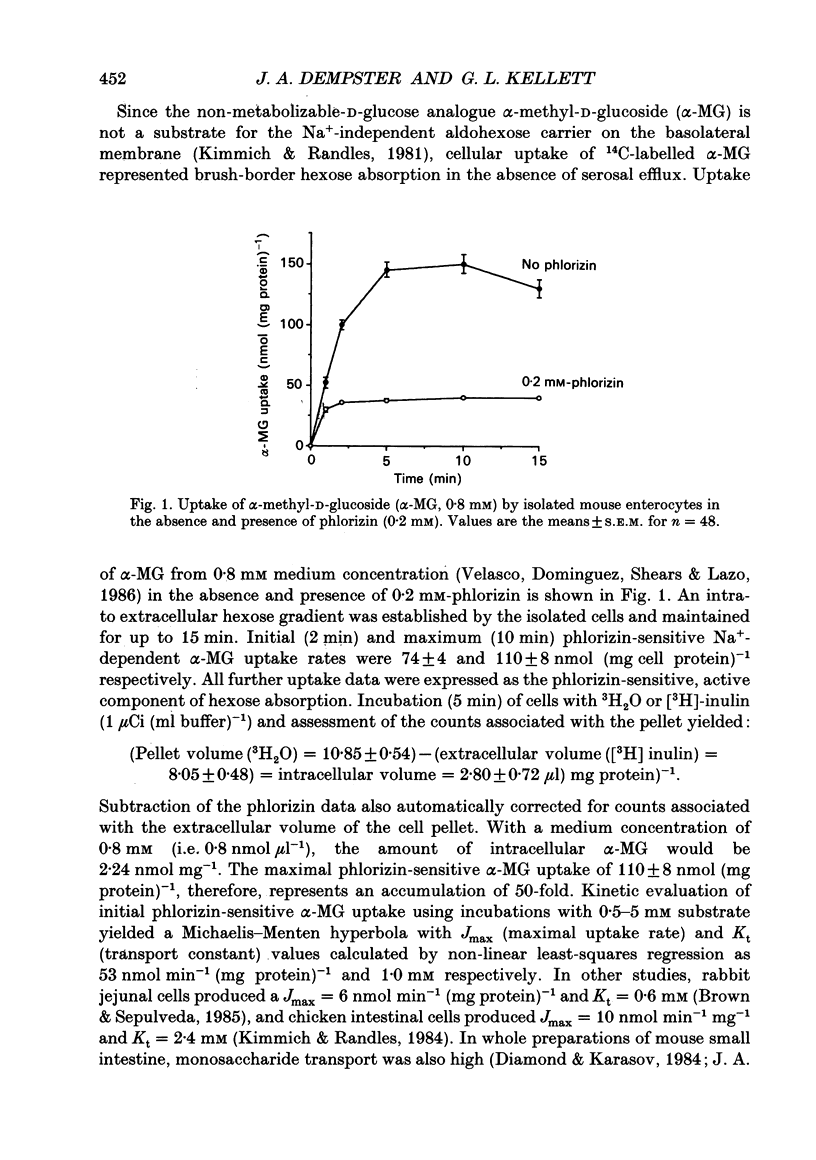
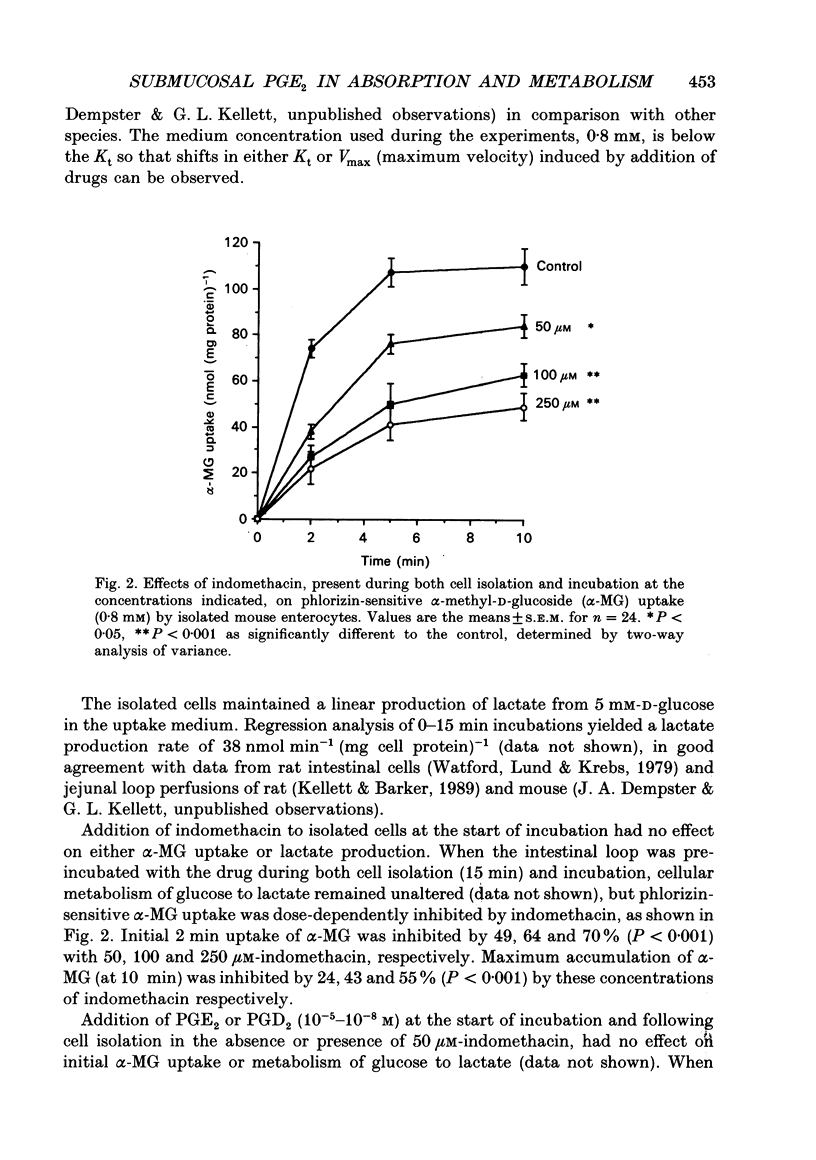
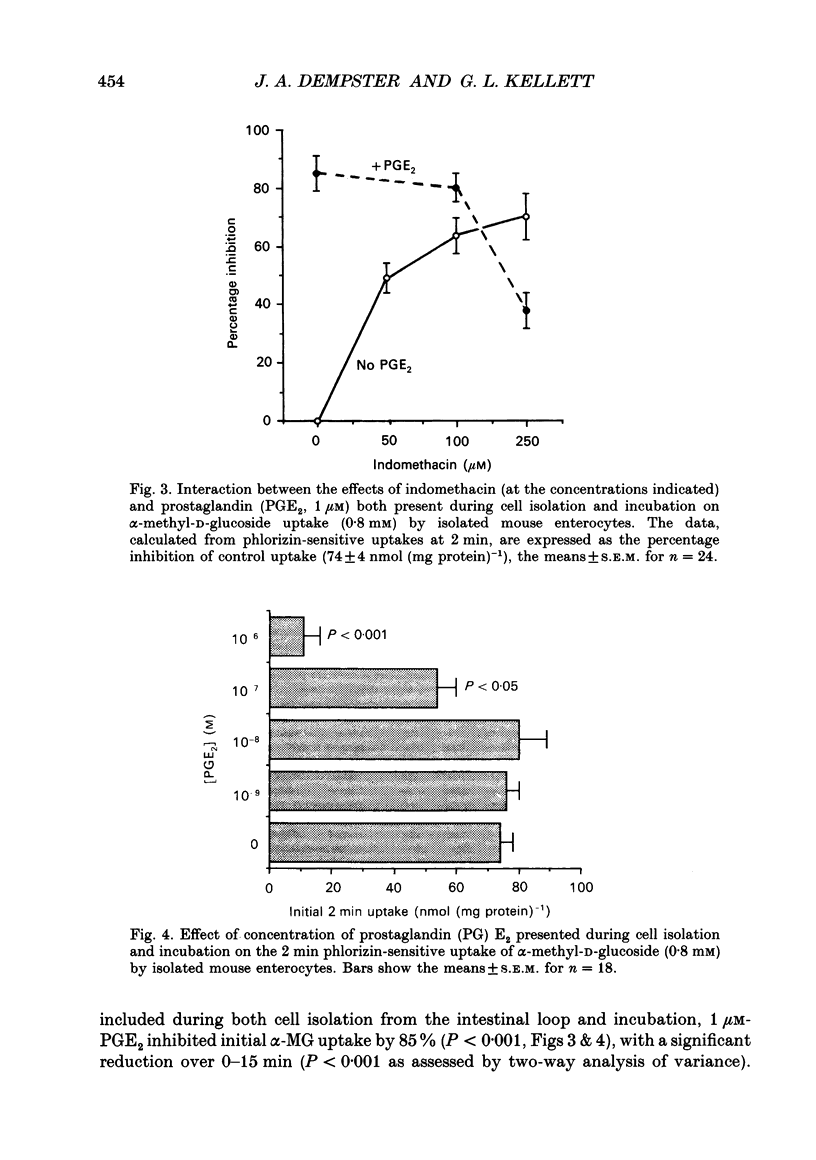
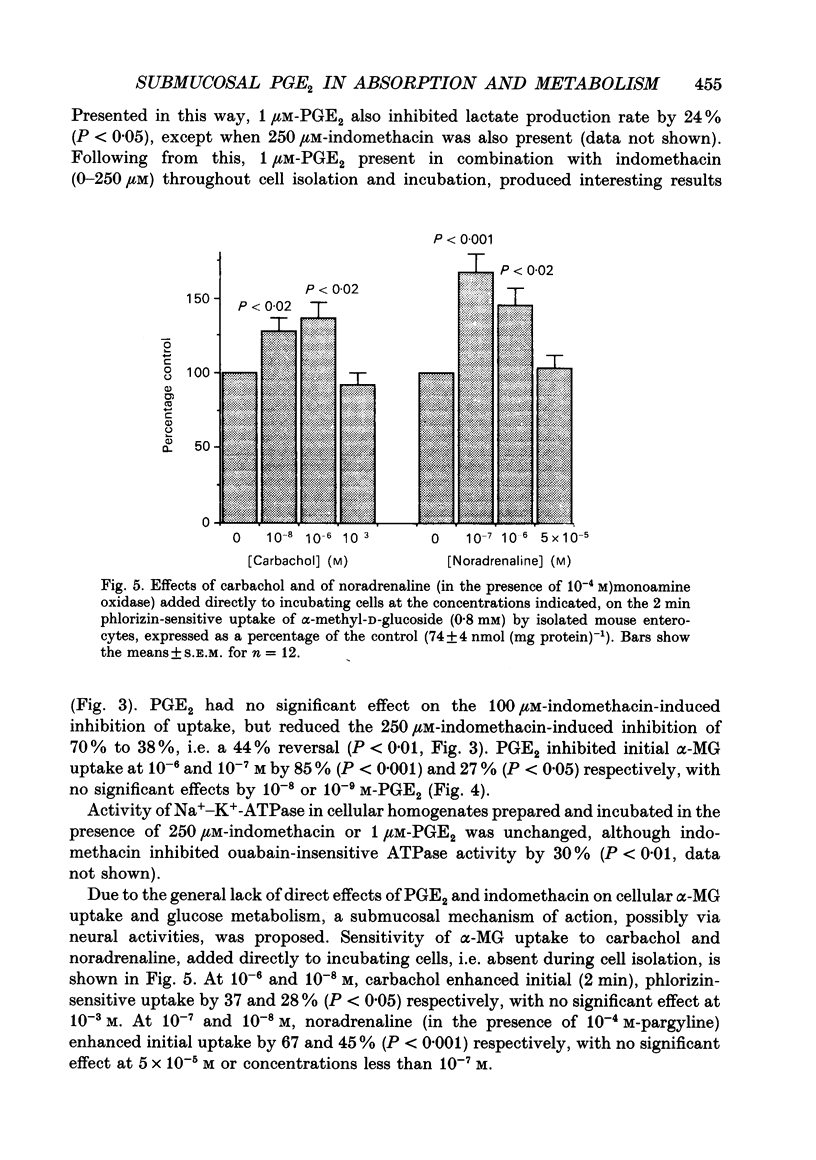
Abstract
1. The involvement of prostaglandin E2 (PGE2) in hexose absorption and metabolism was studied in mouse small intestinal villus cells. 2. Phlorizin-sensitive, Na(+)-dependent alpha-methyl-D-glucoside (alpha-MG) uptake (0.8 mM) during 2 min cell incubations (37 degrees C) was 74 +/- 4 nmol (mg protein)-1. Maximal uptake was 110 +/- 8 nmol (mg protein)-1, representing an accumulation of 50-fold. Metabolism of D-glucose (5 mM) to L-lactate was 38 nmol min-1 (mg protein)-1. 3. Incubation of isolated cells with indomethacin or PGE2 did not affect alpha-MG uptake or D-glucose metabolism. By including indomethacin during cell isolation from whole intestine, alpha-MG uptake was inhibited dose dependently (50-250 microM) by up to 70% (P < 0.001). PGE2 present during both isolation and incubation inhibited by 85% (P < 0.001) at 1 microM and by 27% (P < 0.05) at 0.1 microM, with no effect at lower concentrations. alpha-MG uptake was reduced to 38% (P < 0.01) when 1 microM-PGE2 and 250 microM-indomethacin were presented in combination. When present during cell isolation and incubation, 1 microM-PGE2 inhibited lactate production by 24% (P < 0.05), except when present in combination with 250 microM-indomethacin. Indomethacin, itself, had no effect on lactate production. 