Abstract
The determinants of Xenopus laevis embryos that act before their first cell division are mandatory for the formation of mRNas required to establish the dorsal axis. Although their chemical identities are unknown, a number of their properties have long been recognized. One of the determinants is present in the cytoplasm and is sensitive to UV light. Thus, exposing stage 1 embryos to either standard 254-nm or, as shown here, to 366-nm UV light during the 0.3–0.4 time fraction of their first cycle inactivates the cytoplasmic determinant. As a consequence, both types of irradiated embryos fail to express dorsal markers, e.g., goosecoid and chordin, without affecting formation of ventral markers, e.g., Vent-1. The developmental outcome is dorsal axis-deficient morphology. We report here that biliverdin IXα, a normal constituent of cytoplasmic yolk platelets, is photo-transformed by irradiation with either 254- or 366-nm UV light and that the transformation triggers the dorsal axis deficiency. When the 254- or 366-nm UV-irradiated embryos, fated to dorsal axis deficiency, are incubated solely with μM amounts of biliverdin, they recover and form the axis. In contrast, incubation with either in vitro photo-transformed biliverdin or biliverdin IXα dimethyl ester does not induce recovery. The results define an approach to produce dorsal axis-deficient embryos by photo-transforming its biliverdin by irradiation with 366-nm UV light and identify an unsuspected role for biliverdin IXα in X. laevis embryogenesis.
In 1924, Spemann and Mangold (1) reported that gastrula tissue from the dorso-equatorial region induces an ectopic axis when transplanted into a recipient embryo. They named this axis-inducing region the organizer. This seminal finding established the significance of inductive signals for normal dorsal axis formation and morphogenesis and stimulated the search for the identity of the responsible substances (2–7). Many molecules are now recognized to participate in this complex embryological process. They can be assigned operationally to two groups on the basis of the timing of their actions and location during embryogenesis. The first group is distributed initially to the vegetal cortex and cytoplasm of the egg and stage 1 embryo. Subsequently, they localize to the dorso-vegetal zone. They act early in the period after fertilization but before the first cleavage. The action of these early determinants is essential for the formation and/or activation of many molecules of the second group formed downstream after the midblastula transition in the dorsal-equatorial zone of the embryo. The latter ones have been extensively studied and many are well characterized. They are species-activated or formed in the dorsal (e.g., wnt, β-catenin, siamois, goosecoid, activin, XANF-1, Xnr3, noggin, chordin, transforming growth factor β, follistatin, xSOX3) and ventral (e.g., BMP4, fibroblast growth factor, Vent 1) zones of the embryo (4, 5, 8–11).
In contrast, the chemical identities of the first, early group remain to be characterized. Limited information on their biological activities has emerged by examining the outcomes on developmental milestones after selectively perturbing, removing, inactivating, destroying, or transplanting constituents or segments of oocytes, eggs, or embryos. Thus, stage 1 embryos do not develop a normal dorsal axis when their vegetal-dorsal cortex is ablated (12), their vegetal hemisphere is irradiated by 254-nm UV light before their first mitosis, they are exposed to a cold temperature shock or incubated with colchicine (9, 13–22), or their cytoplasm is removed (23). The “molecule(s)” of the embryo vegetal cytoplasm inactivated by irradiating with UV light can be reconstituted by transferring the cytoplasm from a nonirradiated egg/embryo into the irradiated one (9, 13–22). The recipient irradiated embryo then recovers its capacity to form a dorsal axis (17, 20).
Two molecules have been proposed to be the targets affected when oocytes, eggs, and/or embryos are irradiated with UV light. Microtubules are proposed to carry out rotation of cortical determinants after fertilization. This phenomenon appears to be obviated after exposure to UV light. The latter is believed to destabilize microtubules, leading to failure to place cortical determinants in the dorso-equatorial zone and, thereby, result in dorsal axis deficiency. Therefore, tubulin has been considered to be a target molecule for UV irradiation (14, 15, 19, 20, 24–27). The other proposed target molecule is a cytoplasmic constituent first identified after irradiating oocytes instead of eggs. The affected target is distinct from tubulin because when the irradiated oocytes are subsequently fertilized, the resultant embryos develop a dorsal axis-deficient phenotype yet still exhibit normal cortical rotation (16, 17). The irradiated embryo's dorsalizing capacity is reconstituted by transferring cytoplasm from a donor, control stage 1 embryo. The presence of the latter target molecule also can be detected in stage 1 embryos by direct removal of their cytoplasm. This intervention results in dorsal axis-deficient morphology without inhibiting cortical rotation (23). Thus, in both latter cases, the determinant present in the vegetal cortex is properly localized but the cytoplasmic factor is either inactivated by UV light or physically removed.
