Abstract
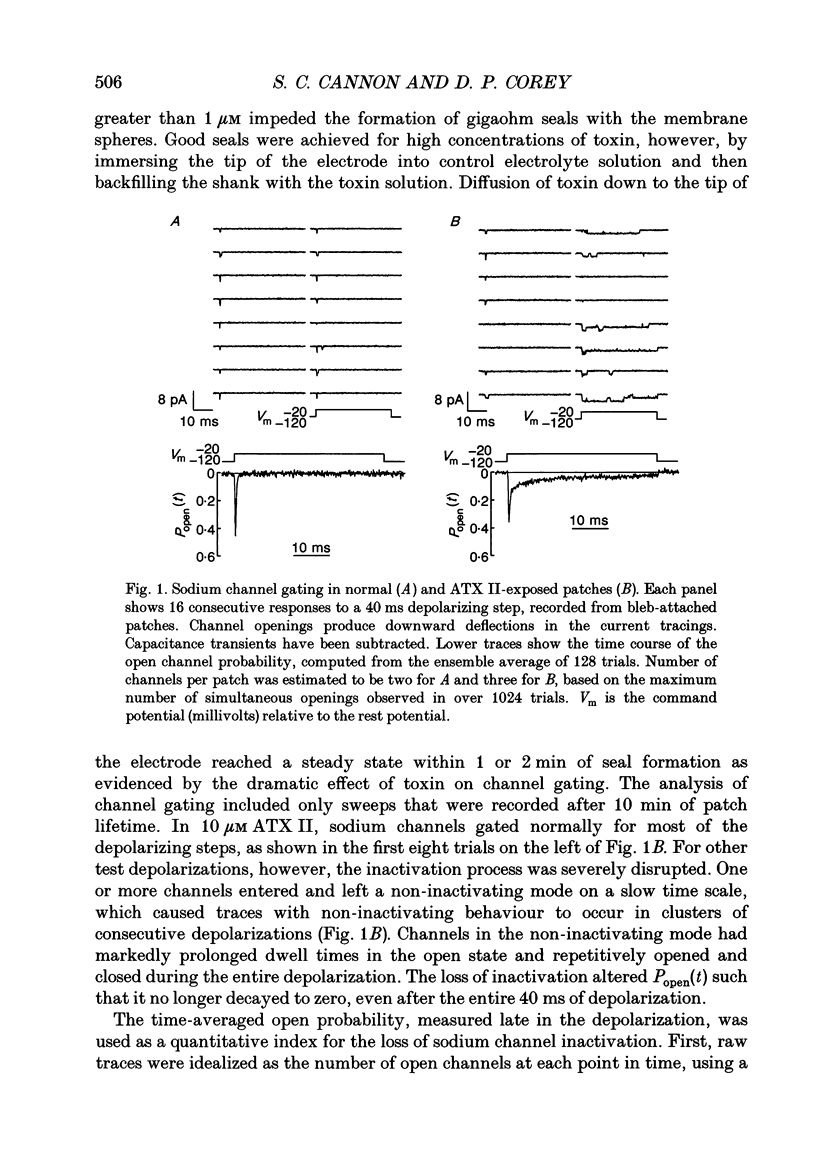
1. Mutations that impair inactivation of the sodium channel in skeletal muscle have recently been postulated to cause several heritable forms of myotonia in man. A peptide toxin from Anemonia sulcata (ATX II) selectively disrupts the inactivation mechanism of sodium channels in a way that mimics these mutations. We applied ATX II to rat skeletal muscle to test the hypothesis that myotonia is inducible by altered sodium channel function. 2. Single-channel sodium currents were measured in blebs of surface membrane that arose from the mechanically disrupted fibres. ATX II impaired inactivation as demonstrated by persistent reopenings of sodium channels at strongly depolarized test potentials. A channel failed to inactivate, however, in only a small proportion of the depolarizing steps. With micromolar amounts of ATX II, the ensemble average open probability at the steady state was 0.01-0.02. 3. Ten micromolar ATX II slowed the relaxation of tension after a single twitch by an order of magnitude. Delayed relaxation is the in vitro analogue of the stiffness experienced by patients with myotonia. However, peak twitch force was not affected within the range of 0-10 microM ATX II. 4. Intracellular injection of a long-duration, constant current pulse elicited a train of action potentials in ATX II-treated fibres. After-depolarizations and repetitive firing often persisted beyond the duration of the stimulus. Trains of action potentials varied spontaneously in amplitude and firing frequency in a similar way to the electromyogram of a myotonic muscle. Both the after-depolarization and the post-stimulus firing were abolished by detubulating the fibres with glycerol. 5. We conclude that a loss of sodium channel inactivation alone, without changes in resting membrane conductance, is sufficient to produce the electrical and mechanical features of myotonia. Furthermore, in support of previous studies on myotonic muscle from patients, this model provides direct evidence that only a small proportion of sodium channels needs to function abnormally to cause myotonia.