4. A submucosal mechanism is proposed to account for the observed inhibitory effects of PGE2 on brush-border uptake of alpha-MG and cellular lactate production. Indomethacin appears to exert not only an effect of its own, possibly via PG-independent actions within the submucosa, but at high concentrations also disrupts the effects of exogenously applied PGE2.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AULSEBROOK K. A. INTESTINAL ABSORPTION OF GLUCOSE AND SODIUM: EFFECTS OF EPINEPHRINE AND NOREPINEPHRINE. Biochem Biophys Res Commun. 1965 Jan 18;18:165–169. doi: 10.1016/0006-291x(65)90734-5. [DOI] [PubMed] [Google Scholar]
- Ahsan M. A., Naftalin R. J., Smith P. M. A submucosal mechanism for catecholamine-induced increases in fluid absorption in rabbit ileum in vitro. J Physiol. 1988 Oct;404:385–405. doi: 10.1113/jphysiol.1988.sp017295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres H., Rock R., Bridges R. J., Rummel W., Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985 Jul;364:301–312. doi: 10.1113/jphysiol.1985.sp015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint G. A., Kiss Z. F., Várkonyi T., Wittmann T., Varró V. Effects of prostaglandin E2 and F2 alpha on the absorption and portal transport of sugar and on the local intestinal circulation. Prostaglandins. 1979 Aug;18(2):265–268. doi: 10.1016/0090-6980(79)90112-6. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Sepúlveda F. V. A rabbit jejunal isolated enterocyte preparation suitable for transport studies. J Physiol. 