Abstract
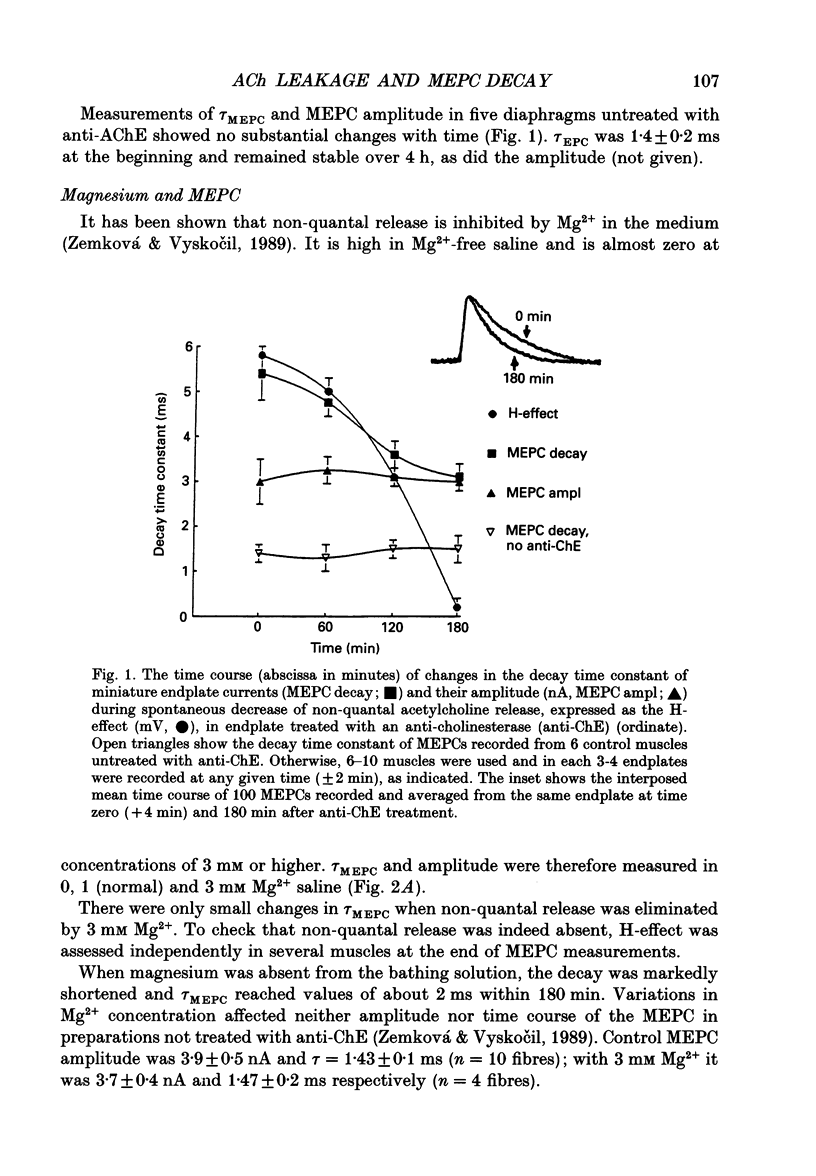
1. The amplitude and exponential decay time constant of miniature endplate currents (MEPCs) were measured in mouse diaphragms treated with anti-cholinesterase under conditions known to modulate non-quantal acetylcholine (ACh) release. 2. Anti-cholinesterase prolonged MEPC decay and the extent of this initial prolongation was not influenced by non-quantal release. When non-quantal release was present, the decays of MEPCs became increasingly faster over several hours. This increased decay did not occur in the absence of non-quantal release. 3. Potentiation of the non-quantal release by zero Mg2+ and 1 x 10(-5) M choline, on the other hand, led to acceleration of MEPC shortening. 4. Increase of temperature from 15 to 26 degrees C and the presence of the desensitization-promoting drug proadifen (5 x 10(-6) M) accelerated the rate of MEPC shortening. 5. These observations are consistent with increased receptor desensitization due to non-quantal release. Repetitive binding of ACh to postsynaptic receptors which prolongs the time course of MEPC in anti-cholinesterase-treated endplates leads to progressive desensitization in the presence of non-quantal release and to the subsequent shortening of the quantal responses.
