Abstract
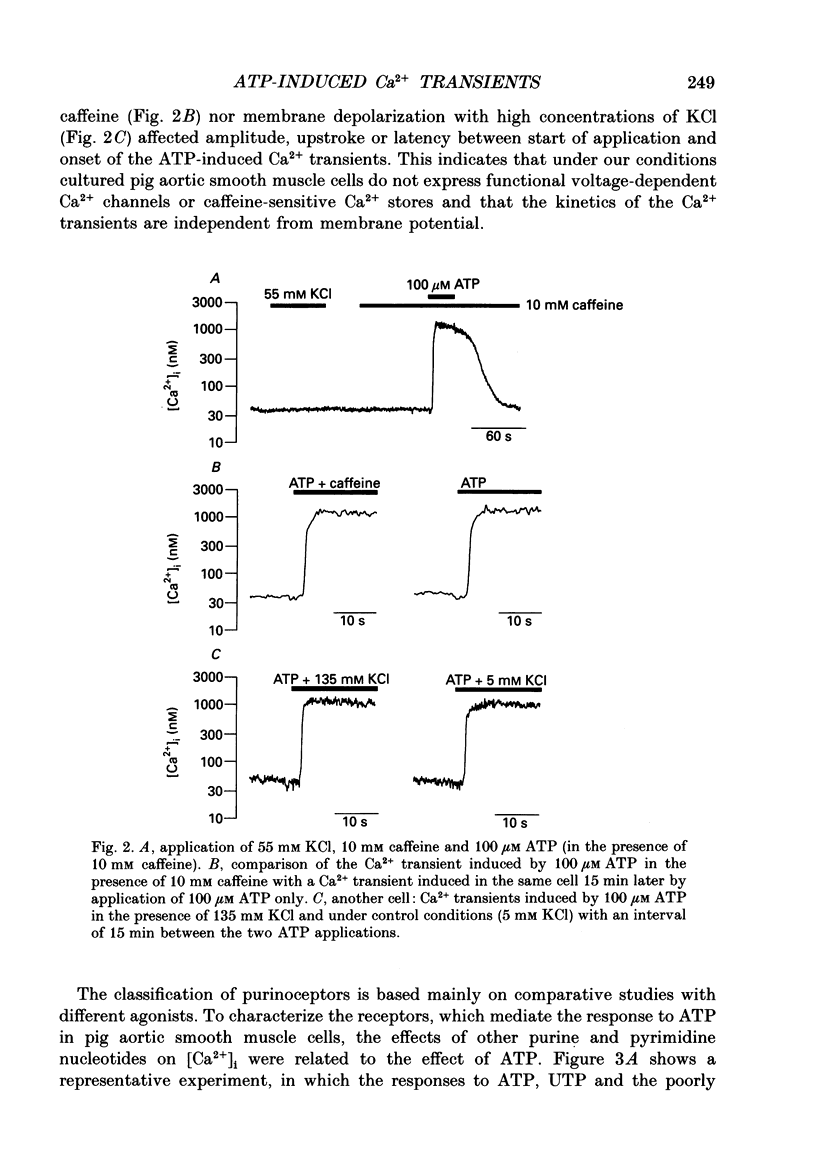
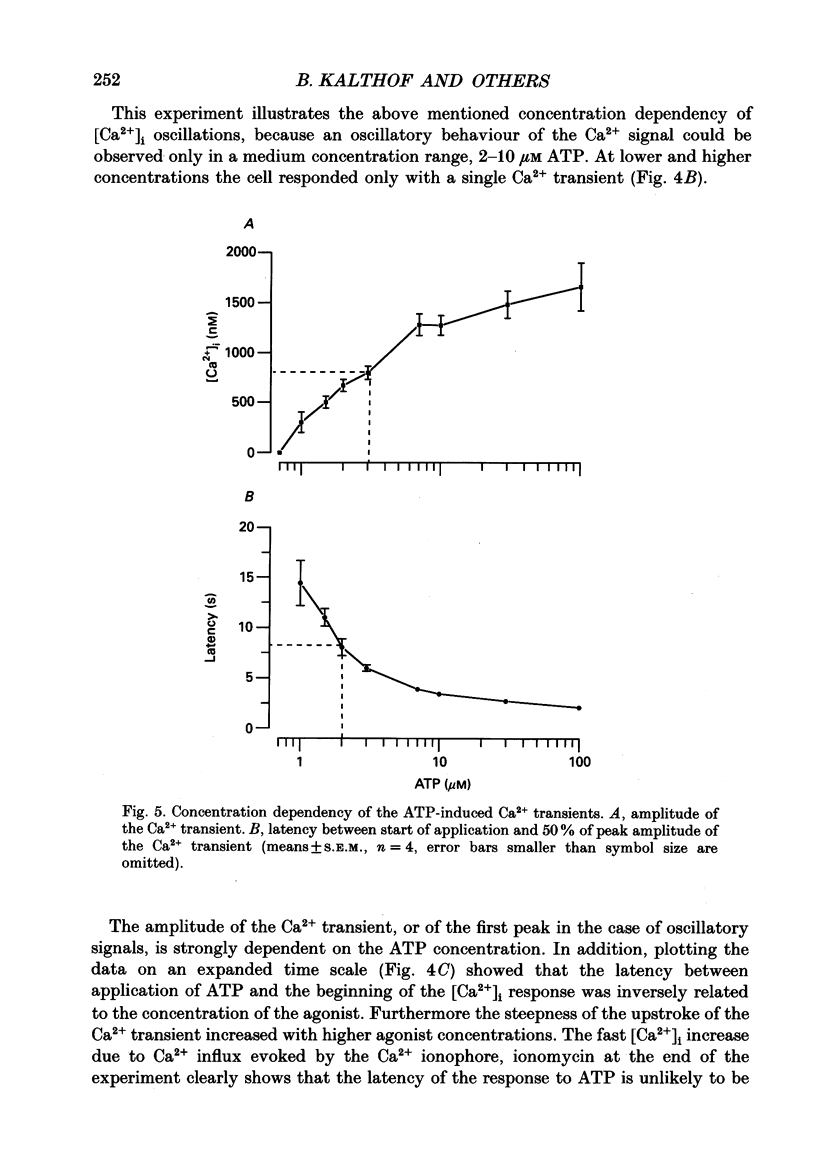
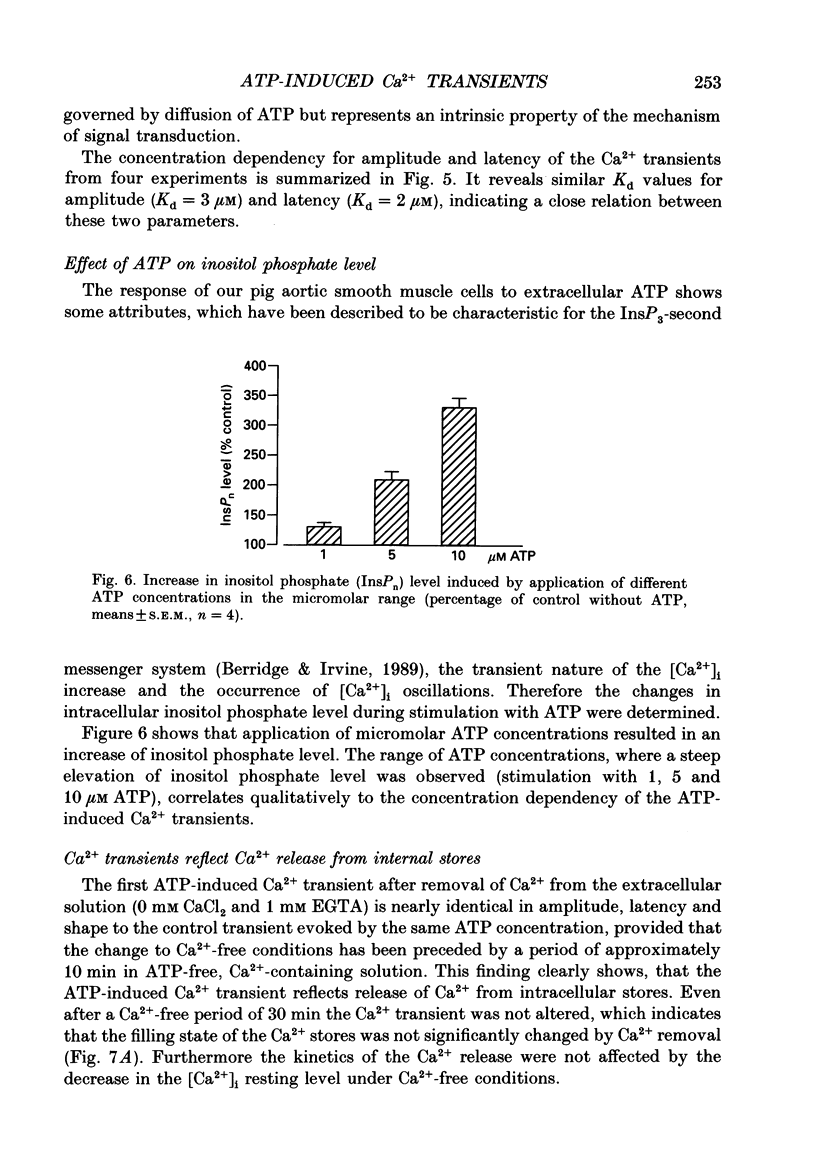
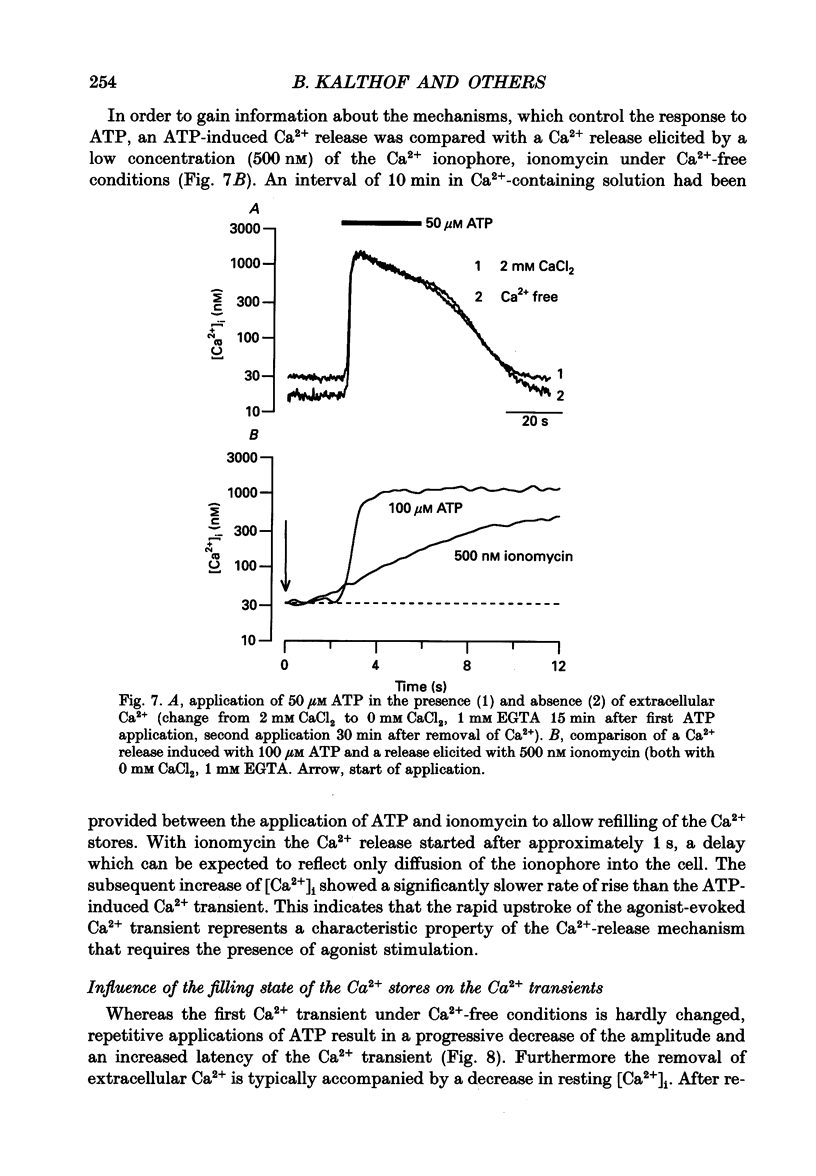
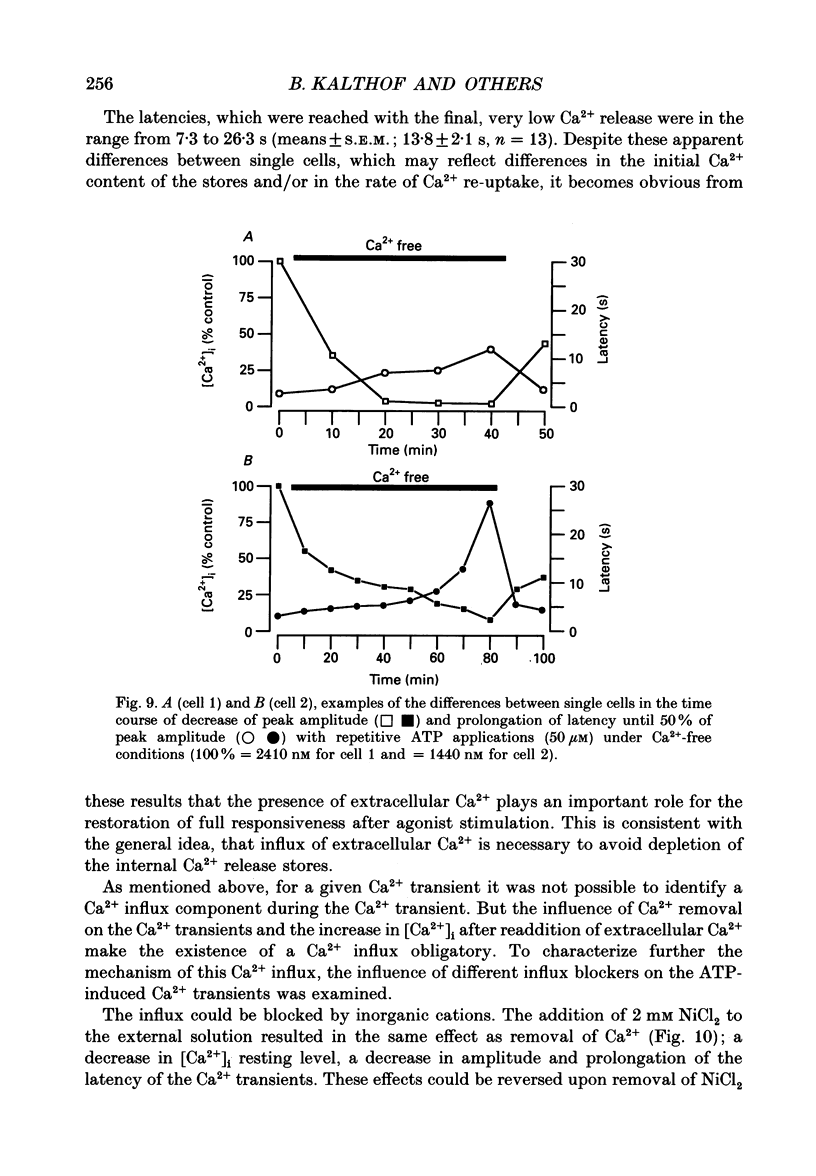
1. Single cultured pig aortic smooth muscle cells were studied using fura-2 and dual excitation wavelength microfluometry. 2. Extracellular ATP in micromolar concentrations induced a transient increase of [Ca2+]i due to Ca2+ release from internal stores. In the same concentration range application of ATP resulted in an increase of intracellular inositol phosphate level. 3. In a medium range of ATP concentrations (2-10 microM) the Ca2+ signal was oscillating, whereas at higher and lower concentrations only a Ca2+ transient with a single peak was elicited. 4. The rank order of potency for the tested purine and pyrimidine nucleotides was: UTP > ATP > ADP >> AMP = adenosine = alpha,beta-methylene ATP = 0. The response to the nucleotides could be abolished by the P2-purinoceptor antagonist suramin. 