We have initiated studies aimed at identifying the chemical nature of this latter cytoplasmic factor of eggs and stage 1 embryos. The sequence of events described above demonstrates the cytoplasmic factor acts in stage 1 embryos to affect mRNA synthesis detected later after the midblastula transition and into gastrula. This sequence of an early action followed by downstream effects on transcription is reminiscent of a ligand-receptor signaling system. This conceptual insight together with the distinction between the two possible targets of UV light allowed us to design an experimental extraction procedure with organic solvents aimed at small, ligand-type molecule(s) that excludes proteins, such as tubulin. We were greatly aided by discovering that the cytoplasmic molecule(s) in question is inactivated not only with 254-nm but also with 366-nm UV light. This susceptibility to longer wavelength UV light allowed for discrimination between the target molecules described above because 366-nm UV light should not affect protein or nucleic acid constituents of the egg or embryo. Here we report that the UV-sensitive cytoplasmic factor is biliverdin. It is present in the oocyte, egg, and embryo cytoplasm, is photo-transformed by both short and long wave UV light, and may be essential for embryo dorsal axis development.
Methods
Embryo Irradiation with Either Short or Long Wavelength UV Lights.
The source of the short wavelength emission (254 nm) was a UV G 11 lamp, while the long wave emission (366 nm) was a UV SL 58 lamp (Ultraviolet Products) (see Extended Experimental Procedures and Figs. 9–11, which are published as supporting information on the PNAS web site, www.pnas.org). For exposure to 254 nm, the embryos were placed on a quartz plate that transmits all UV G 11 lamp emissions. For exposure to 366 nm, the embryos were placed in polystyrene Petri dishes that block transmission of light below 300 nm to avoid possible effects of shorter wavelengths. Several hundred spawned eggs were fertilized in vitro and incubated at 16–18°C. The first mitosis at this ambient temperature of incubation occurred between 100 and 120 min after fertilization. The vegetal surfaces of some of these embryos were irradiated with one or the other sources of UV light within 10 min of fertilization for 20–30 min (14–23, 28). Therefore, the UV light exposure was applied well within the period of maximum effectiveness of UV light (15, 26), in this case between Tfm = 0.3–0.4 (Tfm is the normalized time scale with a value of 1 representing the period from fertilization to the first mitosis). The embryos were not disturbed, touched, or tipped from the moment of fertilization, during UV irradiation or for the duration of development. After control embryos had reached stages 30–35, the dorsal axis development of all experimental embryos was scored by using standard morphological criteria defining their dorsal anterior index (DAI). A score of 5 defines a normal dorsal axis, 4 a microcephalic, 3 a cyclopic, 2 an anoptic, 1 an acephalic, and 0 an adorsal embryo (22).
Purification of an UV-Sensitive Molecule.
An UV-sensitive species was localized to the cytoplasmic yolk platelets (M.M., T.S.D., and K.H.F., unpublished work). Therefore, yolk platelets were isolated and used as starting material (29). Platelets were homogenized in 1 vol of PBS (5 mg/ml) and in ascorbic acid and EDTA, adjusted to pH 7.3 with potassium hydroxide. The homogenate was extracted with acetone, dried, and then dissolved in water. The pH was adjusted to 8, and nonpolar contaminants were removed by ethyl acetate extraction. The desired material was extracted into 1-butanol after saturation of the aqueous layer with sodium chloride. The product was dried and suspended in methanol and applied to a 0.9 × 17-cm Sephadex LH-20 column. Elution from the column with absolute methanol yielded a yellow-orange fraction at 0.3–0.5, a yellow fraction at 0.6–1.0, and a dark blue-green product at 1.4–1.7 column volumes. The fractions were dried by flash evaporation.
The blue-green fraction was dissolved in 1 ml of solvent A (3 mM ammonium acetate, pH 4.5, 20% in acetonitrile), and 250-μl aliquots were injected into a Phenomenex Jupiter 5μ C18 HPLC column (300 Å, 250 × 4.6 mm). The column was connected to a Waters Alliance Chromatography System (Waters 2690 Separations Module interfaced with a Waters 996 Photodiode Array Detector). Solvent B was 100% acetonitrile. The gradient design was: 0% B from 0 to 5 min, 0–100% B linear gradient from 5 to 45 min, and 100% B from 45 to 60 min. The eluate absorbance was recorded at a range of wavelengths from 250 to 550 nm by means of a diode array (see Expanded Experimental Procedures, which are published as supporting information for a more detailed description of the purification).
The cytoplasmic yolk platelet fraction that was photo-transformed by UV light was identified in extracts of about 250 embryos irradiated with 366-nm UV light. The chromatogram of irradiated embryo extracts was compared with that of the nonirradiated ones to identify the photo-transformed fraction. Once the retention time of the UV-sensitive fraction was determined, the parent chemical species was isolated from control egg extracts. To confirm its UV sensitivity, an aliquot of the pertinent HPLC fraction in 44% acetonitrile and 3 mM ammonium acetate, pH 4.5, was irradiated in a cuvette in a Varian-Cary Bio 50 spectrophotometer at 366 nm. The pH was chosen to approximate that of intact yolk platelets. The wavelength was selected on the basis of the absorption spectra of the target fraction. The absorbance change was monitored at 375 nm. The resultant photo-transformation product was then rechromatographed by HPLC. For comparison, a separate aliquot of the intact molecule was irradiated with a monochromatic source at 254 nm from the spectrophotometer and similarly chromatographed.
