Abstract
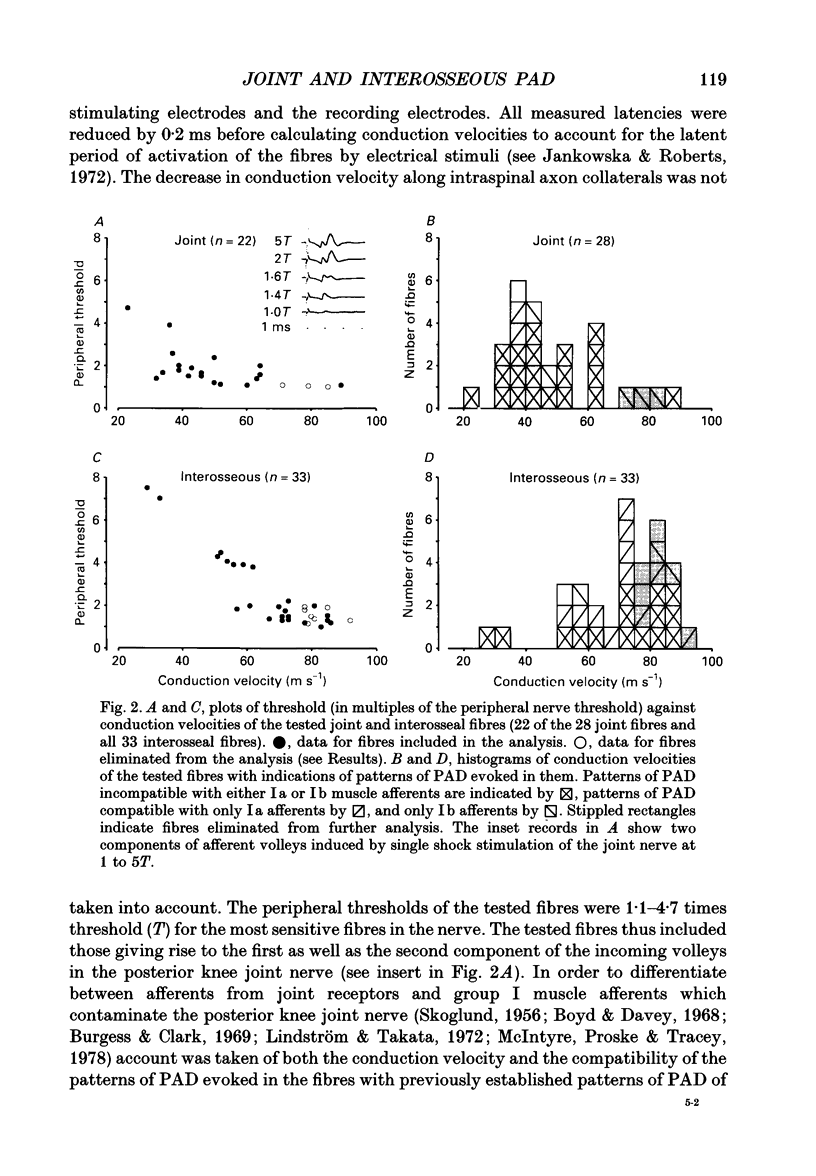
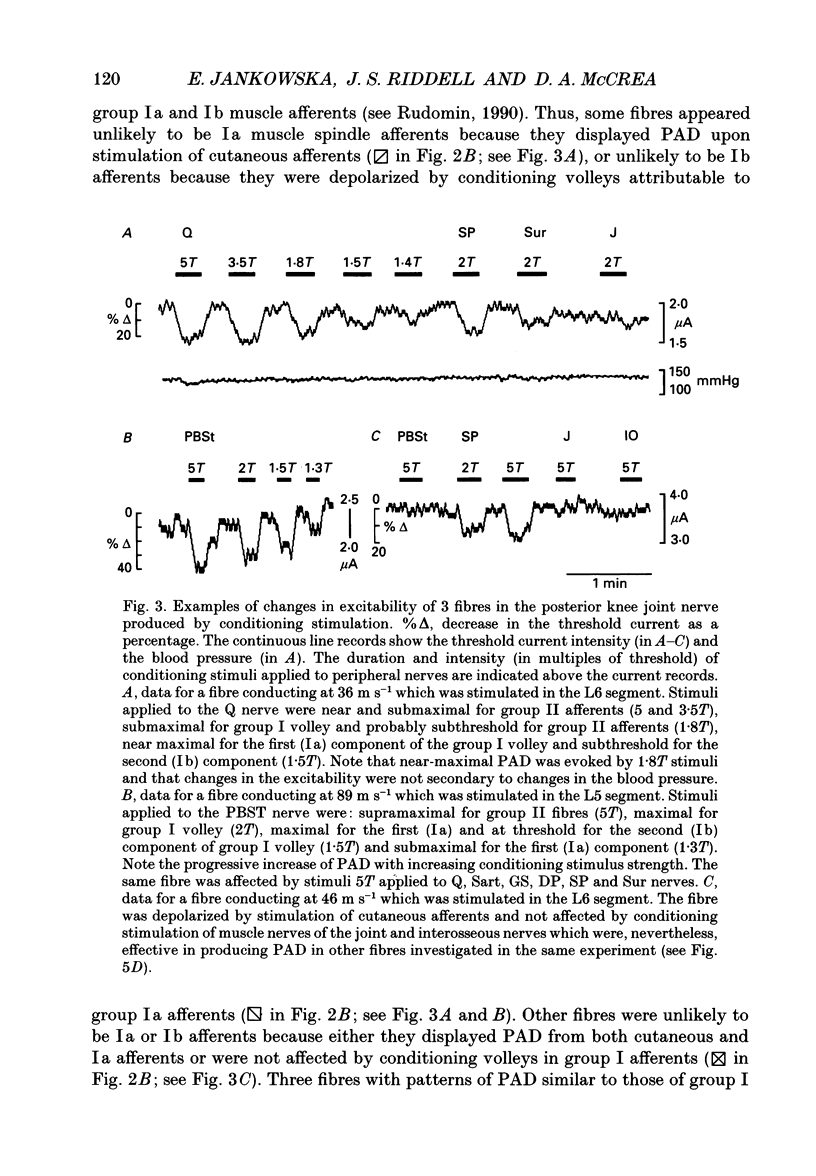
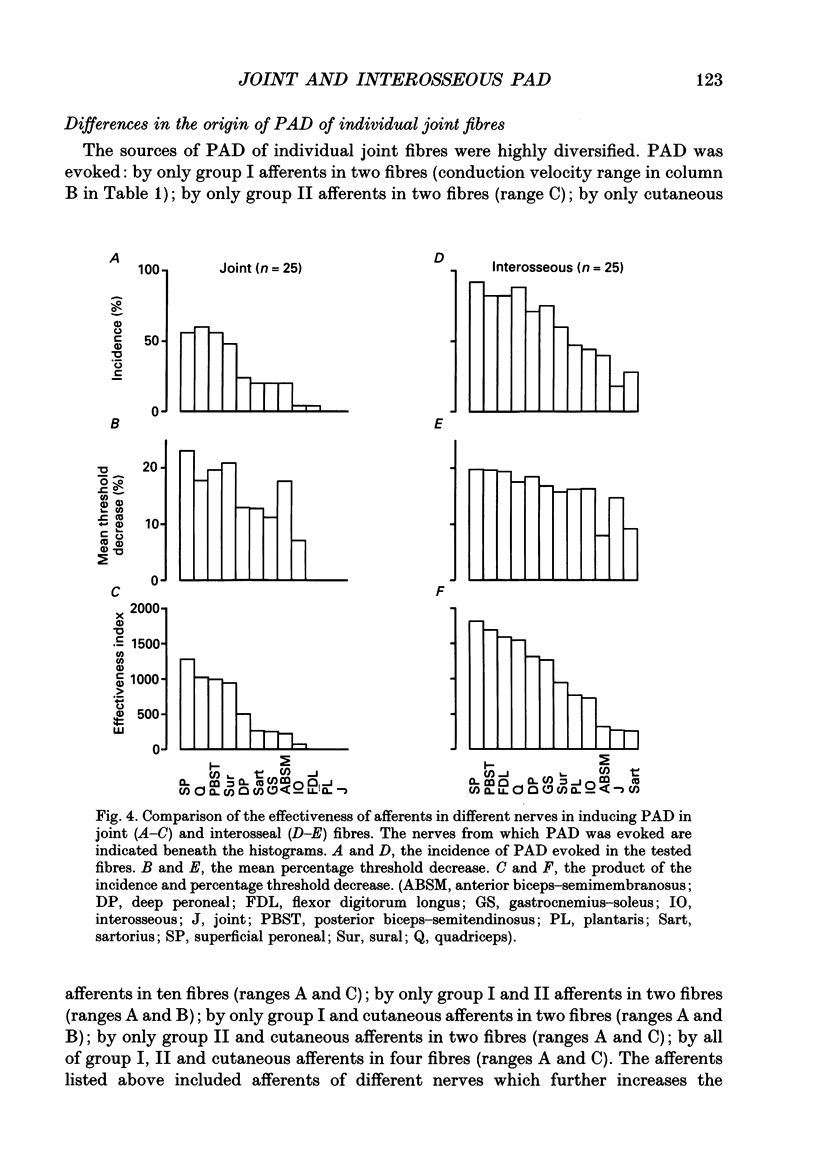
1. Changes in the excitability of the intraspinal terminals of fibres in the posterior knee joint and interosseous nerves were used as a measure of primary afferent depolarization (PAD) which is associated with presynaptic inhibition of transmission from afferent fibres. These were estimated from changes in the intensity of electrical stimuli required to activate the fibres in 50% of trials. In order to avoid the inclusion of group I muscle afferents which contaminate the joint and interosseal nerves, the analysis was restricted to fibres conducting at less than 75 m s-1 and/or displaying patterns of PAD which differed from those of group Ia and Ib muscle afferents in lower lumbar segments of anaesthetized cats. PAD was evoked by electrical stimulation of ipsilateral hindlimb nerves. 2. PAD of fibres in the posterior knee joint nerve was induced from group I (Ia and Ib) and group II muscle afferents and cutaneous afferents but not by stimulation of the joint or the interosseous nerves. The most effective stimuli were those applied to the superficial peroneal, sural, quadriceps and posterior biceps and semitendinosus nerves. 3. PAD of fibres in the interosseous nerve was also induced by stimulation of group I (Ia and Ib) and group II muscle afferents and cutaneous afferents and, in addition, by stimulation of joint and interosseous nerves. The most effective stimuli were those applied to the superficial peroneal, quadriceps, flexor digitorum longus and posterior biceps and semitendinosus nerves. 