5. The latency between agonist application and onset of the Ca2+ transients as well as their amplitude and rate of rise are dependent on ATP concentration. 6. Removal of Ca2+ from the extracellular solution led to a progressive decrease of amplitude and prolonged latency of the Ca2+ transients. This shows that depletion of the Ca2+ stores affects kinetics of the ATP-induced Ca2+ release. 7. The inorganic Ca(2+)-influx blockers Ni2+ and Co2+ affected amplitude and latency in a manner similar to Ca2+ removal, while the Ca2+ antagonist nifedipine was ineffective up to a concentration of 10(-6) M. 8. These results reveal a dual dependency of the InsP3-induced Ca2+ release on agonist concentration and filling state of the Ca2+ stores, which supports the hypothesis of a feedback amplification between InsP3 and released Ca2+.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bals S., Bechem M., Paffhausen W., Pott L. Spontaneous and experimentally evoked [Ca2+]i-transients in cardiac myocytes measured by means of a fast Fura-2 technique. Cell Calcium. 1990 Jun-Jul;11(6):385–396. doi: 10.1016/0143-4160(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R. What controls smooth muscle phenotype? Atherosclerosis. 1981 Nov-Dec;40(3-4):347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Demolle D., Lagneau C., Boeynaems J. M. Stimulation of prostacyclin release from aortic smooth muscle cells by purine and pyrimidine nucleotides. Eur J Pharmacol. 1988 Oct 18;155(3):339–343. doi: 10.1016/0014-2999(88)90526-2. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Callewaert G., Declerck I., Casteels R. ATP-induced Ca2+ release and Cl- current in cultured smooth muscle cells from pig aorta. J Physiol. 