Physical-Chemical Characterization of the UV-Sensitive Molecule.
TLC sheets were silica gel 60F. Absorption spectra were obtained with a Varian Cary Bio 50 Spectrophotometer. Mass spectral analysis was performed in positive ion mode on a ThermoQuest LCQ Classic electrospray ionization/ion trap instrument (MicroMass, Manchester, U.K.). Aliquots incubated in 99.95-atom % methyl d3 alcohol-d (Aldrich) were similarly analyzed to determine the number of exchangeable protons in the molecule. All NMR experiments were run at 25°C on a Varian Unity Inova 500 spectrometer equipped with a 5-mm triple resonance 1H{13C, 15N} probe head. The spectra were processed on a Silicon Graphics O2 workstation using vnmr software (Varian, version 6.1B). The details are described in the Expanded Experimental Procedures, which are published as supporting information.
Biological Activity of the UV-Sensitive Molecule.
The UV-sensitive fraction in the extracts studied is shown here to be biliverdin IXα, a substance that can be obtained commercially. Therefore, it was possible to analyze its biological activity with a fraction purified from oocytes or its commercially available counterpart and compare them to the effects of biliverdin photo-transformed in vitro or of biliverdin dimethyl ester hydrochloride with its modified propionic side chains. Biliverdin IXα and derivatives were obtained from Porphyrin Products (Logan, Utah). Commercially available biliverdin IXα was subjected to the above extraction and chromatographic procedure beginning with the ethyl acetate step. The dimethyl ester required only HPLC purification. Photo-transformed biliverdin was obtained by irradiating an aliquot of embryo culture solution containing biliverdin at the targeted concentration with 366-nm UV light for 12 h. The photo-transformation of the biliverdin was verified spectrophotometrically by loss of the 375-nm absorption peak.
The biological activities of biliverdin and its derivatives were tested by adding each of them to the incubation solution of embryos after the termination of the UV light exposure to either 254- or 366-nm UV light and at selected time periods between Tfm = 0.4 to 2.5. Final concentrations of biliverdin ranged from 0.05 to 5 μM in less than 1% ethanol. The in vitro photo-transformed biliverdin or the biliverdin dimethyl ester hydrochloride were added at a final concentration of 2.2 and 3.7 μM, respectively. An extinction coefficient of 51,000 was used to calculate their concentrations. All Petri dishes were covered with aluminum foil to avoid light exposure. The extent of dorsal axis formation was analyzed by the morphological criteria described above (22).
The presence or absence of dorsal and ventrolateral markers was determined by Northern blot analysis of RNA from control, 254-nm, or 366-nm UV-irradiated embryos (30, 31). Goosecoid, chordin, and Vent-1 cDNAs were labeled with 32P and used as hybridizing probes.
Results
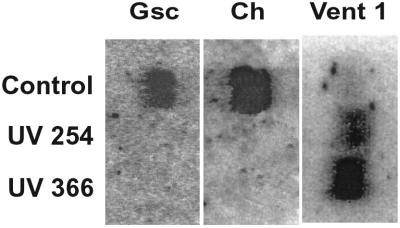
On reaching stage 10.5, control embryos generate abundant amounts of dorsal marker mRNAs, such as e.g., goosecoid and chordin, and relatively lower quantities of ventral ones such as Vent 1 (Fig. 1). The presence and preponderance of dorsal markers is associated with formation of dorsal organs. Thus, over 99% of stage 30–35 embryos form normal dorsal organs including head, body, and tail structures with expected quantity and distribution of pigment characteristic of a DAI score of 5 (Fig. 2A). In contrast, the RNA of embryos irradiated with 254-nm UV light does not hybridize with either goosecoid or chordin cDNAs, indicating their corresponding mRNAs are absent in these embryos. The absence of dorsal markers in 254-nm UV-irradiated embryos is specific because it does not affect ventral markers, such as e.g., Vent 1, that can be detected by hybridization with its cDNA (Fig. 1). Moreover, the amounts of Vent 1 mRNA increase when control embryos reach stage 35, most of these irradiated embryos lack a dorsal axis and are scored with a DAI of 0 (Fig. 2B). The rest reveal a full range of dorsal-deficient DAI scores from 3 to 1 (Fig. 3A). From a total of 277 embryos exposed to 254-nm UV light during five different experiments, over 83% had scores below 3 with most scoring with a DAI of 0. The effects of irradiating with 366-nm UV light are identical in that their RNA also does not hybridize with either goosecoid or chordin but does with Vent 1 cDNAs (Fig. 1). These embryos also do not form dorsal axes. Their morphological appearance is identical to embryos irradiated with 254-nm UV light (Figs. 2C and 3B). Of 805 embryos exposed to 366-nm UV light in nine experiments, over 75% exhibited DAI of less than 2. In four of these separate irradiation experiments, nearly all embryos were scored with a DAI of 0.
