Our approach to CR-BSI prevention involves meticulous standardization at every stage of catheter management. (1) Catheter insertion preparation: Rigorous hand hygiene, maximum sterile barriers, chlorhexidine skin preparation, and maintaining a sterile procedural environment are ensured. During catheter insertion, surgical site disinfection measures, involving the use of 2% chlorhexidine for cleansing and placement of sterile drapes, are consistently applied. A thin sterile adhesive dressing is then applied to protect the insertion site. (2) Catheter maintenance: This phase includes hand hygiene, regular dressing changes, authorized injection caps usage, access site cleaning, and overall line care. Competency standards for catheter dressing changes are collaboratively established by nursing administration and general surgery nursing practitioners. Regular meetings led by infectious disease physicians identify areas for improvement or negligence in CR-BSI cases with severe consequences. (3) Nursing staff training: Group and subsequent team-led small-group demonstrations of daily catheter maintenance procedures and dressing changes ensure uniform practices. Continuous education minimizes practice variations among staff. (4) Pre-intervention chart reviews: Chart reviews identify compliance gaps in daily bathing or showering provision and recording, rectified through targeted education for nursing personnel and patients. Hospital wards prominently display posters emphasizing the importance of CR-BSI prevention. (5) Catheter removal preparation and procedure: Stringent protocols, including hand hygiene, chlorhexidine skin preparation, and a sterile procedural environment, are followed. The same surgical disinfection measures are implemented during catheter removal, and the insertion site is secured with a sterile dressing. Principles from patient safety to reinforce nurses’ adherence to CDC guidelines [21].
Data collection and analysis
CR-BSI diagnosis was conducted according to well-recognized standards, which included culturing a 5-cm segment of the catheter tip and peripheral venous blood [2]. Data collection commenced in the first quarter (Q1) of 2015 and concluded in Q1 of 2017. Electronic medical records (EMR) were utilized to assess the CR-BSI incidence rate and document catheter insertion and daily maintenance. Pre-intervention data were gathered in Q1 of 2015, involving a retrospective review of CR-BSI rates for each quarter from Q1 2015 to Q1 2016. Post-intervention data were gathered quarterly, spanning CR-BSI rates from Q2 2016 to Q1 2017 for each respective quarter. Statistical significance was determined using unpaired Student’s t-tests or proportion tests, with differences considered significant for P-value < 0.05. The analysis was conducted using the SPSS software (version 11.0). No instances of data duplication were detected.
Results
Our interventions improved catheterization performance indicators
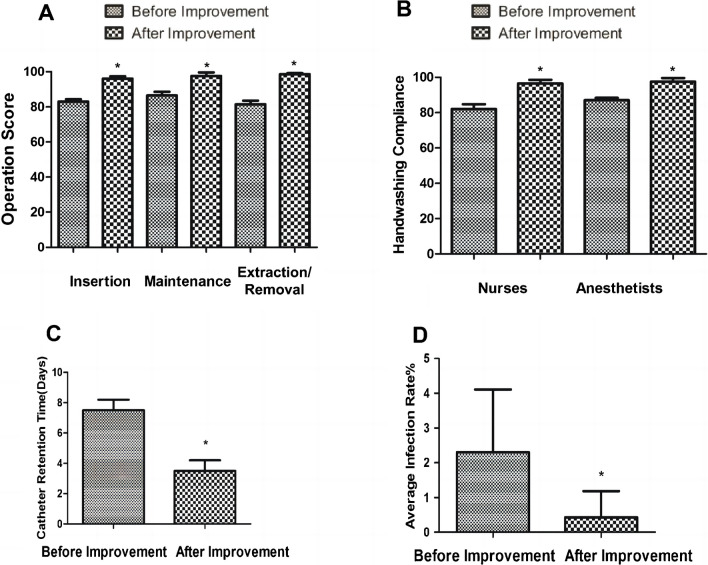
To determine the effectiveness of our interventions, we assessed the catheterization performance indicators. The results revealed that compared to the pre-intervention period (82.50% ± 1.15%, 83.60% ± 1.60%, and 81.60% ± 1.80%), the performance indicators for catheterization, maintenance, and removal significantly increased after the intervention (95.30% ± 1.00%, 96.20% ± 1.62%, and 97.25% ± 0.50%) (Fig. 3A).
Fig. 3.
