Abstract
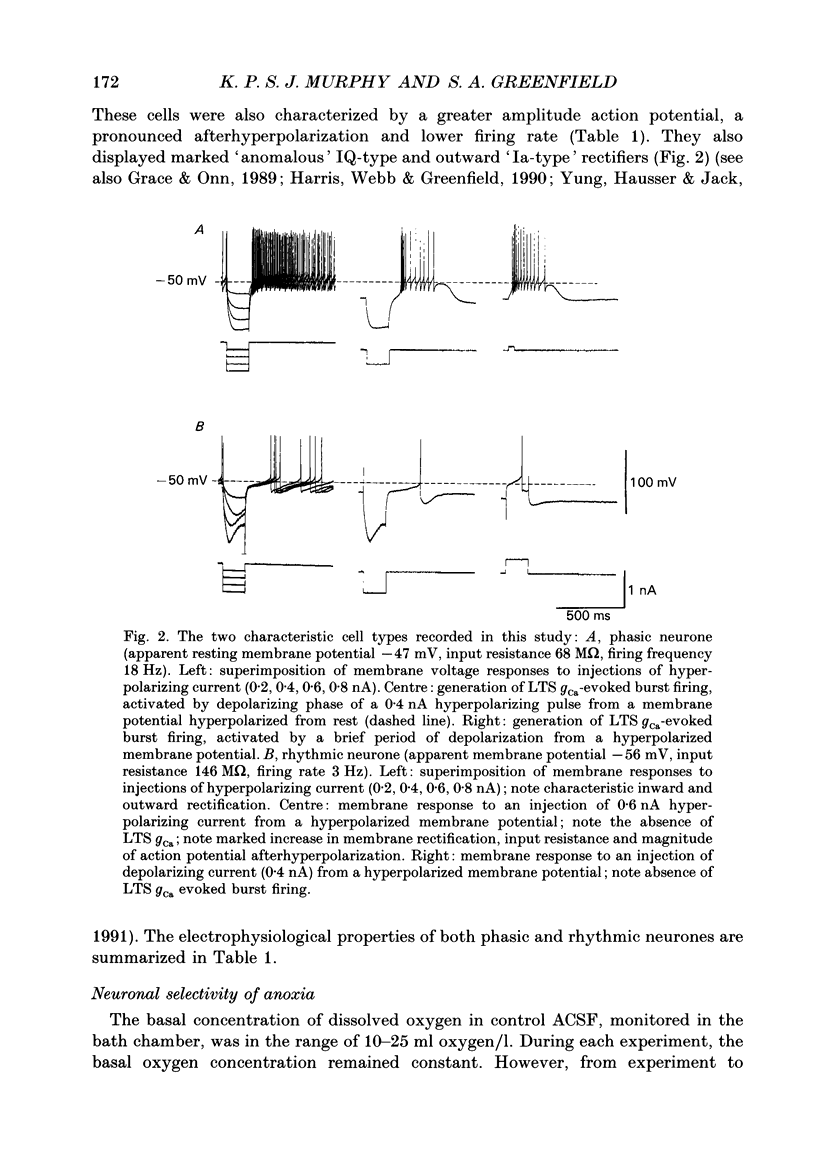
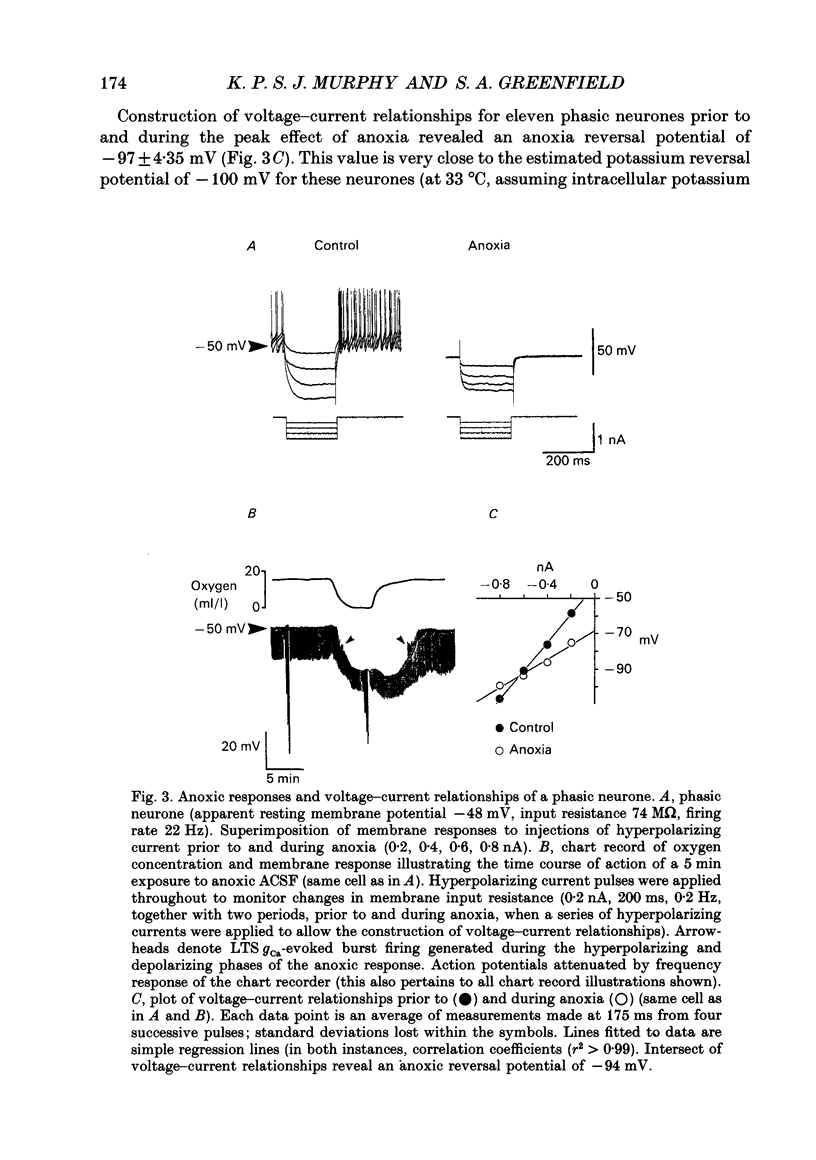
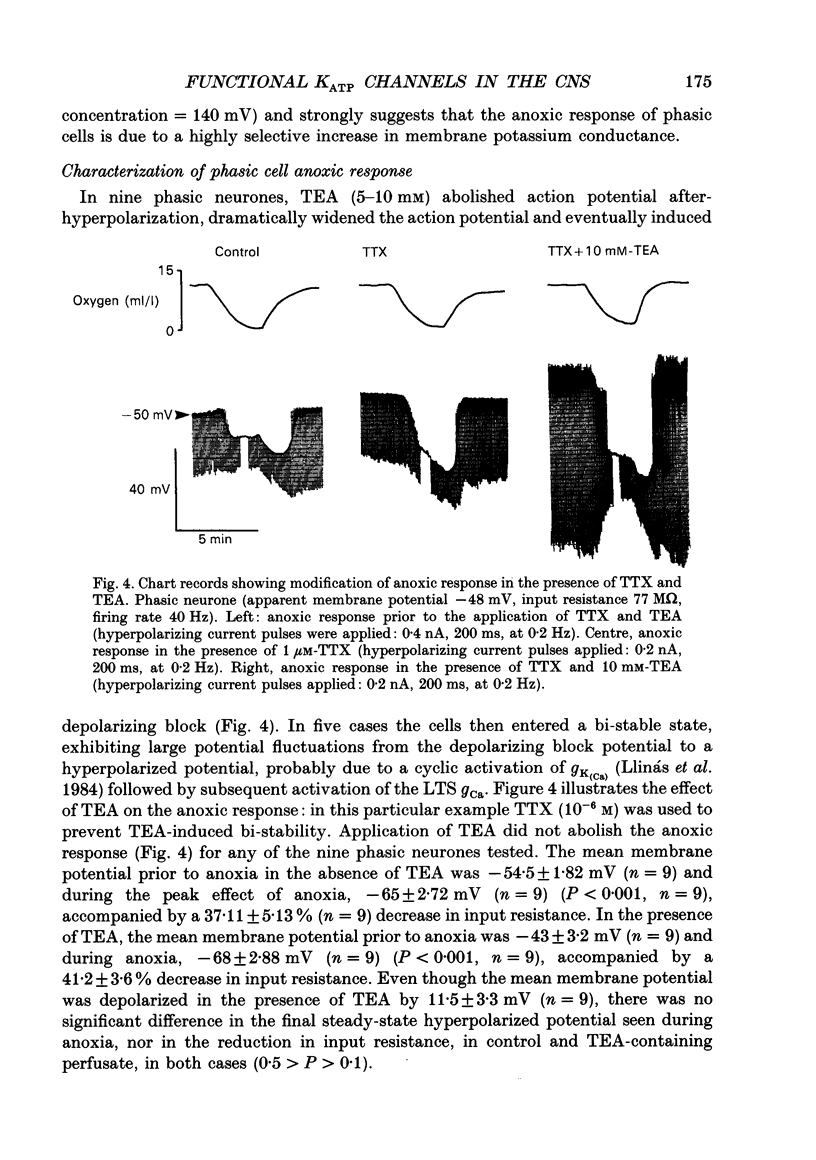
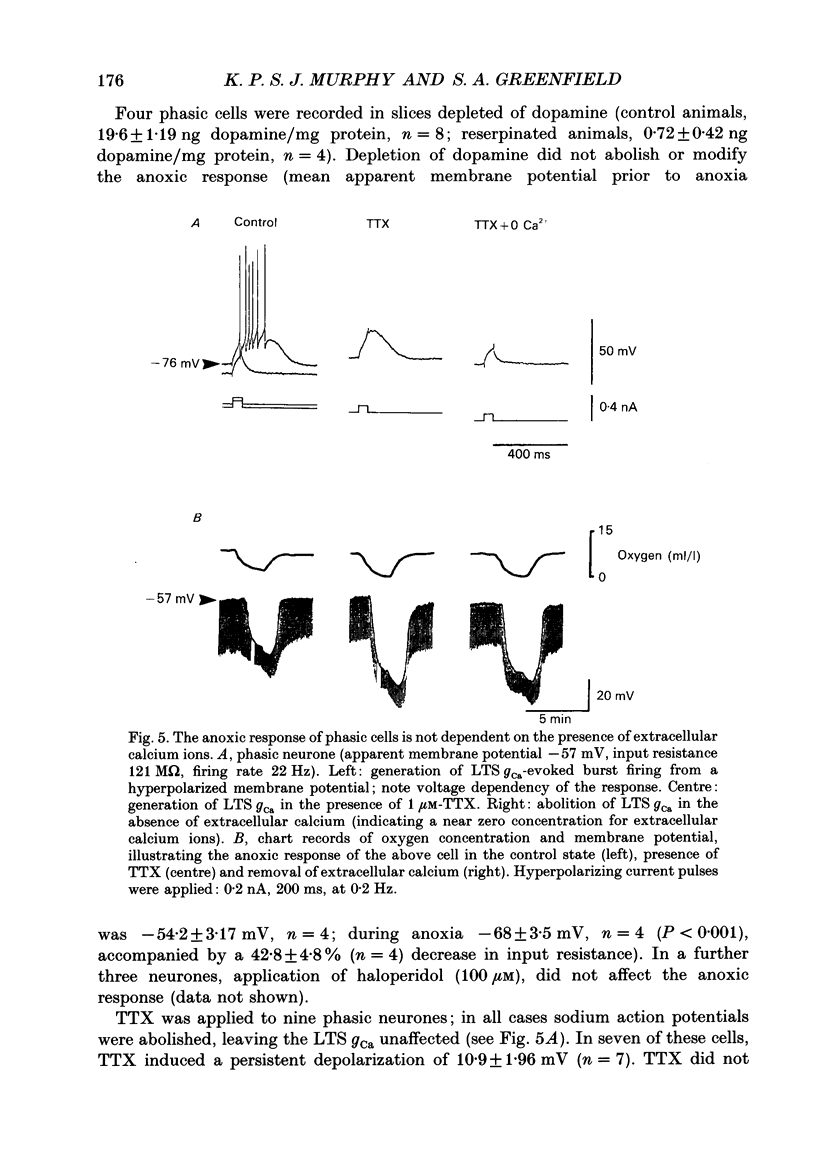
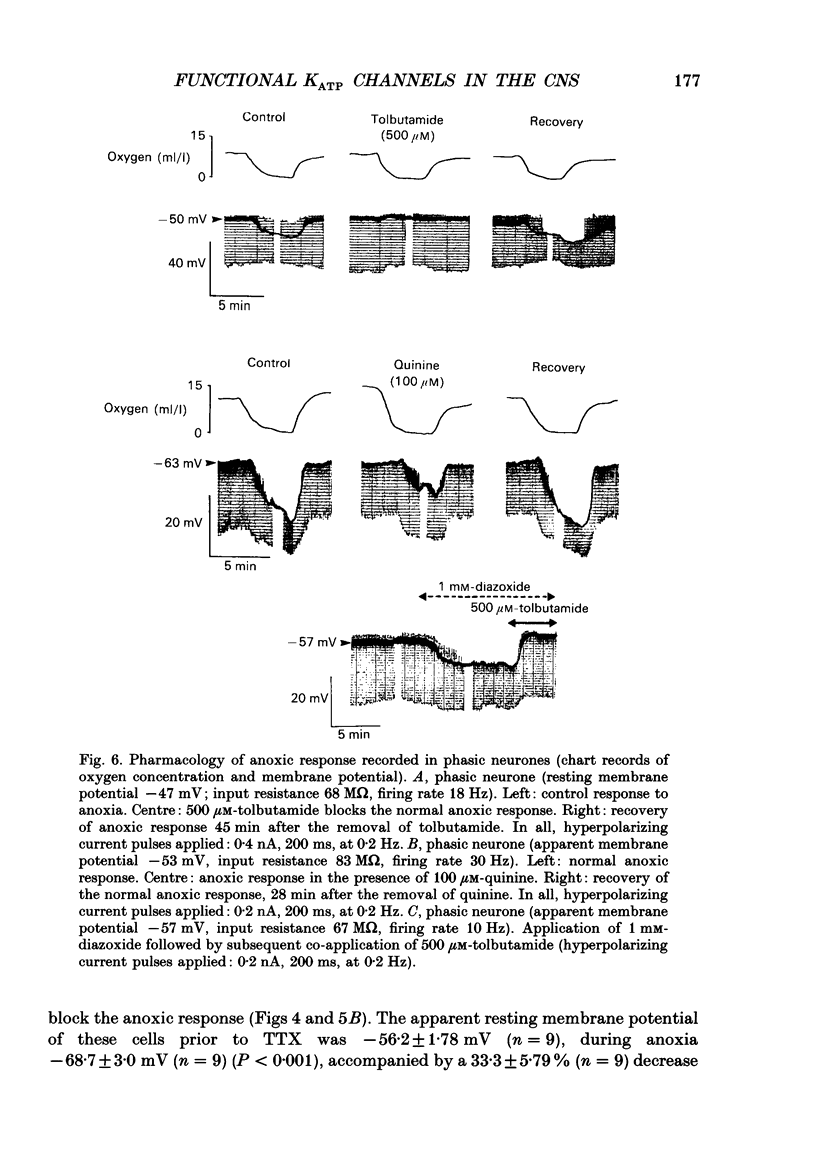
1. Two sub-populations of pars compacta substantia nigra neurones were identified with very different electrophysiological properties and rostral-caudal distribution. Both cell types were identified by biocytin intracellular dye injection and found to be located within pars compacta containing tyrosine hydroxylase-positive cells. These sub-populations displayed distinctly different responses to transient anoxia. 2. The first group ('Phasic' neurones) exhibited a low threshold calcium conductance LTS gCa associated with bursts of action potentials, were located at the level of the mammillary bodies and were highly sensitive to anoxia. The second group ('rhythmic' neurones) fired in a rhythmic pattern, were located at the level of the accessory optic tract and were relatively insensitive to anoxia. 3. The anoxic response of phasic cells was characterized by membrane hyperpolarization (mean 12 mV), a decrease in input resistance (mean 36%) and cessation of action potential firing. The axonic response of these neurones was not blocked by TEA (5-10 mM), haloperidol (100 microM), the removal of extracellular calcium or depletion of endogenous dopamine. However, this effect was blocked by both the sulphonylurea tolbutamide (50-500 microM), and also by quinine (100 microM) and could be mimicked by application of diazoxide (1 mM). 