Figure 1.
Northern blots of RNA from UV-irradiated stage 10.5 embryos. The dorsal markers goosecoid (gsc) and chordin (ch) and ventral one (Vent 1) are present in control RNA. The two dorsal mRNAs are absent, whereas the ventral marker, Vent 1, is present in both 254- and 366-nm irradiated embryos.
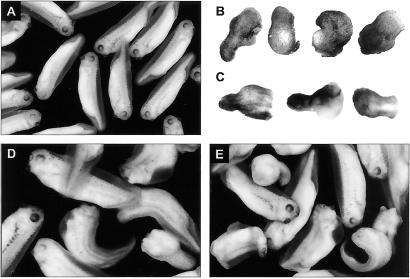
Figure 2.
Morphology of embryos irradiated with UV light. (A) Control embryos after reaching stages 30–35. (B) Adorsal embryos formed after exposure to 254-nm UV light. (C) Adorsal embryos formed after irradiation with 366-nm UV light. (D) Embryos irradiated with either long or short wavelength UV light after addition of frog-biliverdin IXα or (E) commercial biliverdin IXα. (Magnifications: A, ×7; B–D, ×12; E, ×10.)
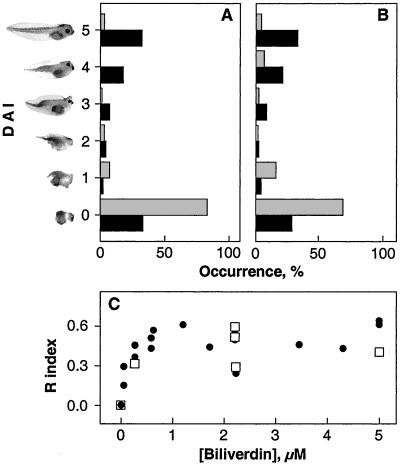
Figure 3.
Biliverdin IXα rescues UV-irradiated embryos from dorsal axis deficiency. (A) Average DAI score of embryos irradiated with 254-nm UV light (gray) is 0.35. About 51% of embryos treated with biliverdin (black) are scored with a DAI between 5 and 4 with an average DAI score of 2.72. (B) Average DAI score of embryos irradiated with 366-nm UV light (gray) is 0.72. Nearly 55% of embryos incubated with biliverdin (black) recover and are scored with 4–5 with an average DAI of 3.08 for the total population. The recoveries are statistically significant (paired t with P < 0.001). (C) The extent of embryo recovery from 254 nm (□) or 366-nm (●) UV light irradiation depends on biliverdin concentration. Recovery was determined with the equation, Ri = (x − uv)/(c − uv), where Ri = recovery index, x = average DAI for embryos incubated with biliverdin, uv = average DAI for embryos exposed to UV, and c = average DAI for control embryos.
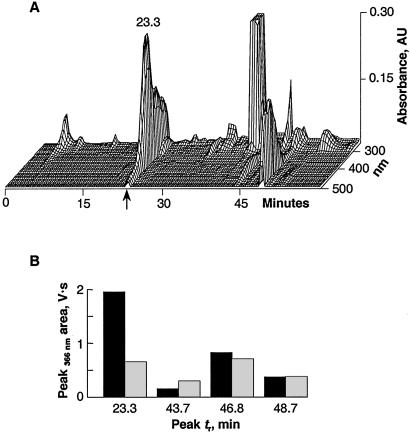
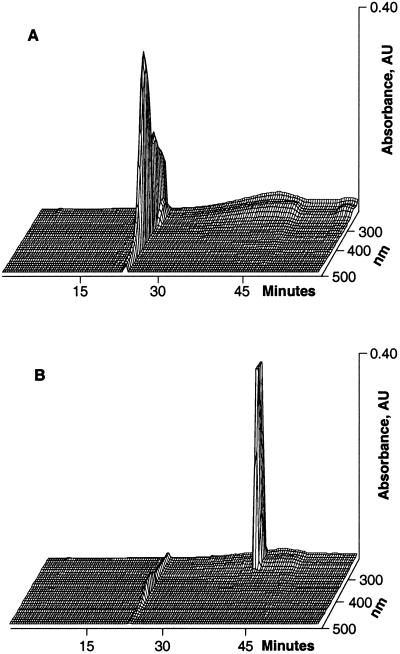
The identical dorsal/ventral marker phenotypes and developmental outcomes of embryos irradiated with either short or long wavelength UV light suggested a common target molecule responsible for the ventralization. The molecule is extractable by organic solvents. The extracts separate in a C18 HPLC column into a number of peaks that differ in their retention times and wavelength absorption characteristics (Fig. 4A). Comparing the chromatographic recordings of the extracts obtained from irradiated and control eggs/embryos demonstrates that the fraction that elutes at 23.3 min with 44% acetonitrile and exhibits an absorption peak at 375 nm is the only one that decreases significantly (Fig. 4B). In a typical experiment, over two-thirds (67%) of the 23.3-min peak area is photo-transformed by UV irradiation. The chromatographic behavior and spectral properties of the transformed peak differs from the parent compound in that it elutes later at 41.6 min, has lost the 375-nm peak, and demonstrates a characteristic absorption peak at 278 nm (Fig. 5). Exposing the 23.3-min fraction to either 254- or 366-nm UV light in a cuvette also photo-transforms it and reduces its absorption comparable to the in vivo observation (see Expanded Experimental Procedures and Fig. 11). The material in the 23.3-min fraction is identified unambiguously as biliverdin IXα by UV-Vis, MS, and NMR (Figs. 6 and 7).