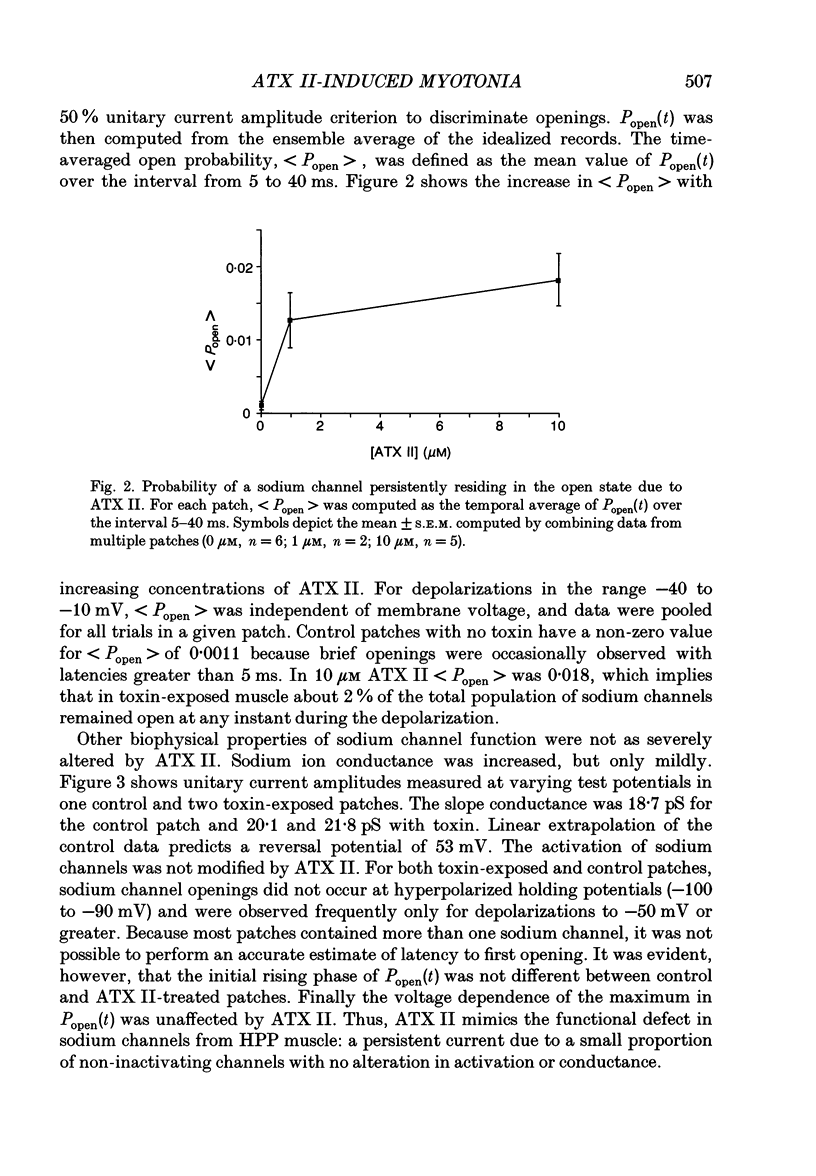
Full text
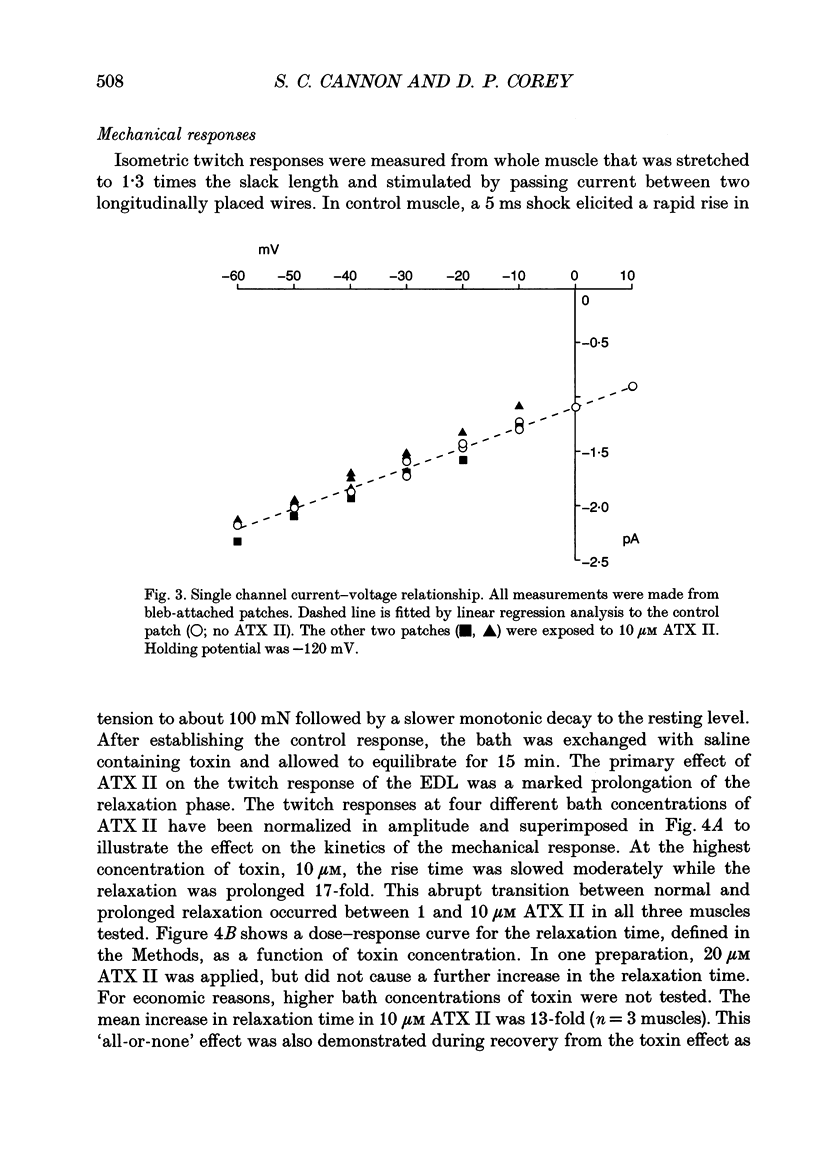
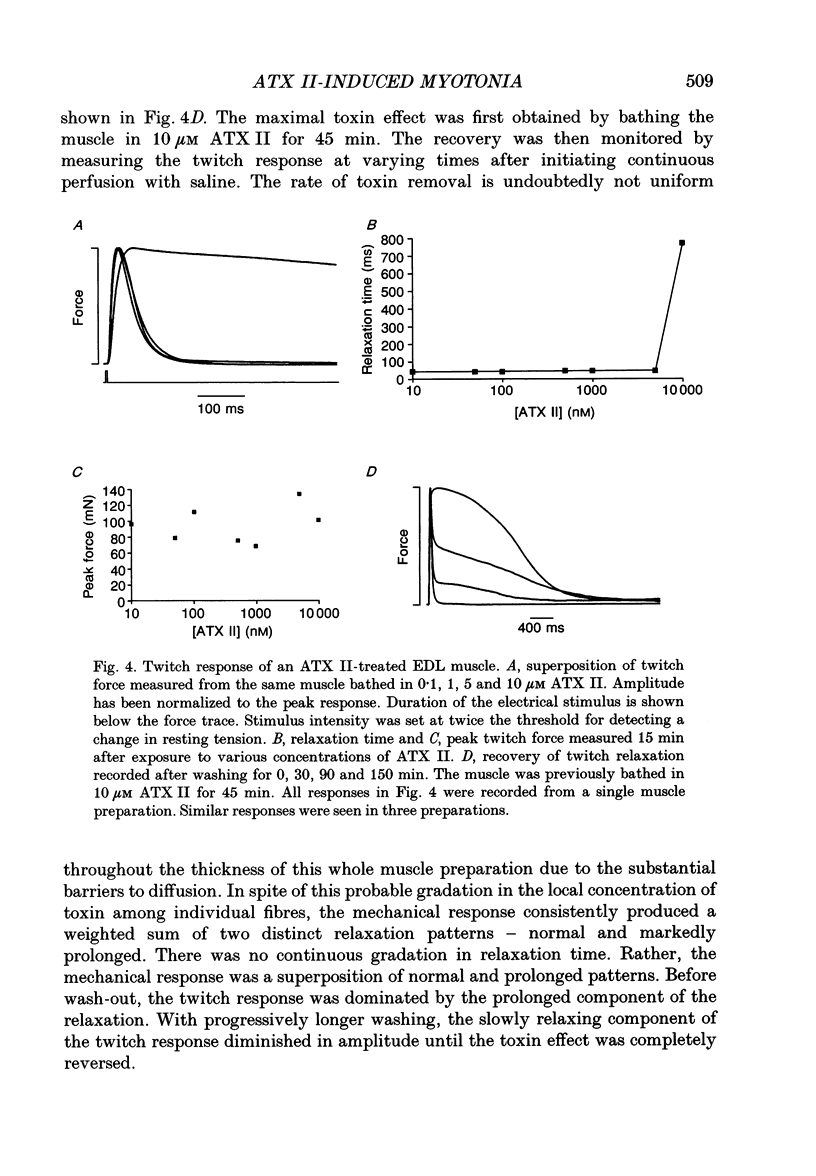
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Bryant S. H. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974 Jul;240(2):505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsen C., Harris J. B., Tesseraux I. Mechanical and electrophysiological effects of sea anemone (Anemonia sulcata) toxins on rat innervated and denervated skeletal muscle. Br J Pharmacol. 1981 Sep;74(1):61–71. doi: 10.1111/j.1476-5381.1981.tb09955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C., Dubois J. M., Rojas E., Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976 Nov 11;455(1):173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991 Apr;6(4):619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Strittmatter S. M. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993 Feb;10(2):317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Differential effects of glycerol treatment on membrane capacity and excitation-contraction coupling in toad sartorius fibres. J Physiol. 1973 Oct;234(2):373–408. doi: 10.1113/jphysiol.1973.sp010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers G. C., George A. L., Barchi R. L., Ting-Passador S. S., Kallen R. G., Lathrop G. M., Beckmann J. S., Hahn A. F., Brown W. F., Campbell R. D. Paramyotonia congenita and hyperkalemic periodic paralysis are linked to the adult muscle sodium channel gene. Ann Neurol. 1991 Dec;30(6):810–816. doi: 10.1002/ana.410300610. [DOI] [PubMed] [Google Scholar]