Effects of CQI. A. Surgical scoring for insertion, maintenance, and removal. The improved surgical scoring is higher compared to the pre-improvement period. The scores are calculated using specialized tables (Supplementary files 2-4). A higher score indicates a more standardized operation. B. Adherence to hand hygiene protocols among nursing staff and anesthesiologists. The level of adherence to hand hygiene practices among nursing personnel and anesthesiologists is higher after the improvement. C. CDT. The improved CDT is shorter than before. Data is expressed as the mean value with its corresponding standard deviation. (*P<0.05). D. Quarterly average infection rate. The improved quarterly average infection rate is lower than before. Data is expressed as the mean value with its corresponding standard deviation. (*P<0.05) GSD, General Surgery Department; OD, Other Departments
Adherence to hand hygiene protocols by nurses and anesthetists showed improvement
We next evaluated the hand hygiene protocol adherence and discovered that compared to pre-intervention (83.00% ± 3.65% and 83.50% ± 1.10%), hand hygiene compliance significantly increased after the intervention (95.21% ± 1.50% and 96.30% ± 2.30%) (Fig. 3B), suggesting the ongoing efforts in CQI resulted in a remarkable increase in compliance with hand hygiene practices among both nurses and anesthetists.
Catheter dwell time (CDT) was reduced upon interventions
Subsequently, we investigated the impact of our interventions on CDT. The data illustrated that compared to the pre-intervention CDT of 7.50 ± 0.85 days, a significant decrease was observed post-intervention, with a dwell time of 3.50 ± 0.75 days (* P < 0.05) (Fig. 3C), indicating that, after surgeons closely monitored indications for catheter removal, the CDT was decreased.
Significant decrease in quarterly infection Rates following improvement
Finally, we measured the quarterly infection rates, and the results showed that compared to the pre-intervention quarterly average infection rate of 2.328% ± 1.85%, CQI led to a noteworthy decrease in infection rates, dropping to 0.305% ± 0.95% (* P < 0.05) (Fig. 3D), demonstrating that the infection rate post-intervention was significantly reduced compared to both the pre-intervention rate and the DNDQI benchmark. These findings collectively suggest that our interventions are effective in reducing CR-BSIs.
Discussion
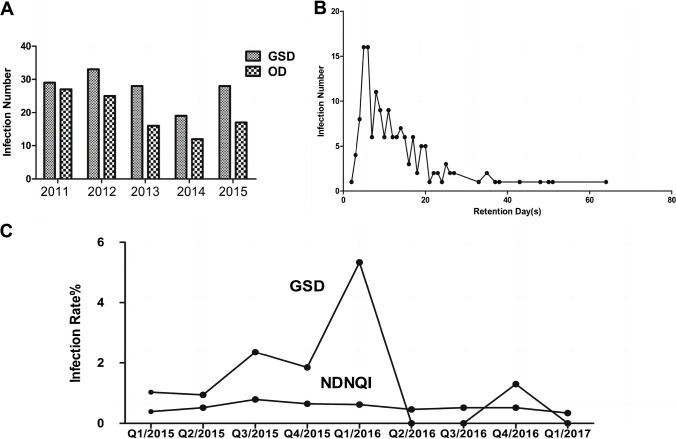
In the last decade, our center has achieved significant improvements in CR-BSIs. However, infection rates within the general surgical nursing units still constitute more than half of all infections in our hospital, ranging from 51 to 68% (Fig. 4A). Notably, the peak occurrence of infections in these units is within 5 to 7 days of catheter placement (Fig. 4B). By Q1 of 2016, the bloodstream infection rate had already declined to a range of 0.94–5.34%. To further mitigate infection rates, we aimed to reassess catheter insertion, maintenance, and removal processes, comparing them to the infection rates from the National Database of Nursing Quality Indicators (NDNQI) (Fig. 4C).
Fig. 4.
Overview of CR-BSI in our hospital A. Number of infections in various departments, including General Surgery. More than half (51–68%) of the infection events occurred in the General Surgery nursing unit B. Association between the number of insertions and bloodstream infections and the duration of CDT. The peak occurrence of infections was within 5 to 7 days of catheter placement C. Reassess catheter insertion, maintenance, and removal processes, comparing them to the infection rates from the National Database of Nursing Quality Indicators. To further mitigate infection rates, we aimed to reassess catheter insertion, maintenance, and removal processes, comparing them to the infection rates from the National Database of Nursing Quality Indicators (NDNQI). Higher infection rates were observed with a dwell time of 3–20 days. Quarterly infection rates for 2015–2017. Before improvement, the infection rate in the General Surgery department was significantly higher than that of NDNQI. After the interventions, the infection rate in the General Surgery department was lower than the NDNQI rate
There is ongoing debate about the feasibility of completely eradicating CR-BSIs, with some suggesting that ICUs should aim to reach the 50th percentile benchmark of national hospital infection monitoring for patient cohorts with similar characteristics [22]. While we and others advocate the pursuit of zero CR-BSI as the primary goal. In this study, we have demonstrated the almost elimination of CR-BSIs.