1985 Jun;363:257–270. doi: 10.1113/jphysiol.1985.sp015708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhave K., Rask-Madsen J. Saturation kinetics applied to in vitro effects of low prostaglandin E2 and F 2 alpha concentrations on ion transport across human jejunal mucosa. Gastroenterology. 1980 Jan;78(1):32–42. [PubMed] [Google Scholar]
- Cheeseman C. I., Maenz D. D. Rapid regulation of D-glucose transport in basolateral membrane of rat jejunum. Am J Physiol. 1989 May;256(5 Pt 1):G878–G883. doi: 10.1152/ajpgi.1989.256.5.G878. [DOI] [PubMed] [Google Scholar]
- Coupar I. M., McColl I. Inhibition of glucose absorption by prostaglandins E 1 , E 2 and F 2 . J Pharm Pharmacol. 1972 Mar;24(3):254–255. doi: 10.1111/j.2042-7158.1972.tb08978.x. [DOI] [PubMed] [Google Scholar]
- Coupar I. M., McColl I. Stimulation of water and sodium secretion and inhibition of glucose absorption from the rat jejunum during intraarterial infusions of prostglandins. Gut. 1975 Oct;16(10):759–765. doi: 10.1136/gut.16.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Moreno M. D., Serrano-Rios M., Prieto J. C. Identification of insulin receptors in epithelial cells from duodenum, jejunum, ileum, caecum, colon and rectum in the rat. Diabete Metab. 1987 Apr;13(2):135–139. [PubMed] [Google Scholar]
- Gingerich R. L., Gilbert W. R., Comens P. G., Gavin J. R., 3rd Identification and characterization of insulin receptors in basolateral membranes of dog intestinal mucosa. Diabetes. 1987 Oct;36(10):1124–1129. doi: 10.2337/diab.36.10.1124. [DOI] [PubMed] [Google Scholar]