- Ellis K. O., Bryant S. H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- Erxleben C., Rathmayer W. Effects of the sea anemone Anemonia sulcata toxin II on skeletal muscle and on neuromuscular transmission. Toxicon. 1984;22(3):387–399. doi: 10.1016/0041-0101(84)90083-7. [DOI] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Khurana T. S., Hoffman E. P., Bruns G. A., Haines J. L., Trofatter J. A., Hanson M. P., Rich J., McFarlane H., Yasek D. M. Hyperkalemic periodic paralysis and the adult muscle sodium channel alpha-subunit gene. Science. 1990 Nov 16;250(4983):1000–1002. doi: 10.1126/science.2173143. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Barchi R. L. 20,25-diazacholesterol myotonia: an electrophysiological study. Ann Neurol. 1981 Sep;10(3):251–260. doi: 10.1002/ana.410100310. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Pollard S., Tesseraux I. The effects of Anemonia sulcata toxin II on vertebrate skeletal muscle. Br J Pharmacol. 1985 Sep;86(1):275–286. doi: 10.1111/j.1476-5381.1985.tb09459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. R., Lemeignan M., Molgo J. Effects of toxin II from the sea anemone Anemonia sulcata on contractile and electrical responses of frog skeletal muscle fibres. Toxicon. 1986;24(4):373–384. doi: 10.1016/0041-0101(86)90197-2. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Kinetic properties of single sodium channels in rat heart and rat brain. J Gen Physiol. 1989 Jan;93(1):85–99. doi: 10.1085/jgp.93.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Iaizzo P. A., Hatt H., Franke C. Altered gating and conductance of Na+ channels in hyperkalemic periodic paralysis. Pflugers Arch. 1991 Apr;418(3):297–299. doi: 10.1007/BF00370530. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. D., Sutro J. B., Hille B. Voltage-dependent gating of veratridine-modified Na channels. J Gen Physiol. 1986 Jan;87(1):25–46. doi: 10.1085/jgp.87.