CVCs serve as essential therapeutic devices facilitating the administration of medications, fluids, and high osmolarity nutrition, as well as vascular monitoring for hospitalized patients, including outpatient populations such as those receiving hemodialysis. However, the inevitable disruption of the skin’s physical barrier by CVCs poses an inherent risk of CR-BSIs [3, 5]. Furthermore, these catheters are prone to microbial colonization, creating an environment conducive to biofilm formation, thus complicating infection treatment. Additionally, catheter use is recognized as a contributing factor to the risk of developing multidrug-resistant bacterial infections and fungal sepsis, often caused by non-albicans Candida species [7]. Given these considerations and the significant prevalence of bloodstream infections associated with catheter usage, the prevention of CR-BSIs emerges as a crucial necessity.
While numerous healthcare settings have established evidence-based clinical practice guidelines, the challenge lies in their effective implementation and closing the gap between ideal evidence and real-world practices [23]. In our general surgical nursing unit, we have implemented straightforward and cost-effective interventions aimed at improving adherence to evidence-based infection control practices and protocols. These efforts are expected to lead to a substantial reduction in CR-BSIs within our department, ultimately alleviating the burden on health outcomes, reducing fatality rates, and curbing healthcare expenditures for patients.
A multidisciplinary team has developed a standardized nursing checklist for catheter insertion, encompassing hand hygiene, maximal sterile barriers, skin preparation, procedural environment, dressings, and standardized procedures. In a collaborative effort, infection control professionals have streamlined the process by digitizing the finalized checklist into a database; thereby generating instantaneous process measurement data [22, 24]. With these process measurement data, the team optimized the effectiveness of the interventions and effectively tackled obstacles in reducing the occurrence of CR-BSIs. A comprehensive study revealed a notable deficit in healthcare personnel’s knowledge regarding the prevention of CR-BSIs, underscoring the need for regular assessment of healthcare personnel’s knowledge and practices [21].
Alternative approaches involve the utilization of antimicrobial-coated medical devices, as well as the utilization of antimicrobial or ethyl alcohol lock strategies, all of which have been employed to mitigate the incidence of CR-BSIs. Furthermore, catheter maintenance bundles that incorporate proper hand sanitation, bandage replacements, routine bathing or cleansing, the implementation of injection caps, cleaning of entry sites, and line upkeep have been introduced into clinical practice to reduce infection rates [25]. Notably, authoritative organizations such as the CDC have also issued guidelines for the prevention of CR-BSIs.
Nevertheless, there remain some limitations associated with this ongoing quality enhancement initiative. Firstly, the checklist does not encompass all aspects of catheter use, such as hand hygiene and scrub cap duration, as well as the number of catheter insertions. Secondly, more precise measurements are required to evaluate nursing practices, accounting for varying levels of experience and adherence to the checklist. Thirdly, the assessment of patients’ nutritional condition was not included, despite the known vulnerability of patients with compromised nutrition to infections. Lastly, our team conducted CQI for only one year; a longer duration is needed to provide a more comprehensive understanding of CR-BSI rates and the sustainability of the improvement project.
Conclusion
In summary, our study confirms that improvements in catheter insertion, maintenance, and removal can effectively diminish the occurrence of CR-BSIs. It underscores the critical role of multidisciplinary teams and checklists in bolstering prevention efforts.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 22.6 KB)
(DOCX 18.2 KB)
(DOC 62.0 KB )
(DOC 54.0 KB)
Acknowledgements
We express our sincere gratitude to the participants in this study, as well as to nurses who assisted with participant recruitment for this research.
Author contributions
Lingli Xu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Roles/Writing - original draft, Writing - review & editing. Leiwen Tang: Methodology, Visualization, Writing - review & editing. Jianfen Qin: Methodology, Validation, Writing - review & editing. Hongying Pan: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - review & editing.
Funding
The study was supported by Medical Science and Technology Project of Zhejiang Province (2023KY806).
Data availability
Data available on request.