1991;440:623–634. doi: 10.1113/jphysiol.1991.sp018728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Huganir R. L., Snyder S. H. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature. 1992 Mar 26;356(6367):350–352. doi: 10.1038/356350a0. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Delbro D., Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985 Jan 2;107(2):161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Linderman J. J., Harris L. J., Slakey L. L., Gross D. J. Charge-coupled device imaging of rapid calcium transients in cultured arterial smooth muscle cells. Cell Calcium. 1990 Feb-Mar;11(2-3):131–144. doi: 10.1016/0143-4160(90)90066-4. [DOI] [PubMed] [Google Scholar]
- Masuo M., Toyo-oka T., Shin W. S., Sugimoto T. Growth-dependent alterations of intracellular Ca(2+)-handling mechanisms of vascular smooth muscle cells. PDGF negatively regulates functional expression of voltage-dependent, IP3-mediated, and CA(2+)-induced Ca2+ release channels. Circ Res. 1991 Nov;69(5):1327–1339. doi: 10.1161/01.res.69.5.1327. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H., Lindeman V., Lindeman S., Kunzelmann K., Späth M., Greger R. Effect of depolarizing and hyperpolarizing agents on the membrane potential difference of primary cultures of rabbit aorta vascular smooth muscle cells. Pflugers Arch. 1991 Aug;419(1):69–75. doi: 10.1007/BF00373749. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12511–12518. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommermeyer H., Schwinzer R., Kaever V., Behl B., Resch K. The G protein coupling T cell antigen receptor/CD3-complex and phospholipase C in the human T cell lymphoma Jurkat is not a target for cholera toxin. Eur J Immunol. 1990 Sep;20(9):1881–1886. doi: 10.1002/eji.1830200902. [DOI] [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. J Biochem. 1987 Dec;102(6):1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]