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H., Salmon J. H. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J Clin Invest. 1971 Oct;50(10):2091–2103. doi: 10.1172/JCI106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Brinkmeier H., Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve. 1988 May;11(5):440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Nagy K. Mechanism of inactivation of single sodium channels after modification by chloramine-T, sea anemone toxin and scorpion toxin. J Membr Biol. 1988 Nov;106(1):29–40. doi: 10.1007/BF01871764. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986 Feb;87(2):305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek L. J., Trimmer J. S., Agnew W. S., Roberts J. W., Petajan J. H., Leppert M. Paramyotonia congenita and hyperkalemic periodic paralysis map to the same sodium-channel gene locus. Am J Hum Genet. 1991 Oct;49(4):851–854. [PMC free article] [PubMed] [Google Scholar]
- Ptácek L. J., George A. L., Jr, Griggs R. C., Tawil R., Kallen R. G., Barchi R. L., Robertson M., Leppert M. F. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991 Nov 29;67(5):1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Ricker K., Camacho L. M., Grafe P., Lehmann-Horn F., Rüdel R. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 1989 Nov;12(11):883–891. doi: 10.1002/mus.880121103. [DOI] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev. 1985 Apr;65(2):310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Stein P., Palade P. Patch clamp of sarcolemmal spheres from stretched skeletal muscle fibers. Am J Physiol. 1989 Feb;256(2 Pt 1):C434–C440. doi: 10.1152/ajpcell.1989.256.2.C434. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]


