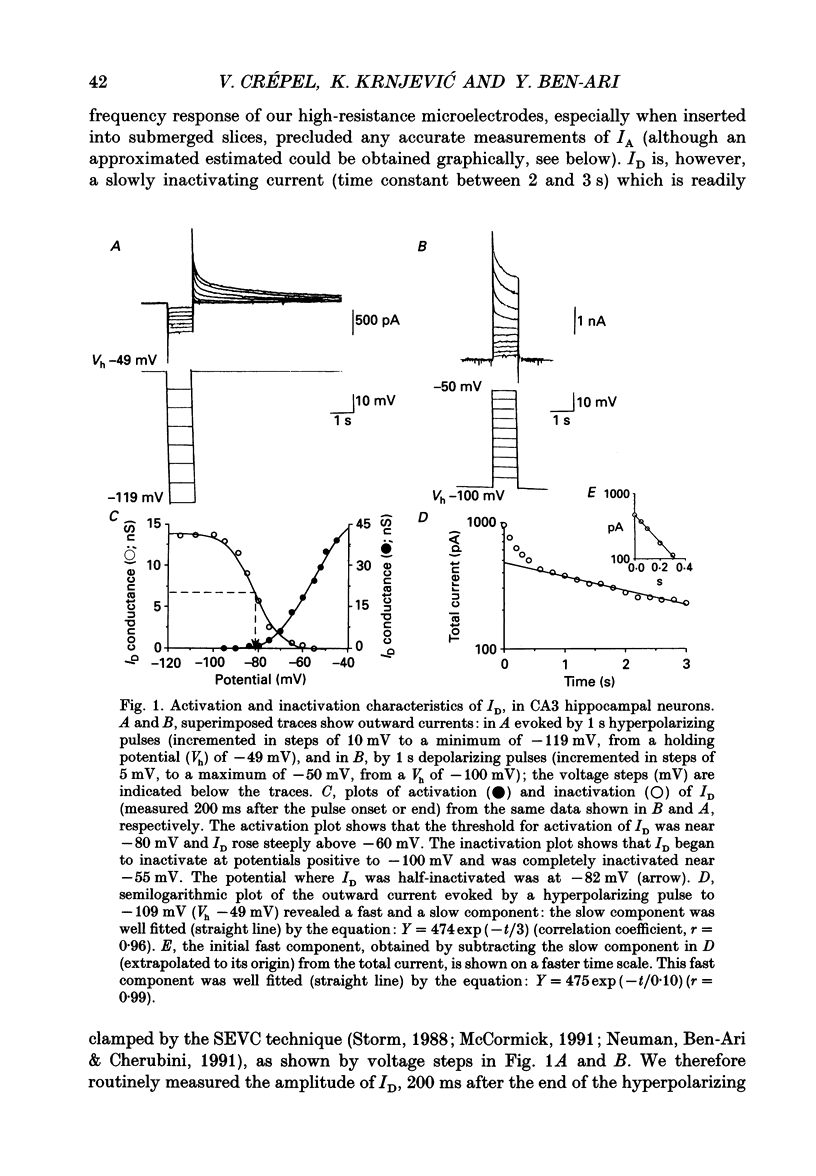
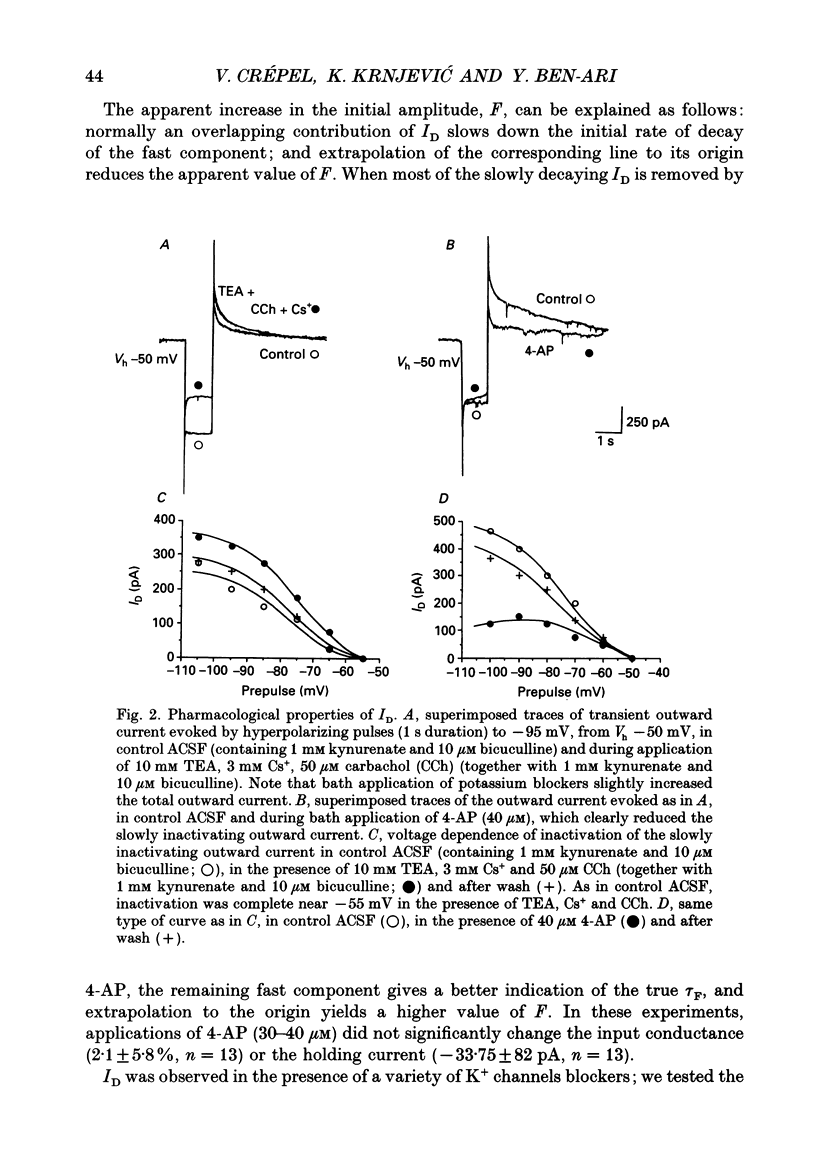
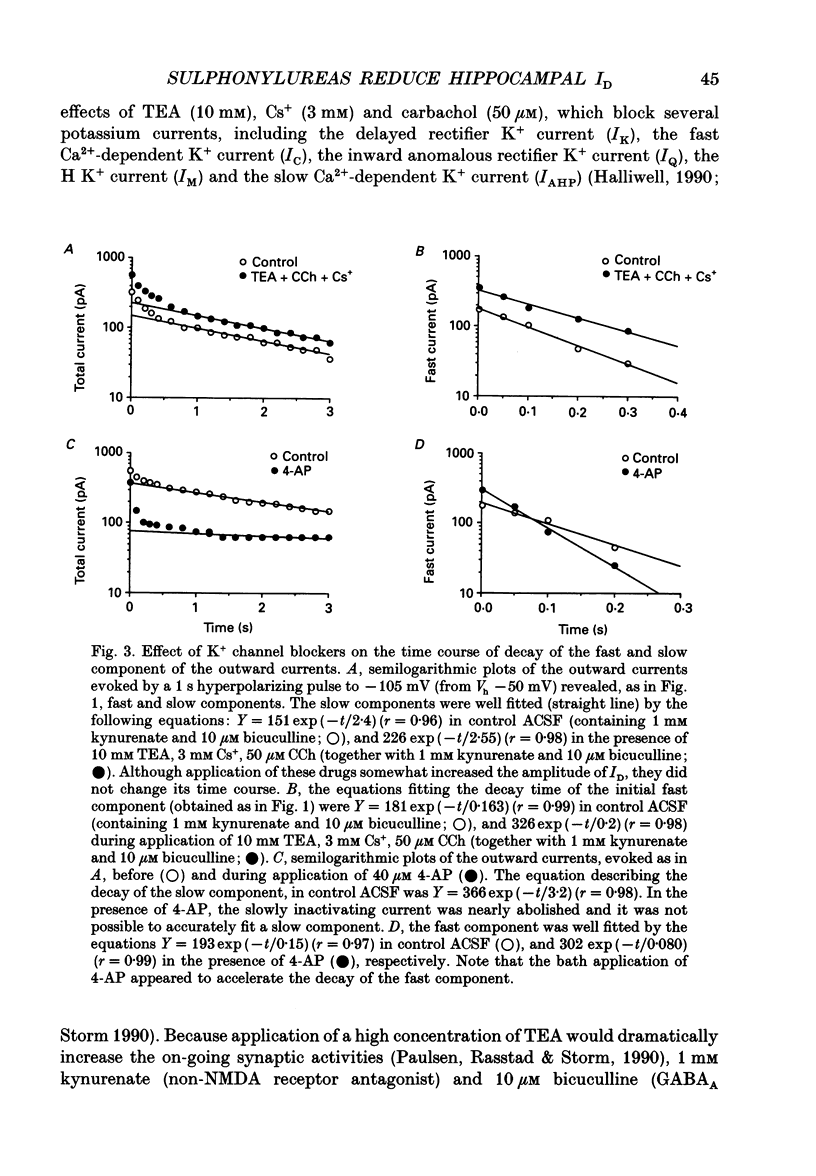
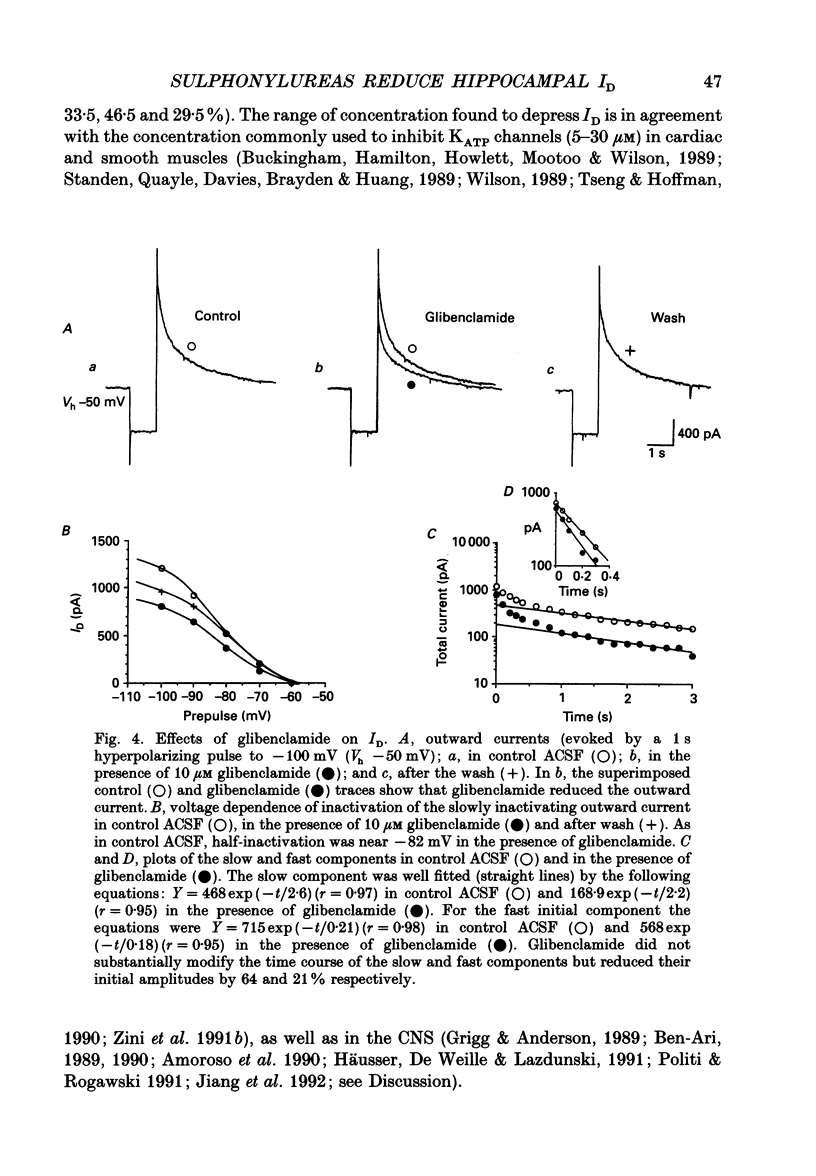
Abstract
1. Using intracellular recording in hippocampal slices, we have examined, in CA3 pyramidal neurons, the effects of sulphonylureas (blockers of ATP-sensitive