4. Rhythmic cells displayed a variable response to anoxia consisting of either modest depolarization, hyperpolarization or no change in membrane potential, in all cases accompanied by little or no change in input resistance. The polarity of the membrane potential shift during anoxia was reversed by TEA (5-10 mM) or the removal of calcium. These cells were also relatively insensitive to diazoxide (1 mM). 5. It is concluded from the neuronal responses to anoxia and the pharmacological modification of these responses, that the ATP-sensitive potassium channel (KATP channel) is functionally operative in the substantia nigra and is primarily distributed on the phasically discharging cells of the rostral pars compacta. The relevance of this recently discovered ionic channel is discussed with regard to the normal and abnormal functioning of the substantia nigra.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoroso S., Schmid-Antomarchi H., Fosset M., Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990 Feb 16;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The effect of precursors of noradrenalin on the response to tyramine and sympathetic stimulation. Br J Pharmacol Chemother. 1960 Mar;15:47–55. doi: 10.1111/j.1476-5381.1960.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M. R., Fillenz M. Absence of increased tyrosine hydroxylation after induction of brain tyrosine hydroxylase following reserpine administration. Biochem Pharmacol. 1979 May 15;28(10):1675–1677. doi: 10.1016/0006-2952(79)90182-5. [DOI] [PubMed] [Google Scholar]