Full text
PDF

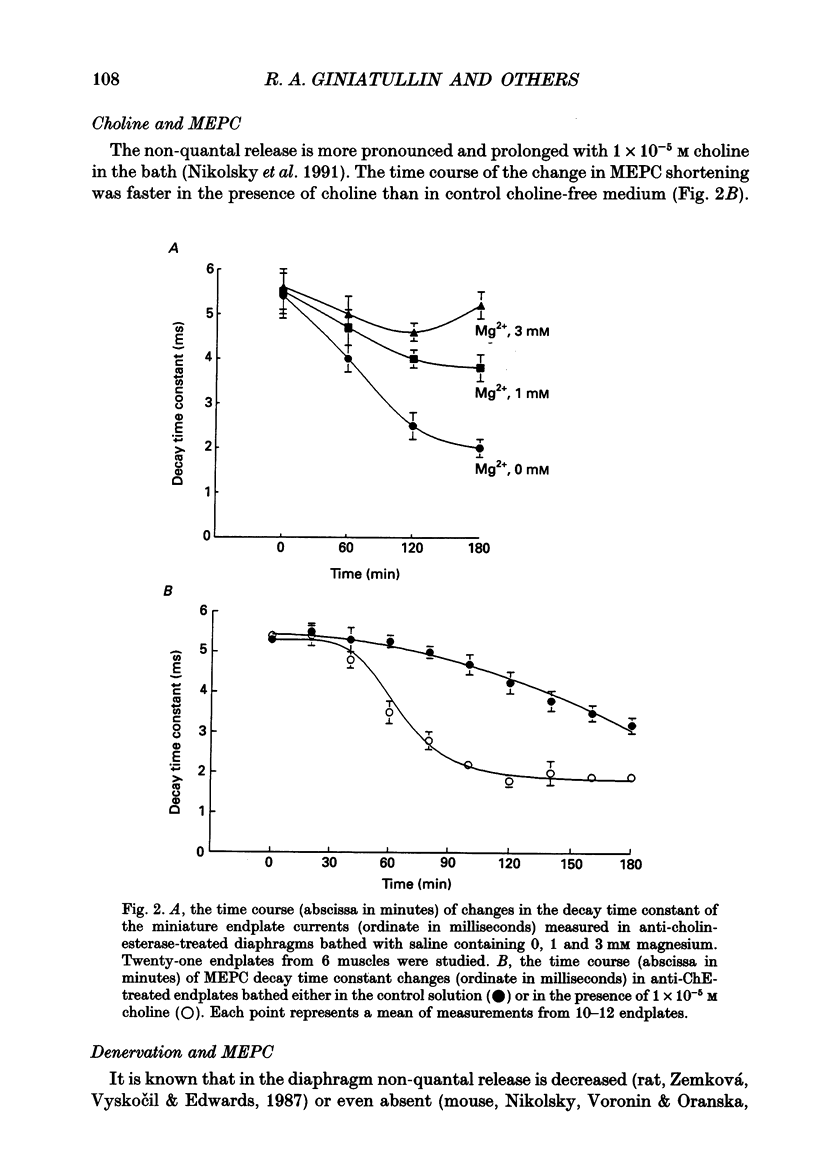




Selected References
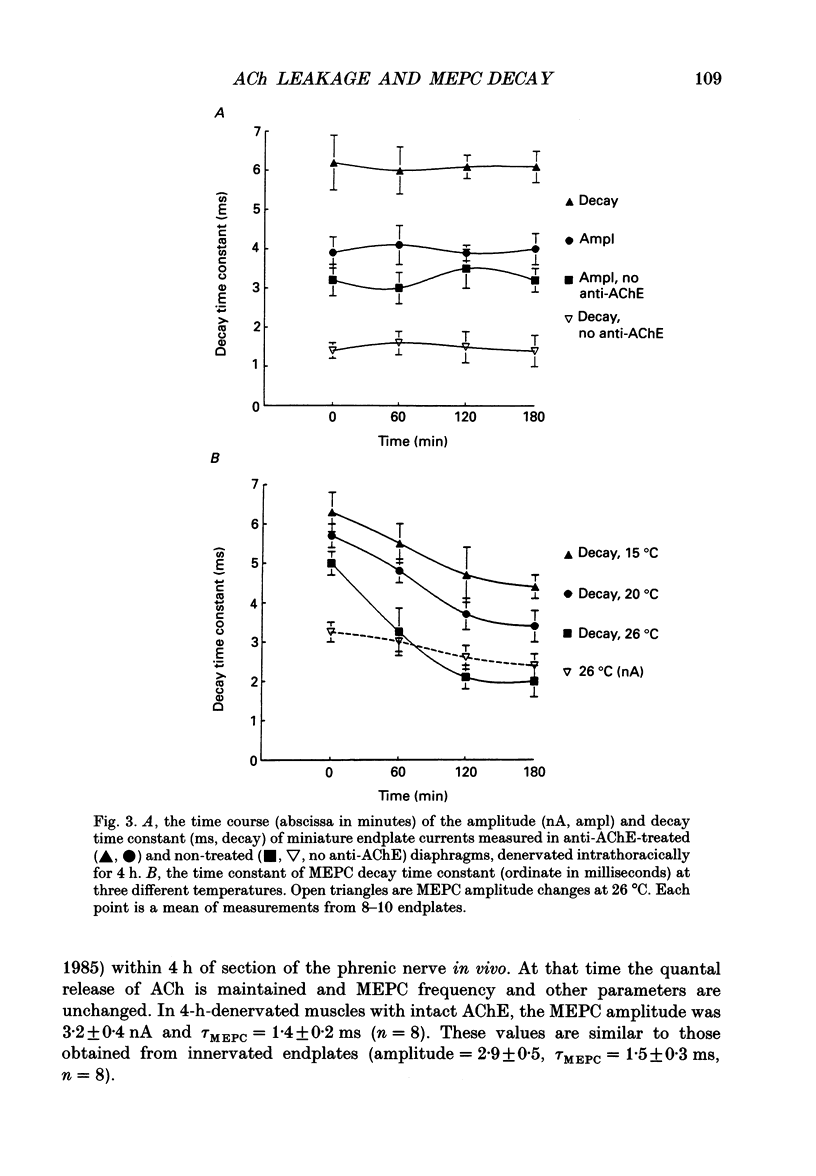
These references are in PubMed. This may not be the complete list of references from this article.
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
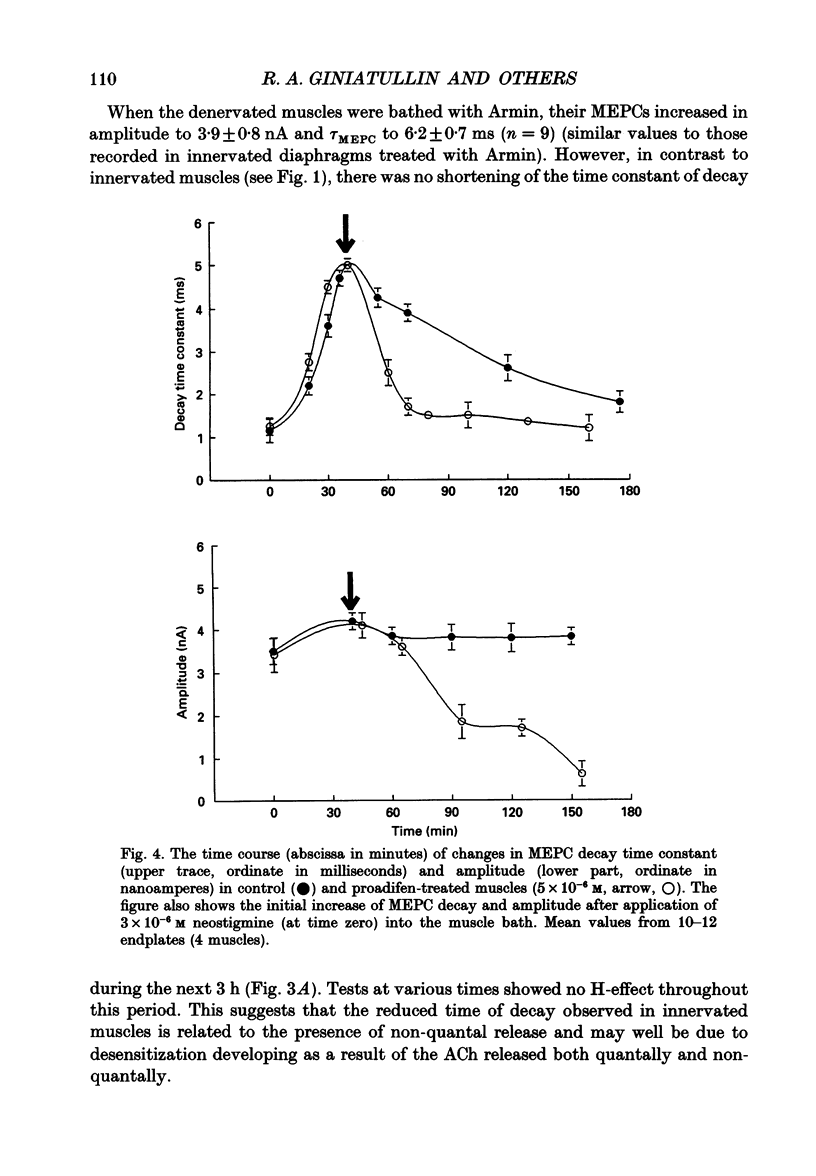
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R. A., Khamitov G., Khazipov R., Magazanik L. G., Nikolsky E. E., Snetkov V. A., Vyskocil F. Development of desensitization during repetitive end-plate activity and single end-plate currents in frog muscle. J Physiol. 1989 May;412:113–122. doi: 10.1113/jphysiol.1989.sp017606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur J Biochem. 1979 Feb 15;94(1):255–279. doi: 10.1111/j.1432-1033.1979.tb12893.