4. Individual fibres of the joint and the interosseous nerves were depolarized by only some of the conditioning stimuli. Even the most effective stimuli did not produce PAD in all of the fibres tested. Individual fibres of the joint and the interosseous nerves were depolarized by diverse combinations of afferents of different functional types and of different peripheral nerves. The differences in the sources of PAD were not associated with the conduction velocities and hence are unlikely to be related to differences in the receptor origin of the tested fibres. The diversity in the sources of PAD of individual fibres is interpreted as providing a high degree of differentiation in the control of transmission from receptors in joints and interosseal membranes.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A. The histological structure of the receptors in the knee-joint of the cat correlated with their physiological response. J Physiol. 1954 Jun 28;124(3):476–488. doi: 10.1113/jphysiol.1954.sp005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A., Kalu K. U. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 1979 Apr;289:277–297. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Jankowska E., Skoog B. Convergence onto interneurons subserving primary afferent depolarization of group I afferents. J Neurophysiol. 1984 Mar;51(3):432–449. doi: 10.1152/jn.1984.51.3.432. [DOI] [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Characteristics of knee joint receptors in the cat. J Physiol. 1969 Aug;203(2):317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER D., ENGBERG I., FUNKENSTEIN H., LUNDBERG A. DECEREBRATE CONTROL OF REFLEXES TO PRIMARY AFFERENTS. Acta Physiol Scand. 1963 Dec;59:424–437. doi: 10.1111/j.1748-1716.1963.tb02758.x. [DOI] [PubMed] [Google Scholar]
- Calvillo O., Madrid J., Rudomín P. Presynaptic depolarization of unmyelinated primary afferent fibers in the spinal cord of the cat. Neuroscience. 1982 Jun;7(6):1389–1409. doi: 10.1016/0306-4522(82)90252-4. [DOI] [PubMed] [Google Scholar]
- Clark F. J., Burgess P. R. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol. 1975 Nov;38(6):1448–1463. doi: 10.1152/jn.1975.38.6.1448. [DOI] [PubMed] [Google Scholar]
- Craig A. D., Heppelmann B., Schaible H. G. The projection of the medial and posterior articular nerves of the cat's knee to the spinal cord. J Comp Neurol. 1988 Oct 8;276(2):279–288. doi: 10.1002/cne.902760210. [DOI] [PubMed] [Google Scholar]
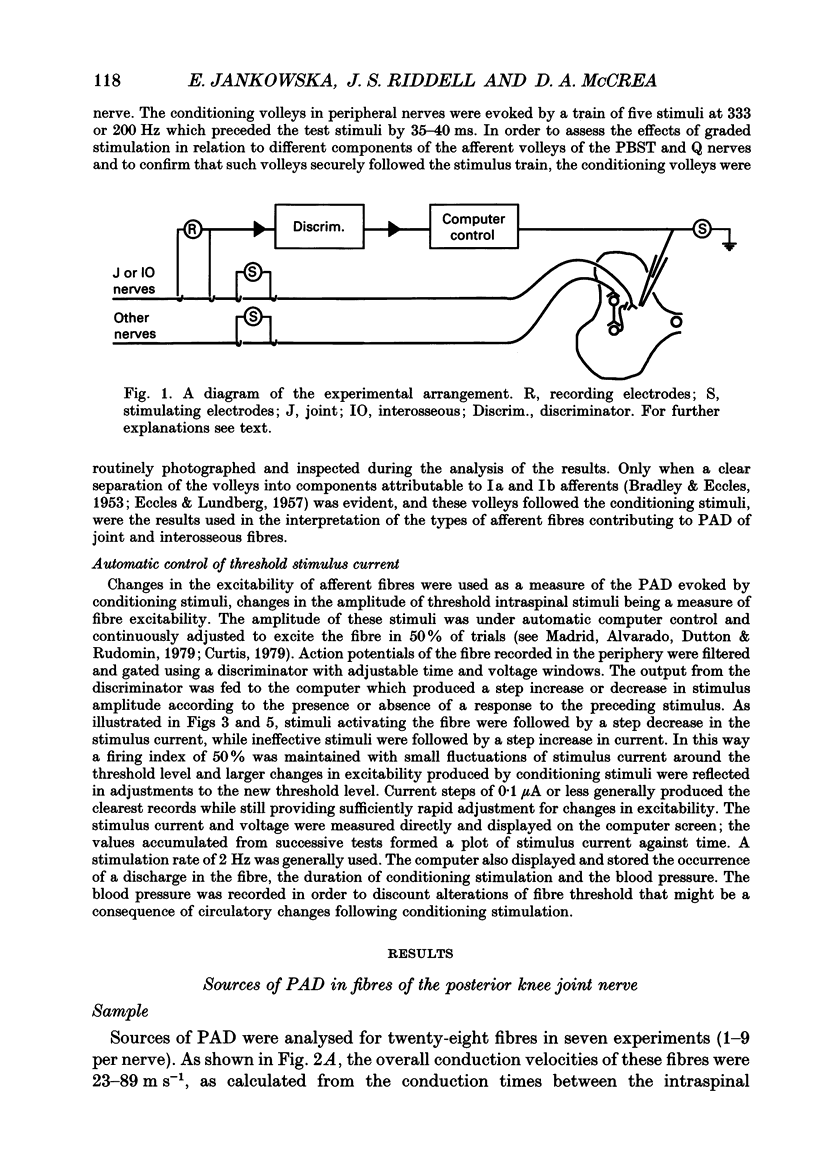
- Curtis D. R. A method for continuously monitoring the electrical threshold of single intraspinal nerve fibers. Electroencephalogr Clin Neurophysiol. 1979 Oct;47(4):503–506. doi: 10.1016/0013-4694(79)90167-6. [DOI] [PubMed] [Google Scholar]