- Chiodo L. A., Antelman S. M., Caggiula A. R., Lineberry C. G. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980 May 12;189(2):544–549. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E., Kursky M. D., Vorobetz Z. D. Intracellular protein kinase and calcium inward currents in perfused neurones of the snail Helix pomatia. Neuroscience. 1984 Jan;11(1):263–267. doi: 10.1016/0306-4522(84)90229-x. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Ullrich S., Wollheim C. B., Petersen O. H. Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin-secreting cells. FEBS Lett. 1985 Jun 3;185(1):4–8. doi: 10.1016/0014-5793(85)80729-8. [DOI] [PubMed] [Google Scholar]
- GATES E. W., HYMAN I. Use of tolbutamide in paralysis agitans: preliminary report. J Am Med Assoc. 1960 Mar 26;172:1351–1354. doi: 10.1001/jama.1960.03020130009003. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Baimbridge K. G., Thibault J. The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci. 1987 Dec;7(12):3935–3944. doi: 10.1523/JNEUROSCI.07-12-03935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Herkenham M., Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987 Dec;7(12):3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991 May;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Hirsch E. C., Agid Y. A. Differences in tyrosine hydroxylase-like immunoreactivity characterize the mesostriatal innervation of striosomes and extrastriosomal matrix at maturity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):303–307. doi: 10.1073/pnas.84.