Abstract
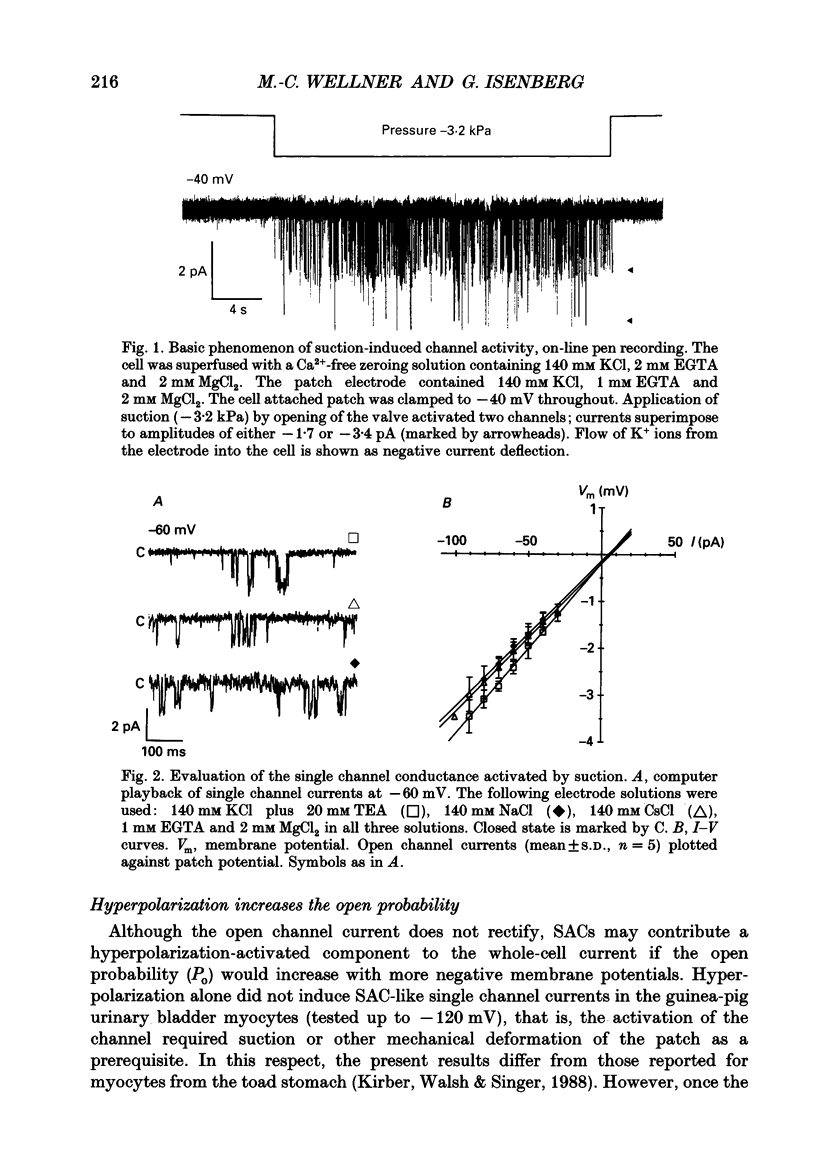
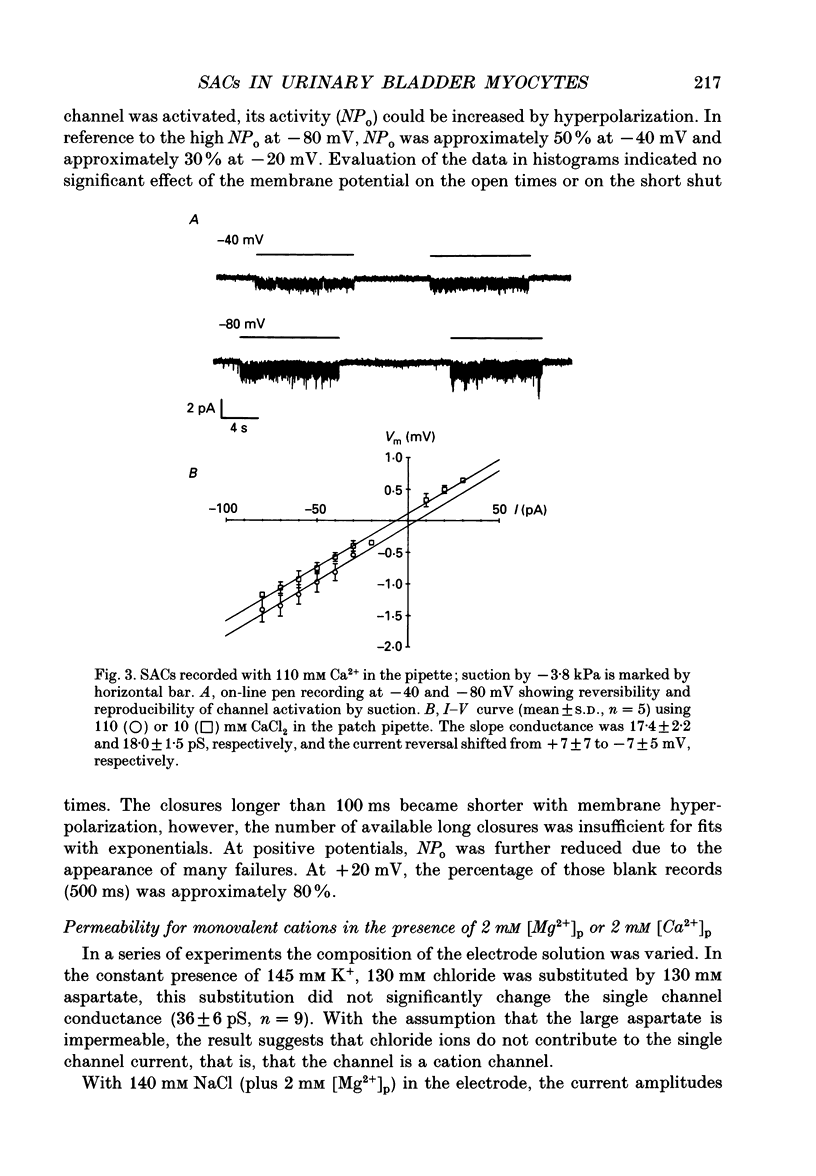
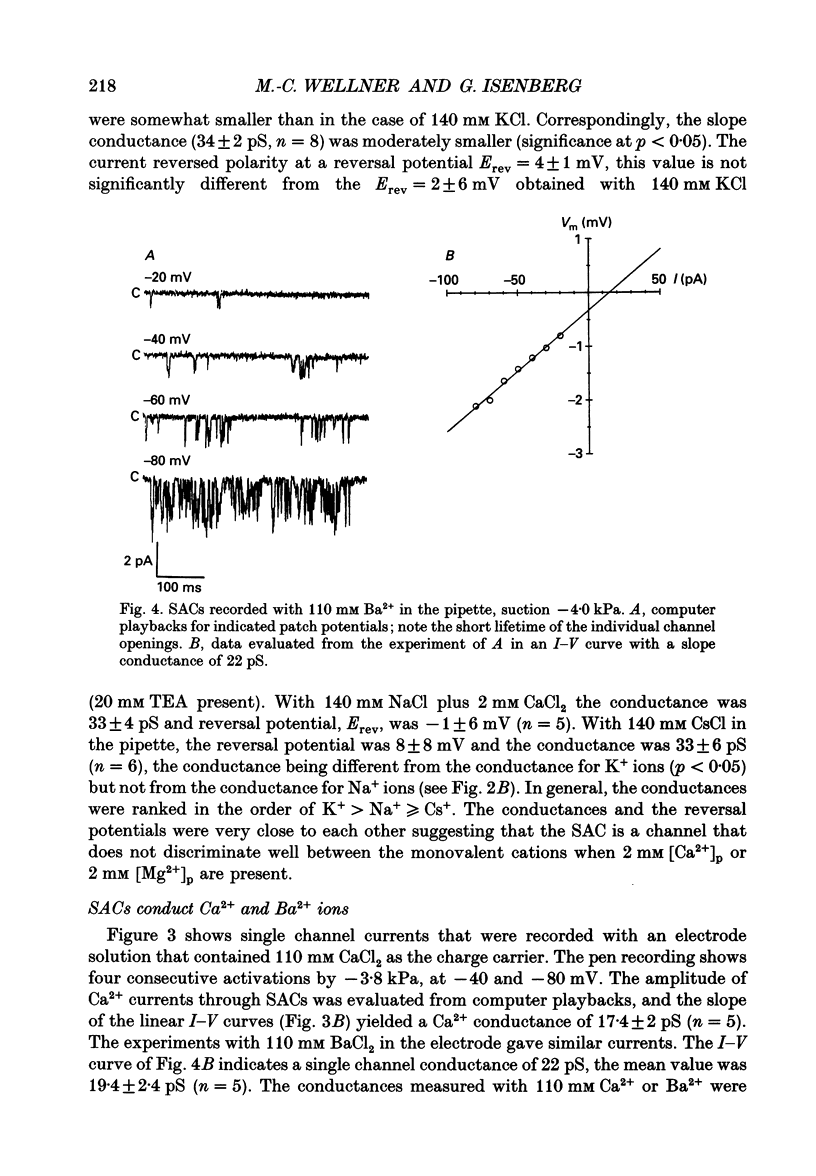
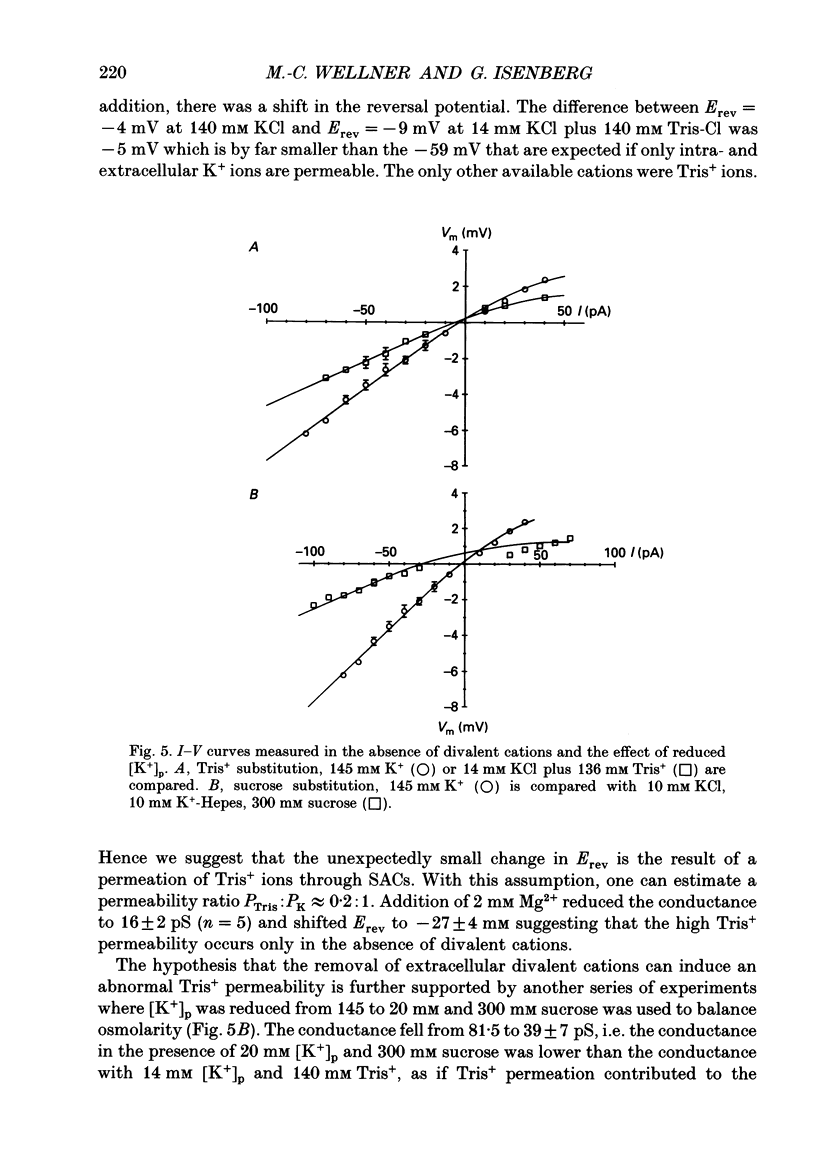
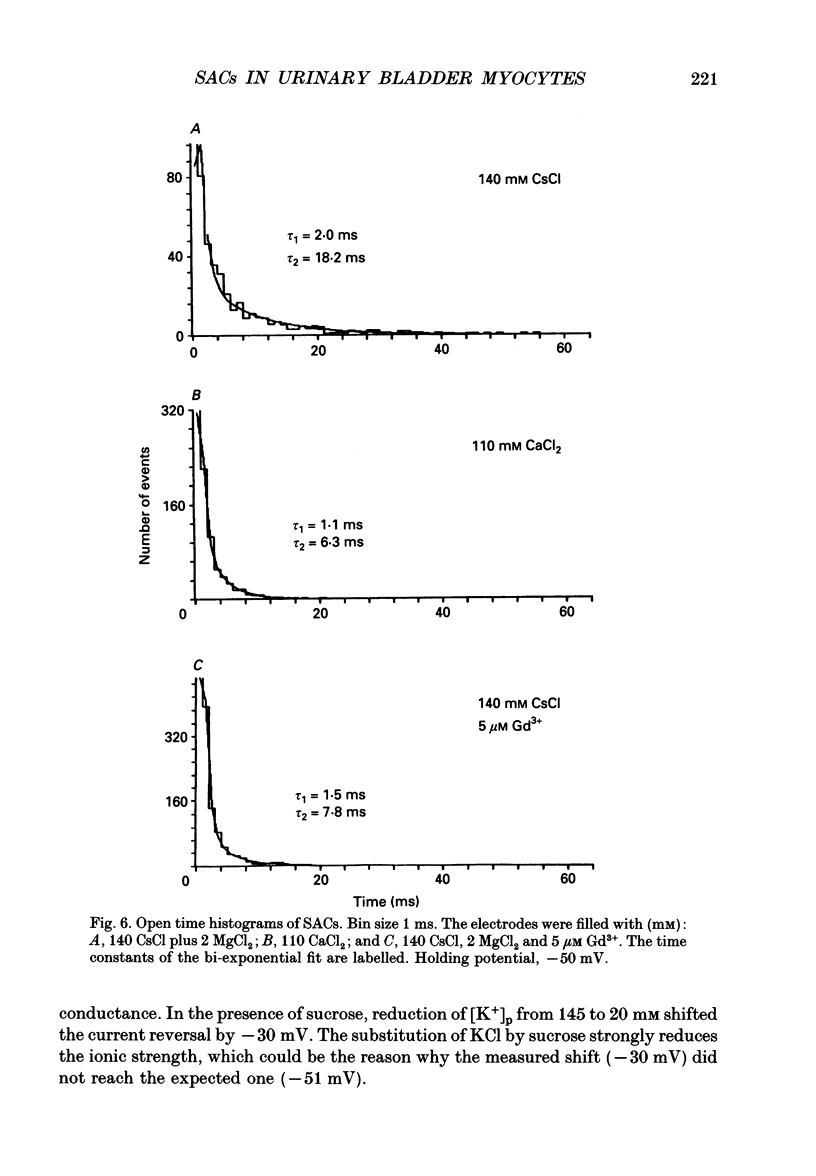
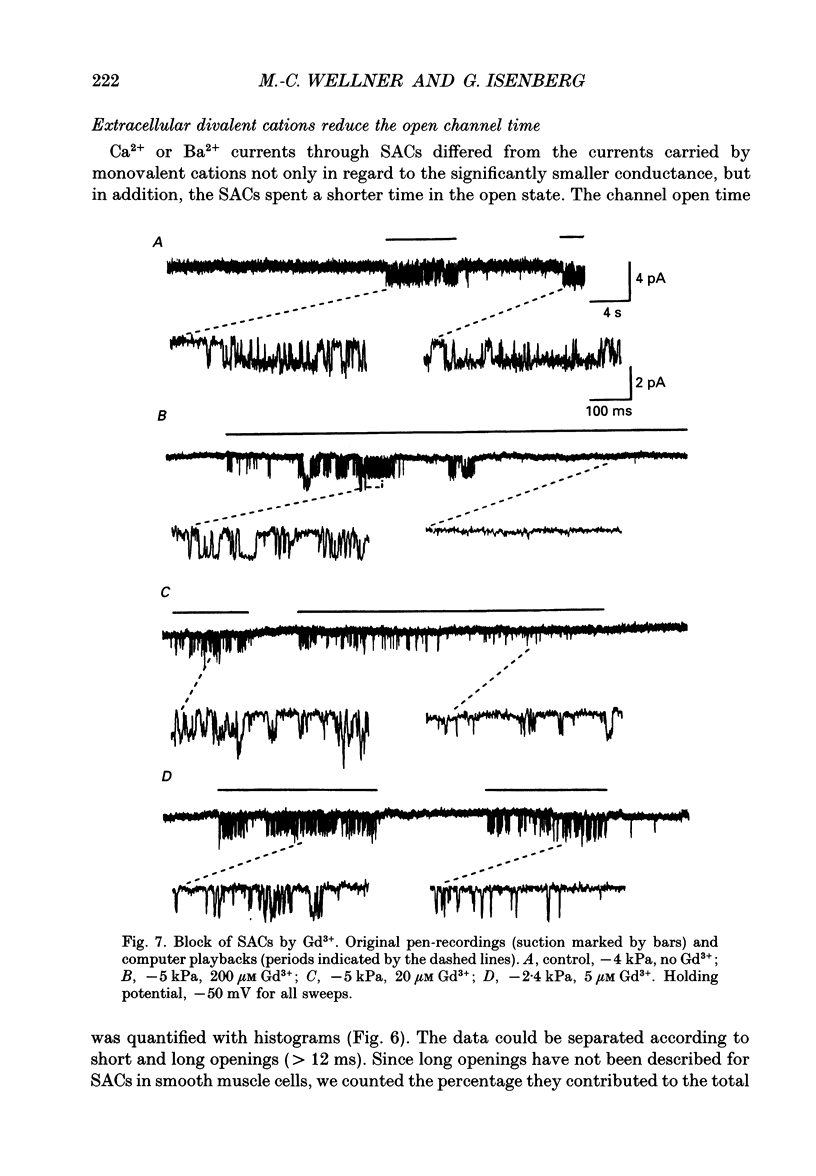
1. Stretch-activated channels (SACs) were analysed on patches attached to myocytes isolated from the guinea-pig urinary bladder. At 22 degrees C application of -2 to -4 kPa to the patch electrode induced SACs at a density of one to two per patch (3-5 M omega electrodes). 2. With electrodes containing 145 mM K+, 20 mM TEA and 2 mM Mg2+, the single channel current followed a linear I-V curve with a slope conductance of 39 +/- 5 pS (mean +/- S.D.) and a reversal potential of 2 +/- 6 mV. Substitution of chloride by aspartate ions left both parameters unchanged suggesting that the anions do not contribute to the currents. 