- Holliday R., Tarrant G. M. Altered enzymes in ageing human fibroblasts. Nature. 1972 Jul 7;238(5358):26–30. doi: 10.1038/238026a0. [DOI] [PubMed] [Google Scholar]
- Kellett G. L., Barker E. D. The effect of vanadate on glucose transport and metabolism in rat small intestine. Biochim Biophys Acta. 1989 Mar 13;979(3):311–315. doi: 10.1016/0005-2736(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. Sodium-sugar coupling stoichiometry in chick intestinal cells. Am J Physiol. 1984 Jul;247(1 Pt 1):C74–C82. doi: 10.1152/ajpcell.1984.247.1.C74. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. alpha-Methylglucoside satisfies only Na+-dependent transport system of intestinal epithelium. Am J Physiol. 1981 Nov;241(5):C227–C232. doi: 10.1152/ajpcell.1981.241.5.C227. [DOI] [PubMed] [Google Scholar]
- Lawson L. D., Powell D. W. Bradykinin-stimulated eicosanoid synthesis and secretion by rabbit ileal components. Am J Physiol. 1987 Jun;252(6 Pt 1):G783–G790. doi: 10.1152/ajpgi.1987.252.6.G783. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Automated fluorometric analysis of micromolar quantities of ATP, glucose, and lactic acid. Anal Biochem. 1972 Jan;45(1):211–221. doi: 10.1016/0003-2697(72)90021-8. [DOI] [PubMed] [Google Scholar]