K+ channels) on the slowly inactivating D-type K+ current (ID). 2. In the presence of TTX (1 microM) to block Na+ currents, ID had the following characteristics: activation by large depolarizing pulses from membrane potentials negative to -75 mV, slow inactivation kinetics, high sensitivity to 4-aminopyridine (4-AP, 3-40 microM), insensitivity to tetraethylammonium (TEA, 10 mM), Cs+ (3 mM) and carbachol (50 microM). 3. Applications of glibenclamide (10 microM) did not modify the input conductance of the cell, but reduced the amplitude of ID by 31.2 +/- 5.6% (n = 16), without altering its voltage dependence and inactivation kinetics. The effects were usually reversible. 4. Glibenclamide also reduced ID in the presence of TEA (10 mM), Cs+ (3 mM) and carbachol (50 microM), to block several K+ currents (IK, IC, IQ, IM), as well as kynurenate (1 mM) and bicuculline (10 microM) to block on-going synaptic currents mediated by activation of non-NMDA (N-methyl-D-aspartate) and GABA (gamma-aminobutyrate)-A receptors, respectively. 5. Comparable depressions of ID were produced by two other sulphonylureas: gliquidone (10 microM), 42.6 +/- 7.9% (n = 13) and tolbutamide (500 microM), 39.1 +/- 12.8 (n = 8). 6. It is concluded that, in the central nervous system, sulphonylureas can modulate K+ currents which are not generated by ATP-sensitive K+ channels.
Full text
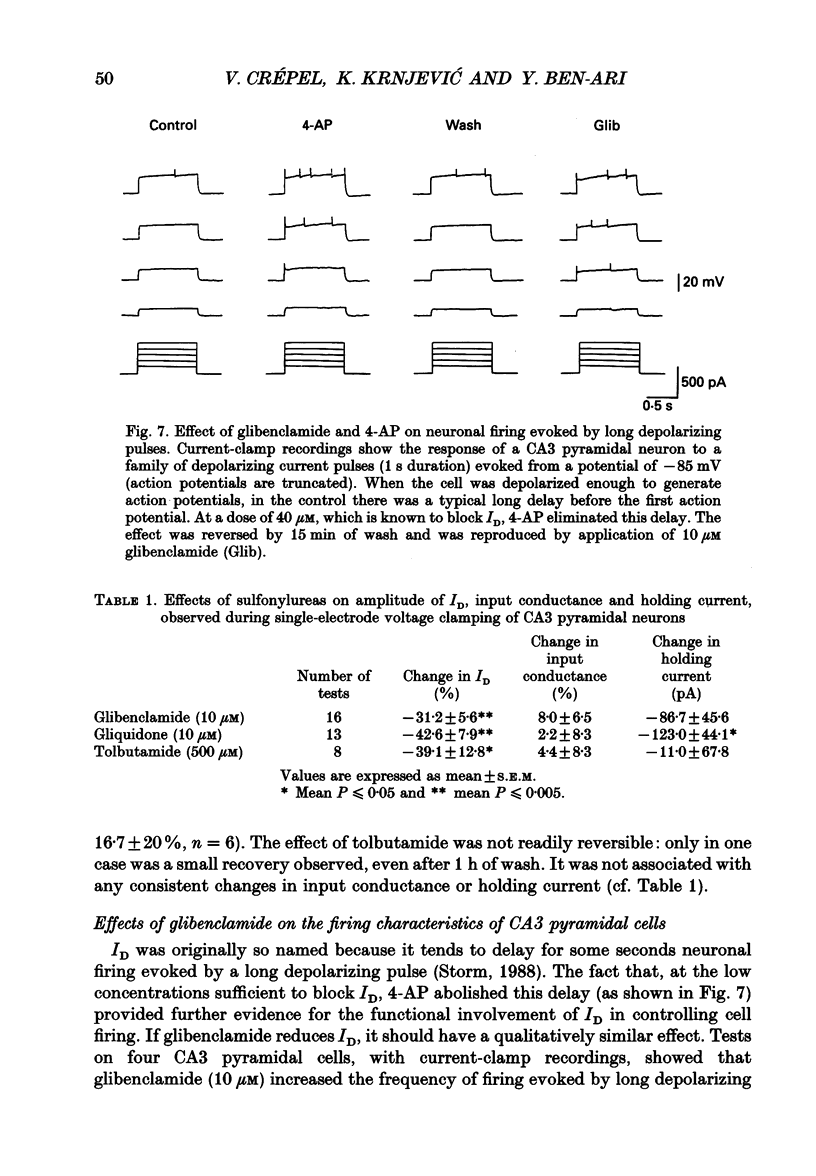
PDF




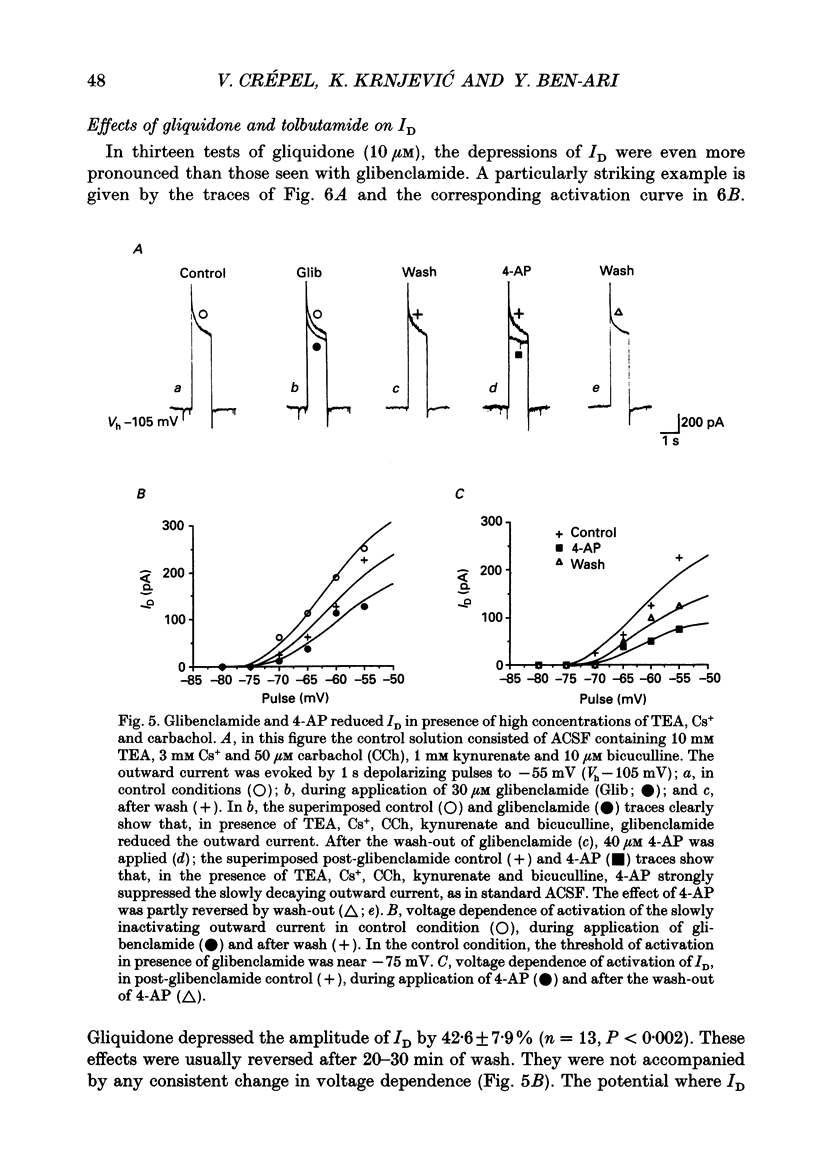




Selected References
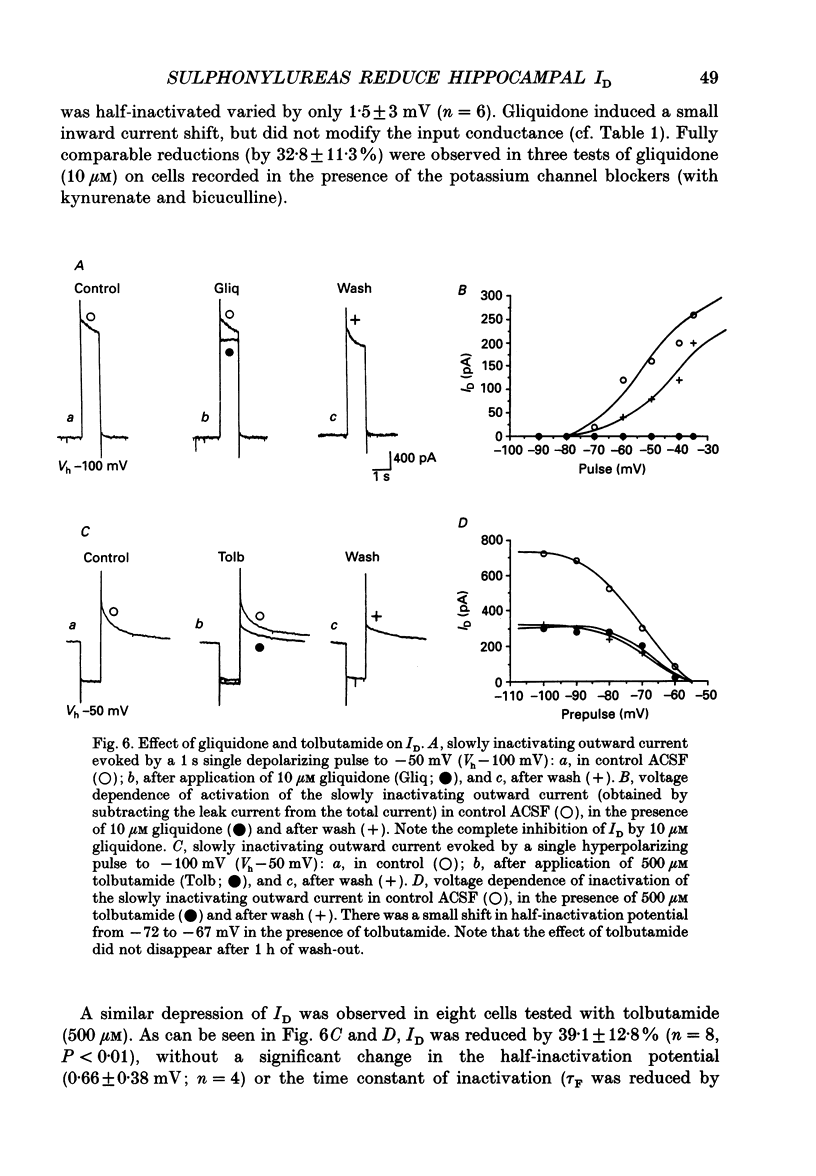
These references are in PubMed. This may not be the complete list of references from this article.