3. Hyperpolarization from -30 to -80 mV did not open channels by itself but increased channel activity (NPo; where N is the number of channels in the patch and Po is the probability of the channel being open) twofold. The hyperpolarization-induced increase in NPo can be attributed to a reduction of long closures. At positive patch potentials numerous blank records strongly diminished NPo. 4. Inward currents through SACs can be carried by a variety of cations. In the presence of 2 mM Mg2+, the respective channel conductance was 40 +/- 4 pS for 140 mM K+ > 34 +/- 2 pS for 140 mM Na+ > or = 33 +/- 6 pS for 140 mM Cs+ > 19 +/- 2 pS for 110 mM Ba2+ > 17 +/- 2 pS for 110 mM Ca2+. 5. Reduction of CaCl2 from 110 to 10 mM did not change the conductance but shifted the reversal potential from +7 to -7 mV; the reversal potentials suggest that SACs are slightly more permeable for Ca2+ than for K+. 6. In the absence of divalent cations, the conductance of K+ was 82 +/- 4 pS for inward but 45 pS for outward currents. Addition of either 2 mM Ca2+ or 2 mM Mg2+ reduced the conductance for inward currents to 40 pS. 7. The change from 140 to 14 mM KCl plus 136 mM Tris-Cl reduced the conductance from 82 to 56 pS whereas the reversal potential shifted only from -4 to -9 mV. When 20 mM K+ and 300 mM sucrose were applied, the conductance fell to 39 pS and the reversal potential shifted by -30 mV. The results suggest that Tris+ can