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S. A., Appleyard M. E., Bloomfield M. R. 6-Hydroxydopamine-induced turning behaviour in the rat: the significance of acetylcholinesterase in cerebrospinal fluid. Behav Brain Res. 1986 Jul;21(1):47–54. doi: 10.1016/0166-4328(86)90059-8. [DOI] [PubMed] [Google Scholar]
- Hainsworth A. H., Röper J., Kapoor R., Ashcroft F. M. Identification and electrophysiology of isolated pars compacta neurons from guinea-pig substantia nigra. Neuroscience. 1991;43(1):81–93. doi: 10.1016/0306-4522(91)90419-o. [DOI] [PubMed] [Google Scholar]
- Hansen J. M., Kristensen M. Tolbutamde in the treatment of Parkinson's disease--a double blind trial. Dan Med Bull. 1965 Dec;12(7):181–184. [PubMed] [Google Scholar]
- Harris N. C., Webb C., Greenfield S. A. The effects of gamma-hydroxybutyrate on the membrane properties of guinea-pig pars compacta neurons in the substantia nigra in vitro. Neuroscience. 1989;31(2):363–370. doi: 10.1016/0306-4522(89)90380-1. [DOI] [PubMed] [Google Scholar]
- Häusser M. A., de Weille J. R., Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochem Biophys Res Commun. 1991 Jan 31;174(2):909–914. doi: 10.1016/0006-291x(91)91504-6. [DOI] [PubMed] [Google Scholar]