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E., Vyskocil F. Effect of the desensitization-potentiating agent SKF-525a on frog end-plate currents. Eur J Pharmacol. 1982 May 7;80(1):115–119. doi: 10.1016/0014-2999(82)90185-6. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Snetkov V. A., Giniatullin R. A., Khazipov R. N. Changes in the time course of miniature endplate currents induced by bath-applied acetylcholine. Neurosci Lett. 1990 Jun 8;113(3):281–285. doi: 10.1016/0304-3940(90)90598-4. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolsky E. E., Voronin V. A., Oranska T. I., Vyskocil F. The dependence of non-quantal acetylcholine release on the choline-uptake system in the mouse diaphragm. Pflugers Arch. 1991 Mar;418(1-2):74–78. doi: 10.1007/BF00370454. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Electrophysiological examination of transmitter release in non-quantal form in the mouse diaphragm and the activity of membrane ATP-ase. Physiol Bohemoslov. 1978;27(5):449–455. [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F., Edwards C. A study on early post-denervation changes of non-quantal and quantal acetylcholine release in the rat diaphragm. Pflugers Arch. 1987 Aug;409(4-5):540–546. doi: 10.1007/BF00583813. [DOI] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F., Edwards C. The effects of nerve terminal activity on non-quantal release of acetylcholine at the mouse neuromuscular junction. J Physiol. 1990 Apr;423:631–640. doi: 10.1113/jphysiol.1990.sp018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemková H., Vyskocil F. Effect of Mg2+ on non-quantal acetylcholine release at the mouse neuromuscular junction. Neurosci Lett. 1989 Sep 11;103(3):293–297. doi: 10.1016/0304-3940(89)90115-8. [DOI] [PubMed] [Google Scholar]