- Matuchansky C., Bernier J. J. Effect of prostaglandin E 1 on glucose, water, and electrolyte absorption in the human jejunum. Gastroenterology. 1973 Jun;64(6):1111–1118. [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Smith G. S., Warhurst G., Turnberg L. A. Synthesis and degradation of prostaglandin E2 in the epithelial and sub-epithelial layers of the rat intestine. Biochim Biophys Acta. 1982 Dec 13;713(3):684–687. doi: 10.1016/0005-2760(82)90331-9. [DOI] [PubMed] [Google Scholar]
- Tapper E. J., Bloom A. S., Lewand D. L. Endogenous norepinephrine release induced by tyramine modulates intestinal ion transport. Am J Physiol. 1981 Sep;241(3):G264–G269. doi: 10.1152/ajpgi.1981.241.3.G264. [DOI] [PubMed] [Google Scholar]
- Tapper E. J., Powell D. W., Morris S. M. Cholinergic-adrenergic interactions on intestinal ion transport. Am J Physiol. 1978 Oct;235(4):E402–E409. doi: 10.1152/ajpendo.1978.235.4.E402. [DOI] [PubMed] [Google Scholar]
- Towler C. M., Pugh-Humphreys G. P., Porteous J. W. Characterization of columnar absorptive epithelial cells isolated from rat jejunum. J Cell Sci. 1978 Feb;29:53–75. doi: 10.1242/jcs.29.1.53. [DOI] [PubMed] [Google Scholar]
- Velasco G., Domínguez P., Shears S. B., Lazo P. S. Permeability properties of isolated enterocytes from rat small intestine. Biochim Biophys Acta. 1986 Dec 19;889(3):361–365. doi: 10.1016/0167-4889(86)90199-0. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollen N., Kellett G. L. Regulation of glucose homeostasis in rat jejunum by despentapeptide-insulin in vitro. Gut. 1988 Aug;29(8):1064–1069. doi: 10.1136/gut.29.8.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]