Figure 4.
HPLC elution profile of an extract of eggs or stage 1 embryos. (A) The chromatographic profile exhibits a number of peaks with distinctive characteristics. The peak pertinent to this report elutes with a retention time of 23.3 min (arrow). (B) Comparison of peak areas of the HPLC profiles of extracts from control (black) and 366-nm UV light irradiated embryos (gray) demonstrate that the 23.3-min peak is the only one that is markedly reduced.
Figure 5.
Rechromatography of purified 23.3-min fraction before and after UV light irradiation. (A) Before irradiation. (B) Chromatography of the same 23.3-min fraction after 366-nm UV light irradiation in vitro. The 23.3-min fraction is virtually abolished, and a major new species is generated. The photo-transformation product differs in its elution time (41.6 min) and spectral properties.
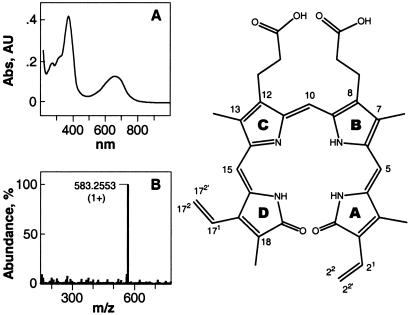
Figure 6.
Physical chemical features of the HPLC fraction 23.3 min identifies it as biliverdin IXα. The numbers in the structure indicate the position of the protons and the carbons described in Fig. 7. (A) The 23.3-min HPLC fraction has a unique UV-Vis absorption spectrum with characteristic peaks at 375 and 665 nm in ethanol. (B) MS positive ion mode analyses of the 23.3-min HPLC fraction yields a single high abundance peak with a mass-to-charge ratio of (1+) 583.2553. The molecule is predicted to have 19 double-bond equivalents, including all rings and carboxyl groups. There are five exchangeable protons ascertained by the mass increase in the presence of deuterium. These characteristics are identical to those of (1+) biliverdin. This identification was reinforced by the identical TLC Rf values (0.85 in a 3:1 chloroform/methanol mixture), cochromatographic behavior of a commercial biliverdin sample and the yolk platelet material purified on C18 with HPLC, and superposition of both absorption and NMR spectra (see Fig. 7).
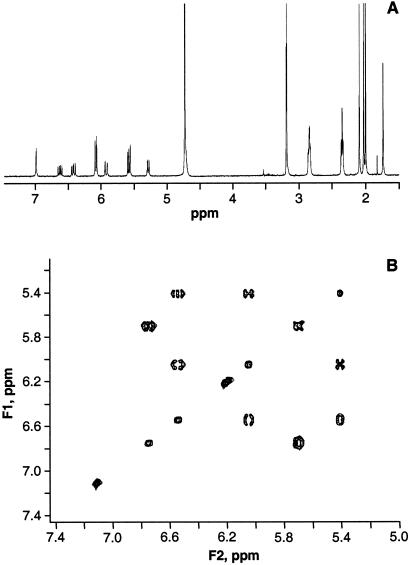
Figure 7.
NMR one-dimensional 1H spectrum and total correlation spectroscopy (TOCSY) spectra of the 23.3-min HPLC fraction identified it as biliverdin IXα. (A) The one-dimensional 1H spectrum of the pure 23.3-min HPLC fraction is identical to that of commercial biliverdin IXα. Chemical shifts are relative to trimethylsilyl propionate at 0.00 ppm: 1H NMR (methanol-d4) δ 6.54(m, 1H, H-21), 5.41(d, 1H, H-22), 6.05(d, 1H, H-22′), 6.22(s, 1H, H-5), 7.11(s, 1H, H-10), 6.19(s, 1H, H-15), 6.75(m, 1H, H-171), 5.72(d, 1H, H-172), 5.68(m, 1H, H-172′), 2.47(t, 4H, H-82, H-122), 2.96(t, 4H, H-81, H-121), 2.09, 2.02, 2.00, 1.73(s, 12H, H-31, H-71, H-131, H-181); 13C NMR (methanol-d4) δ 173.3(C-1, C-19), 127.1(C-21), 119.9(C-22), 100.0(C-5, C-15), 117.1(C-10), 127.6(C-171), 123.1(C-172), 21.5(C-81, C-121), 38.2(C-82, C-122) 177.8(C-83, C-123), 139.5(C-8, C-12). (B) Plot of the vinyl region from TOCSY spectrum of the oocyte molecule. The chemical shifts and coupling patterns are identical to commercial biliverdin IXα. Additionally, the coupling between the carbonyl carbon and the α and β methylene protons of the propionic acid side chains were verified from the distortionless enhancement by polarization transfer-heteronuclear multiple quantum correlation and heteronuclear multiple bond correlation spectra (data not shown).