- DEVANANDAN M. S., HOLMQVIST B., YOKOTA T. PRESYNAPTIC DEPOLARIZATION OF GROUP I MUSCLE AFFERENTS BY CONTRALATERAL AFFERENT VOLLEYS. Acta Physiol Scand. 1965 Jan-Feb;63:46–54. doi: 10.1111/j.1748-1716.1965.tb04040.x. [DOI] [PubMed] [Google Scholar]
- Devanandan M. S., Eccles R. M., Stenhouse D. Presynaptic inhibition evoked by muscle contraction. J Physiol. 1966 Jul;185(2):471–485. doi: 10.1113/jphysiol.1966.sp007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOZAK W., MAGNI F. Dorsal root reflexes of muscle group I afferent fibres. J Physiol. 1961 Nov;159:128–146. doi: 10.1113/jphysiol.1961.sp006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Woolf C. J. Effects of cutaneous nerve and intraspinal conditioning of C-fibre afferent terminal excitability in decerebrate spinal rats. J Physiol. 1981 Sep;318:25–39. doi: 10.1113/jphysiol.1981.sp013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967 Jun;101(Pt 3):505–532. [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., McIntyre A. K., Proske U. Tendon organ afferents in the knee joint nerve of the cat. Neurosci Lett. 1989 Sep 11;103(3):287–292. doi: 10.1016/0304-3940(89)90114-6. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Primary afferent depolarization of central terminals of group II muscle afferents in the cat spinal cord. J Physiol. 1989 Apr;411:71–83. doi: 10.1113/jphysiol.1989.sp017561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Johannisson T. Segmental actions of afferents of the interosseous nerve in the cat. J Physiol. 1983 Dec;345:373–389. doi: 10.1113/jphysiol.1983.sp014983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. 