permeate through SACs when extracellular divalent cations are absent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear C. E. A nonselective cation channel in rat liver cells is activated by membrane stretch. Am J Physiol. 1990 Mar;258(3 Pt 1):C421–C428. doi: 10.1152/ajpcell.1990.258.3.C421. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Denbigh J. S., Lang R. J. Inward rectification in freshly isolated single smooth muscle cells of the rabbit jejunum. J Physiol. 1987 Feb;383:461–476. doi: 10.1113/jphysiol.1987.sp016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. E., Tang J. M., Rae J. L., Eisenberg R. S. A cation channel in frog lens epithelia responsive to pressure and calcium. J Membr Biol. 1986;93(3):259–269. doi: 10.1007/BF01871180. [DOI] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):115–126. doi: 10.1007/BF00586904. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T., Ordway R. W., Kirber M. T., Singer J. J., Walsh J. V., Jr Hyperpolarization-activated cationic channels in smooth muscle cells are stretch sensitive. Pflugers Arch. 1991 Jan;417(5):493–499. doi: 10.1007/BF00370945. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Walsh J. V., Jr, Singer J. J. Stretch-activated ion channels in smooth muscle: a mechanism for the initiation of stretch-induced contraction. Pflugers Arch. 1988 Sep;412(4):339–345. doi: 10.1007/BF01907549. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Markwardt F., Isenberg G. Gating of maxi K+ channels studied by Ca2+ concentration jumps in excised inside-out multi-channel patches (myocytes from guinea pig urinary bladder). J Gen Physiol. 1992 Jun;99(6):841–862. doi: 10.1085/jgp.99.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M., Sachs F., Jing Z. Q. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J. 1991 Mar;59(3):722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglietti V., Toselli M. A study of stretch-activated channels in the membrane of frog oocytes: interactions with Ca2+ ions. J Physiol. 1988 Dec;407:311–328. doi: 10.1113/jphysiol.1988.sp017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Characterization of stretch-activated ion channels in Xenopus oocytes. J Physiol. 1990 Dec;431:103–122. doi: 10.1113/jphysiol.1990.sp018322. [DOI] [PMC free article] [PubMed] [Google Scholar]