To demonstrate the correlation between biliverdin photo-transformation and dorsal axis deficiency, the UV-exposed embryos were incubated with the intact tetrapyrrole. The DAI score of the resultant embryos is shifted from the predominant adorsal, DAI = 0 expected from irradiated embryos (Fig. 2 B and C), toward higher values closer to normal (Figs. 2D, 3A, and 3B). The rescued embryos demonstrate various degrees of dorsal axis development, including fully developed head, eyes, and tail structures. About 55% tadpoles develop with DAI scores of 5 or 4. Some of the embryos develop less completely, exhibiting a range of DAI scores from 3 to 1. From 20% to 30% still do not develop dorsal structures at all and are scored with a DAI of 0. The degree of recovery of dorsal axis formation achieved by incubating embryos with commercially available biliverdin is comparable (Fig. 2E). This effect of biliverdin pertains to embryos irradiated with either 254- or 366-nm UV light (Fig. 3). It is concentration dependent because a greater amount leads to greater degrees of recovery with a plateau of recovery reached at 1.2 μM biliverdin (Fig. 3C). In contrast, there is no recovery with 2.2 μM photo-transformed biliverdin or 3.7 μM biliverdin dimethyl ester hydrochloride (not shown).
The time during development when biliverdin rescues irradiated embryos is maximal during the period encompassed by the first cleavage (normalized time, fertilization-first mitosis Tfm = 1). The effectiveness decreases rapidly by Tfm = 1.75 and disappears by 3.
Intact biliverdin, photo-transformed, or dimethyl ester are not dysmorphogens. When control-fertilized oocytes unexposed to UV light are incubated with any of these compounds they develop normally with an average DAI of 5.
Discussion
Dorsal organ formation requires transcription and translation of specific mRNAs (1–7). The appearance of these mRNAs depends on the activities and interactions of maternal determinants present in the vegetal cortex and cytoplasm that act during the period between fertilization and the first cleavage. The perturbation of the cytoplasmic early determinant in stage 1 embryos results in absence of these mRNAs by gastrula stage and in dorsal axis deficiency by stage 35 (4, 9, 13–22, 30, 31). A frequently used method to perturb this cytoplasmic determinant is irradiation with 254-nm UV light (15, 17, 19, 20, 24–26). We have shown that irradiation with 366-nm UV light also is effective because it affects the formation of dorsal markers and generates identical dorsal axis deficiency (Figs. 1–3).
We have isolated, characterized, inactivated, and tested the ability of biliverdin to rescue UV-irradiated embryos from developing dorsal axis deficiency (Figs. 1–7). Thus, this normal constituent of frog oocytes and eggs (34) here shown to be present in its IXα isomeric form is chemically transformed when embryos are irradiated with either 254- or 366-nm UV light. Moreover, when embryos rendered incompetent to generate a dorsal axis after irradiation with either long and short wavelength UV light are supplemented with biliverdin IXα they recover the lost capability and form a dorsal axis. The intact IXα isoform and at least one of the carboxyl groups of its propionic side chains are essential for the biological activity because photo-transformed biliverdin or dimethyl ester biliverdin do not induce irradiated embryos to form dorsal structures. Therefore, we conclude that biliverdin IXα may be required for dorsal axis formation of Xenopus laevis embryos and its photo-transformation by UV light is responsible for their failure to develop a dorsal axis (Figs. 1–7).
While the inactivation of the cytoplasmic determinant by UV light must occur before the first cleavage (15, 16), the rescue by biliverdin encompasses the period between the second and third cleavages. Moreover, the restoration of the capability of irradiated embryos to form a normal dorsal axis by addition of biliverdin together with the absence of multiple ectopic axes (Fig. 2 D and E) suggests that whereas both 254- and 366-nm UV light affects the biliverdin in the cytoplasmic yolk platelet neither affect the localization of the cortical determinant to the future dorsal zone. A single normal dorsal axis in biliverdin-rescued embryos can take place only if the cortical determinant is properly localized to the dorso-vegetal zone (12). Currently, it is believed that the cortical factor attains its ultimate position by means of rotation of the cortex dependent on microtubules. It has also been proposed that 254-nm UV light inhibits this microtubule-driven rotation and, consequently, leads to dorsal axis deficiency (14, 15, 20, 24–27). However, the identical adorsal teratology produced by either 254- or 366-nm UV irradiation together with the rescue of dorsal axes by biliverdin, suggest that the UV light perturbation of cortical rotation may be more complex, perhaps differ from the current model, and call for revisiting the subject experimentally.