Declarations
Conflict of interests
The authors declared no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lutwick L et al (2019) Managing and preventing vascular catheter infections: a position paper of the international society for infectious diseases. Int J Infect Dis 84:22–29 [DOI] [PubMed] [Google Scholar]
- 2.Mermel LA et al (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pronovost PJ, Marsteller JA, Goeschel CA (2011) Preventing bloodstream infections: a measurable national success story in quality improvement. Health Aff 30:628–634 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y et al (2023) Incidence rate, pathogens and Economic Burden of catheter-related bloodstream infection: a Single-Center, Retrospective Case-Control Study. Infect Drug Resist 16:3551–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, Haniffa R (2014) Critical care and severe sepsis in resource poor settings. Trans R Soc Trop Med Hyg 108:453–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetz AM, Wagener MM, Miller JM, Muder RR (1998) Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol 19:842–845 [DOI] [PubMed] [Google Scholar]
- 7.Raad II et al (1994) Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol 15:231–238 [PubMed] [Google Scholar]
- 8.Mermel LA, McCormick RD, Springman SR, Maki DG (1991) The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-ganz catheters: a prospective study utilizing molecular subtyping. Am J Med 91:197s–205s [DOI] [PubMed] [Google Scholar]
- 9.Maki DG, Ringer M, Alvarado CJ (1991) Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet (London England) 338:339–343 [DOI] [PubMed] [Google Scholar]
- 10.O’Grady NP et al (2011) Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Diseases: Official Publication Infect Dis Soc Am 52:e162–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mermel LA (2000) Prevention of intravascular catheter-related infections. Ann Intern Med 132:391–402 [DOI] [PubMed] [Google Scholar]
- 12.Buttigieg SC, Gauci D, Dey P (2016) Continuous quality improvement in a Maltese hospital using logical framework analysis. J Health Organ Manag 30:1026–1046 [DOI] [PubMed] [Google Scholar]
- 13.Silver SA et al (2016) How to sustain change and support continuous quality improvement. Clin J Am Soc Nephrology: CJASN 11:916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakyo TA, Xiao LD (2017) Nurse managers’ experiences in continuous quality improvement in resource-poor healthcare settings. Nurs Health Sci 19:244–249 [DOI] [PubMed] [Google Scholar]
- 15.Saxena S, Ramer L, Shulman IA (2004) A comprehensive assessment program to improve blood-administering practices using the FOCUS-PDCA model. Transfusion 44:1350–1356 [DOI] [PubMed] [Google Scholar]
- 16.Maraiki F, Farooq F, Ahmed M (2016) Eliminating the use of intravenous glass bottles using a FOCUS-PDCA model and providing a practical stability reference guide. Int J Pharm Pract 24:271–282 [DOI] [PubMed] [Google Scholar]
- 17.Reilly JB, Myers JS, Salvador D, Trowbridge RL (2014) Use of a novel, modified fishbone diagram to analyze diagnostic errors. Diagnosis (Berlin Germany) 1:167–171 [DOI] [PubMed] [Google Scholar]
- 18.Chang H (2015) Evaluation Framework for Telemedicine using the logical Framework Approach and a Fishbone Diagram. Healthc Inf Res 21:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorski LA (2017) The 2016 infusion Therapy standards of Practice. Home Healthc Now 35:10–18 [DOI] [PubMed] [Google Scholar]
- 20.Gorski LA et al (2021) Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs 44, S1-S224 [DOI] [PubMed]
- 21.Alkubati SA, Ahmed NT, Mohamed ON, Fayed AM, Asfour HI (2015) Health care workers’ knowledge and practices regarding the prevention of central venous catheter-related infection. Am J Infect Control 43:26–30 [DOI] [PubMed] [Google Scholar]
- 22.Wall RJ et al (2005) Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care 14:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabana MD et al (1999) Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 282:1458–1465 [DOI] [PubMed] [Google Scholar]
- 24.Duffy EA, Rodgers CC, Shever LL, Hockenberry MJ (2015) Implementing a daily maintenance care bundle to prevent Central Line-Associated Bloodstream infections in Pediatric Oncology patients. J Pediatr Oncol Nursing: Official J Association Pediatr Oncol Nurses 32:394–400 [DOI] [PubMed] [Google Scholar]
- 25.Walz JM et al (2015) The bundle plus: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg 120:868–876 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 22.6 KB)
(DOCX 18.2 KB)
(DOC 62.0 KB )
(DOC 54.0 KB)
Data Availability Statement
Data available on request.