- Kapoor R., Webb C., Greenfield S. A. Endogenous dopamine modifies electroresponsiveness of pars compacta cells in the guinea pig substantia nigra in vitro. Exp Brain Res. 1989;74(3):653–657. doi: 10.1007/BF00247370. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviel V., Chéramy A., Glowinski J. Role of the dendritic release of dopamine in the reciprocal control of the two nigro-striatal dopaminergic pathways. Nature. 1979 Jul 19;280(5719):236–239. doi: 10.1038/280236a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Mourre C., Ben Ari Y., Bernardi H., Fosset M., Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989 May 1;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Mourre C., Smith M. L., Siesjö B. K., Lazdunski M. Brain ischemia alters the density of binding sites for glibenclamide, a specific blocker of ATP-sensitive K+ channels. Brain Res. 1990 Aug 27;526(1):147–152. doi: 10.1016/0006-8993(90)90262-a. [DOI] [PubMed] [Google Scholar]
- Mourre C., Widmann C., Lazdunski M. Sulfonylurea binding sites associated with ATP-regulated K+ channels in the central nervous system: autoradiographic analysis of their distribution and ontogenesis, and of their localization in mutant mice cerebellum. Brain Res. 1990 Jun 11;519(1-2):29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Greenfield S. A. ATP-sensitive potassium channels counteract anoxia in neurones of the substantia nigra. Exp Brain Res. 1991;84(2):355–358. doi: 10.1007/BF00231456. [DOI] [PubMed] [Google Scholar]