After sperm entry, the yolk platelets are concentrated to the entire vegetal hemisphere of the fertilized eggs. We propose that biliverdin is released from the organelles to interact with the cortical factor (Fig. 8). The biliverdin-cortical factor complex, localized to the future dorso-vegetal zone, can act as a switch-on mode to initiate a cascade of events that establish the Nieuwkoop center and the Spemann–Mangold organizer (3–7). The dorsalizing signals that are synthesized and/or activated after midblastula transition determine the configuration of the dorsal axis and inhibit the activity of other ventralizing signals (4). Photo-transformation of biliverdin by UV light generates an ineffective product. Therefore, the chemical switch remains off, the Nieuwkoop center and the Spemann–Mangold organizer are not formed and dorsalizing signals such as the gene products goosecoid and chordin (Fig. 1) as well as many others, e.g., xSOX3 and siamois, are absent or reduced (10, 30–33). Concurrently, the ventral signals act unrestrained (4). The result is an adorsal embryo.
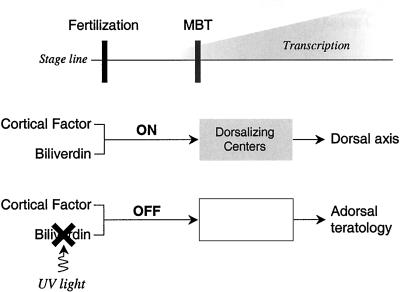
Figure 8.
Molecular switch for induction of dorsal axis. Biliverdin is proposed to interact with a cortical factor to trigger or switch on the downstream activation of genes. When the chemical switch is turned on, the Nieuwkoop center and the Spemann–Mangold organizer are sequentially formed. The UV irradiation of biliverdin renders an ineffective photo-product, the chemical switch remains off, and the dorsalizing gene products that participate in the configuration of the dorsal axis are not formed. The result is adorsal teratology. MBT, midblastula transition.
The cortical factor molecule(s) that might interact with biliverdin remain to be elucidated. Biliverdin is known to bind to proteins such as myoglobin and the aryl hydrocarbon receptor, among others (35, 36). The binding to myoglobin appears to occur through the carboxyls of the propionic side chains. For this reason we examined whether the propionic side chains were involved in the recovery from the UV light exposure. We propose that native biliverdin IXα binds to its proposed molecular partner, and if a protein, might do so through the same components of its structure that is used with other such macromolecules. This expectation is supported by the finding that biliverdin dimethyl ester, a molecule whose propionic acid side-chain carboxyl groups are esterified, fails to restore the capability of UV light-irradiated embryos to form dorsal axis.
The present findings, therefore, identify a function for biliverdin in biology and specifically as a cytoplasmic determinant of embryo development. Hitherto, biliverdin has been considered to be a breakdown product in the heme degradation pathway. Now, it must be understood as a primary molecule with a critical function in embryogenesis. Moreover, the long-held “concept” of the cytoplasmic early determinant can now be understood as a molecule. This finding opens the door to the asking of specific questions regarding its synthesis, transport, storage, utilization, molecular partners, mechanism of possible gene regulation, among others.
Supplementary Material
Acknowledgments
We are grateful to Dr. Gerhard Wagner for providing NMR spectrometer time; Dr. Andrew Tyler for MS support; and Drs. Bert L. Vallee, James Riordan, S. James Adelstein, Edmond Fisher, and Earl Davie for their valuable advice. The dorsal and ventrolateral markers were a generous gift from Drs. Sergei Sokol and Jeremy Green. We are grateful to Drs. Jose Halperin and Xuc Bin for help with the hybridization studies. This work was supported by the Endowment for Research in Human Biology, Brigham and Women's Hospital Research Fund 645912, a generous gift from Mrs. Susan Deland, and National Institutes of Health Grant GM47467-09.