- Alzheimer C., ten Bruggencate G. Actions of BRL 34915 (Cromakalim) upon convulsive discharges in guinea pig hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1988 Apr;337(4):429–434. doi: 10.1007/BF00169535. [DOI] [PubMed] [Google Scholar]
- Amoroso S., Schmid-Antomarchi H., Fosset M., Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990 Feb 16;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y. Effect of glibenclamide, a selective blocker of an ATP-K+ channel, on the anoxic response of hippocampal neurones. Pflugers Arch. 1989;414 (Suppl 1):S111–S114. doi: 10.1007/BF00582258. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Galanin and Glibenclamide Modulate the Anoxic Release of Glutamate in Rat CA3 Hippocampal Neurons. Eur J Neurosci. 1990 Jan;2(1):62–68. doi: 10.1111/j.1460-9568.1990.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Buckingham R. E., Hamilton T. C., Howlett D. R., Mootoo S., Wilson C. Inhibition by glibenclamide of the vasorelaxant action of cromakalim in the rat. Br J Pharmacol. 1989 May;97(1):57–64. doi: 10.1111/j.1476-5381.1989.tb11923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Crépel V., Krnjević K., Ben-Ari Y. Glibenclamide depresses the slowly inactivating outward current (ID) in hippocampal neurons. Can J Physiol Pharmacol. 1992 Feb;70(2):306–307. doi: 10.1139/y92-038. [DOI] [PubMed] [Google Scholar]
- Ferrer R., Atwater I., Omer E. M., Gonçalves A. A., Croghan P. C., Rojas E. Electrophysiological evidence for the inhibition of potassium permeability in pancreatic beta-cells by glibenclamide. Q J Exp Physiol. 1984 Oct;69(4):831–839. doi: 10.1113/expphysiol.1984.sp002872. [DOI] [PubMed] [Google Scholar]
- French J. F., Riera L. C., Mullins U. L., Sarmiento J. G. Modulation of [3H]glibenclamide binding to cardiac and insulinoma membranes. Eur J Pharmacol. 1991 May 25;207(1):23–28. doi: 10.1016/s0922-4106(05)80033-1. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M., Johnson D. E., Janis R. A., Triggle D. J. Characterization of binding of the ATP-sensitive potassium channel ligand, [3H]glyburide, to neuronal and muscle preparations. J Pharmacol Exp Ther. 1991 Jun;257(3):1162–1171. [PubMed] [Google Scholar]
- Grigg J. J., Anderson E. G. Glucose and sulfonylureas modify different phases of the membrane potential change during hypoxia in rat hippocampal slices. Brain Res. 1989 Jun 12;489(2):302–310. doi: 10.1016/0006-8993(89)90863-9. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Howell S. L. The mechanism of insulin secretion. Diabetologia. 1984 May;26(5):319–327. doi: 10.1007/BF00266030. [DOI] [PubMed] [Google Scholar]
- Häusser M. A., de Weille J. R., Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochem Biophys Res Commun. 1991 Jan 31;174(2):909–914. doi: 10.1016/0006-291x(91)91504-6. [DOI] [PubMed] [Google Scholar]
- Jiang C., Xia Y., Haddad G. G. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol. 1992 Mar;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A. Adenosine-5'-triphosphate-sensitive single potassium channel in the atrioventricular node cell of the rabbit heart. J Physiol. 1984 Jul;352:265–284. doi: 10.1113/jphysiol.1984.sp015290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H. J., Heinemann U. Hypoxia-induced functional alterations in adult rat neocortex. J Neurophysiol. 1992 Apr;67(4):798–811. doi: 10.1152/jn.1992.67.4.798. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991 Oct;66(4):1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- Mourre C., Ben Ari Y., Bernardi H., Fosset M., Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989 May 1;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Greenfield S. A. Neuronal selectivity of ATP-sensitive potassium channels in guinea-pig substantia nigra revealed by responses to anoxia. J Physiol. 1992;453:167–183. doi: 10.1113/jphysiol.1992.sp019222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman R., Ari Y. Ben, Cherubini E. Mast Cell Degranulating Peptide Increases the Frequency of Spontaneous Miniature Postsynaptic Currents in CA3 Rat Hippocampal Neurons. Eur J Neurosci. 1991 Jun;3(6):523–530. doi: 10.1111/j.1460-9568.1991.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Politi D. M., Rogawski M. A. Glyburide-sensitive K+ channels in cultured rat hippocampal neurons: activation by cromakalim and energy-depleting conditions. Mol Pharmacol. 1991 Aug;40(2):308–315. [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve H. L., Vaughan P. F., Peers C. Glibenclamide inhibits a voltage-gated K+ current in the human neuroblastoma cell line SH-SY5Y. Neurosci Lett. 1992 Jan 20;135(1):37–40. doi: 10.1016/0304-3940(92)90130-y. [DOI] [PubMed] [Google Scholar]
- Roeper J., Hainsworth A. H., Ashcroft F. M. Tolbutamide reverses membrane hyperpolarisation induced by activation of D2 receptors and GABAB receptors in isolated substantia nigra neurones. Pflugers Arch. 1990 Jun;416(4):473–475. doi: 10.1007/BF00370758. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Schmid-Antomarchi H., de Weille J., Fosset M., Lazdunski M. The antidiabetic sulfonylurea glibenclamide is a potent blocker of the ATP-modulated K+ channel in insulin secreting cells. Biochem Biophys Res Commun. 1987 Jul 15;146(1):21–25. doi: 10.1016/0006-291x(87)90684-x. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treherne J. M., Ashford M. L. The regional distribution of sulphonylurea binding sites in rat brain. Neuroscience. 1991;40(2):523–531. doi: 10.1016/0306-4522(91)90138-e. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Zini S., Ben-Ari Y. Autoradiographic study of the cellular localization of [3H]glibenclamide binding sites in the rat hippocampus. Neurosci Lett. 1991 Jun 10;127(1):21–24. doi: 10.1016/0304-3940(91)90884-v. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Actions of pinacidil on membrane currents in canine ventricular myocytes and their modulation by intracellular ATP and cAMP. Pflugers Arch. 1990 Jan;415(4):414–424. doi: 10.1007/BF00373618. [DOI] [PubMed] [Google Scholar]
- Verspohl E. J., Ammon H. P., Mark M. Evidence for more than one binding site for sulfonylureas in insulin-secreting cells. J Pharm Pharmacol. 1990 Apr;42(4):230–235. doi: 10.1111/j.2042-7158.1990.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Wilson C. Inhibition by sulphonylureas of vasorelaxation induced by K+ channel activators in vitro. J Auton Pharmacol. 1989 Feb;9(1):71–78. doi: 10.1111/j.1474-8673.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Zini S., Ben-Ari Y., Ashford M. L. Characterization of sulfonylurea receptors and the action of potassium channel openers on cholinergic neurotransmission in guinea pig isolated small intestine. J Pharmacol Exp Ther. 1991 Nov;259(2):566–573. [PubMed] [Google Scholar]
- Zini S., Tremblay E., Roisin M. P., Ben-Ari Y. Two binding sites for [3H]glibenclamide in the rat brain. Brain Res. 1991 Feb 22;542(1):151–154. doi: 10.1016/0006-8993(91)91010-x. [DOI] [PubMed] [Google Scholar]