- Nedergaard S., Webb C., Greenfield S. A. A possible ionic basis for dendritic release of dopamine in the guinea-pig substantia nigra. Acta Physiol Scand. 1989 Jan;135(1):67–68. doi: 10.1111/j.1748-1716.1989.tb08552.x. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977 Mar 24;266(5600):375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- Ostergaard K., Schou J. P., Zimmer J. Rat ventral mesencephalon grown as organotypic slice cultures and co-cultured with striatum, hippocampus, and cerebellum. Exp Brain Res. 1990;82(3):547–565. doi: 10.1007/BF00228796. [DOI] [PubMed] [Google Scholar]
- Roeper J., Hainsworth A. H., Ashcroft F. M. Tolbutamide reverses membrane hyperpolarisation induced by activation of D2 receptors and GABAB receptors in isolated substantia nigra neurones. Pflugers Arch. 1990 Jun;416(4):473–475. doi: 10.1007/BF00370758. [DOI] [PubMed] [Google Scholar]
- Shepard P. D., German D. C. Electrophysiological and pharmacological evidence for the existence of distinct subpopulations of nigrostriatal dopaminergic neuron in the rat. Neuroscience. 1988 Nov;27(2):537–546. doi: 10.1016/0306-4522(88)90287-4. [DOI] [PubMed] [Google Scholar]
- Smits R. P., Steinbusch H. W., Mulder A. H. Distribution of dopamine-immunoreactive cell bodies in the guinea-pig brain. J Chem Neuroanat. 1990 Mar-Apr;3(2):101–123. [PubMed] [Google Scholar]
- Treherne J. M., Ashford M. L. The regional distribution of sulphonylurea binding sites in rat brain. Neuroscience. 1991;40(2):523–531. doi: 10.1016/0306-4522(91)90138-e. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Yung W. H., Häusser M. A., Jack J. J. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991 May;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]