Abbreviation
- DAI
dorsal anterior index
References
- 1.Spemann H, Mangold H. Wilhelm Roux Arch Entwicklungsmech Org. 1924;100:599–638. doi: 10.1007/BF02080953. [DOI] [PubMed] [Google Scholar]
- 2.Witkowski J. Trends Biochem Sci. 1985;10:379–381. [Google Scholar]
- 3.Nieuwkoop P D. Wilhelm Roux Arch Entwicklungsmech Org. 1969;163:298–315. doi: 10.1007/BF00577017. [DOI] [PubMed] [Google Scholar]
- 4.Heasman J. Development (Cambridge, UK) 1997;124:4179–4191. doi: 10.1242/dev.124.21.4179. [DOI] [PubMed] [Google Scholar]
- 5.Moon R T, Kimelman D. BioEssays. 1998;20:536–545. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Nieto M A. Cell. 1999;98:417–425. doi: 10.1016/s0092-8674(00)81971-6. [DOI] [PubMed] [Google Scholar]
- 7.Smith J C, Slack J M. J Embryol Exp Morphol. 1983;78:299–317. [PubMed] [Google Scholar]
- 8.Darras S, Marikawa Y, Elinson R P, Lemaire P. Development (Cambridge, UK) 1997;124:4275–4286. doi: 10.1242/dev.124.21.4275. [DOI] [PubMed] [Google Scholar]
- 9.Kessler D, Melton D A. Science. 1994;266:596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- 10.Penzel R, Oschwald R, Chen Y, Tacke L, Grunz H. Int J Dev Biol. 1997;41:667–677. [PubMed] [Google Scholar]
- 11.Harland R, Gerhart J C. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 12.Kageura H. Development (Cambridge, UK) 1997;124:1543–1551. doi: 10.1242/dev.124.8.1543. [DOI] [PubMed] [Google Scholar]
- 13.Grant P, Wacaster J F. Dev Biol. 1972;28:454–471. doi: 10.1016/0012-1606(72)90029-2. [DOI] [PubMed] [Google Scholar]
- 14.Scharf S R, Gerhart J C. Dev Biol. 1980;79:181–198. doi: 10.1016/0012-1606(80)90082-2. [DOI] [PubMed] [Google Scholar]
- 15.Scharf S R, Gerhart J C. Dev Biol. 1983;99:75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- 16.Holwill S, Heasman J, Crawley C R, Wylie C C. Development (Cambridge, UK) 1987;100:735–743. [Google Scholar]
- 17.Elinson R P, Pasceri P. Development (Cambridge, UK) 1989;106:511–518. doi: 10.1242/dev.106.3.511. [DOI] [PubMed] [Google Scholar]
- 18.Yuge M, Kobayakawa Y, Fujisue M, Yamana K. Development (Cambridge, UK) 1990;110:1051–1056. doi: 10.1242/dev.110.4.1051. [DOI] [PubMed] [Google Scholar]
- 19.Fujisue M, Kobayakawa Y, Yamana K. Development (Cambridge, UK) 1993;118:163–170. doi: 10.1242/dev.118.1.163. [DOI] [PubMed] [Google Scholar]
- 20.Holowacz T, Elinson R P. Development (Cambridge, UK) 1993;119:277–285. doi: 10.1242/dev.119.1.277. [DOI] [PubMed] [Google Scholar]
- 21.Holowacz T, Elinson R P. Development (Cambridge, UK) 1995;121:2789–2798. doi: 10.1242/dev.121.9.2789. [DOI] [PubMed] [Google Scholar]
- 22.Kao K R, Elinson R P. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- 23.Kikkawa M, Kazuhiro T, Shinagawa A. Development (Cambridge, UK) 1996;122:3687–3696. doi: 10.1242/dev.122.12.3687. [DOI] [PubMed] [Google Scholar]
- 24.Vincent J P, Gerhart J C. Dev Biol. 1987;123:566–539. doi: 10.1016/0012-1606(87)90411-8. [DOI] [PubMed] [Google Scholar]
- 25.Sakai M. Development (Cambridge, UK) 1996;122:2207–2214. doi: 10.1242/dev.122.7.2207. [DOI] [PubMed] [Google Scholar]
- 26.Larabell C A, Rowning B A, Wells J, Wu M, Gerhart J C. Development (Cambridge, UK) 1996;122:1281–1289. doi: 10.1242/dev.122.4.1281. [DOI] [PubMed] [Google Scholar]
- 27.Medina A, Wendler S R, Steinbeisser H. Int J Dev Biol. 1997;41:741–745. [PubMed] [Google Scholar]
- 28.Thomas V, Heasman J, Ford C, Nagajski D, Wylie C C. J Embryol Exp Morphol. 1983;76:67–81. [PubMed] [Google Scholar]
- 29.Falchuk K H, Montorzi M, Vallee B L. Biochemistry. 1995;34:16524–16531. doi: 10.1021/bi00050a037. [DOI] [PubMed] [Google Scholar]
- 30.Cho K W, Blumberg B, Steinbeisser H, De Robertis E M. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbeisser H, De Robertis E M, Ku M, Kessler D S, Melton D A. Development (Cambridge, UK) 1993;118:499–507. doi: 10.1242/dev.118.2.499. [DOI] [PubMed] [Google Scholar]
- 32.Sokol S, Christian J L, Moon R T, Melton D A. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 33.Brannon M, Kimelman D. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- 34.Marinetti G V, Bagnara J T. Science. 1983;219:985–987. doi: 10.1126/science.6681678. [DOI] [PubMed] [Google Scholar]
- 35.Phelan D, Winter G M, Rogers W J, Lam J C, Denison M S. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 36.Wagner U G, Muller N, Schmitzberger W, Falk H, Kratky C. J Mol Biol. 1995;24:326–337. doi: 10.1006/jmbi.1994.0142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.