3. Effects on primary afferent terminals. Exp Brain Res. 1972;15(1):39–53. doi: 10.1007/BF00234957. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Pierrot-Deseilligny E., Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987 Aug;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992 Jul;72(3):623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38(4):335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- Lindström S., Takata M. Monosynaptic excitation of dorsal spinocerebellar tract neurones from low threshold joint afferents. Acta Physiol Scand. 1972 Mar;84(3):430–432. [PubMed] [Google Scholar]
- Madrid J., Alvarado J., Dutton H., Rudomín P. A method for the dynamic continuous estimation of excitability changes of single fiber terminals in the central nervous system. Neurosci Lett. 1979 Mar;11(3):253–258. doi: 10.1016/0304-3940(79)90003-x. [DOI] [PubMed] [Google Scholar]
- McIntyre A. K., Proske U., Tracey D. J. Afferent fibres from muscle receptors in the posterior nerve of the cat's knee joint. Exp Brain Res. 1978 Nov 15;33(3-4):415–424. doi: 10.1007/BF00235563. [DOI] [PubMed] [Google Scholar]
- SKOGLUND S. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol Scand Suppl. 1956;36(124):1–101. [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Tomlinson R. D., Robinson D. A. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol. 1984 Jun;51(6):1121–1136. doi: 10.1152/jn.1984.51.6.1121. [DOI] [PubMed] [Google Scholar]


