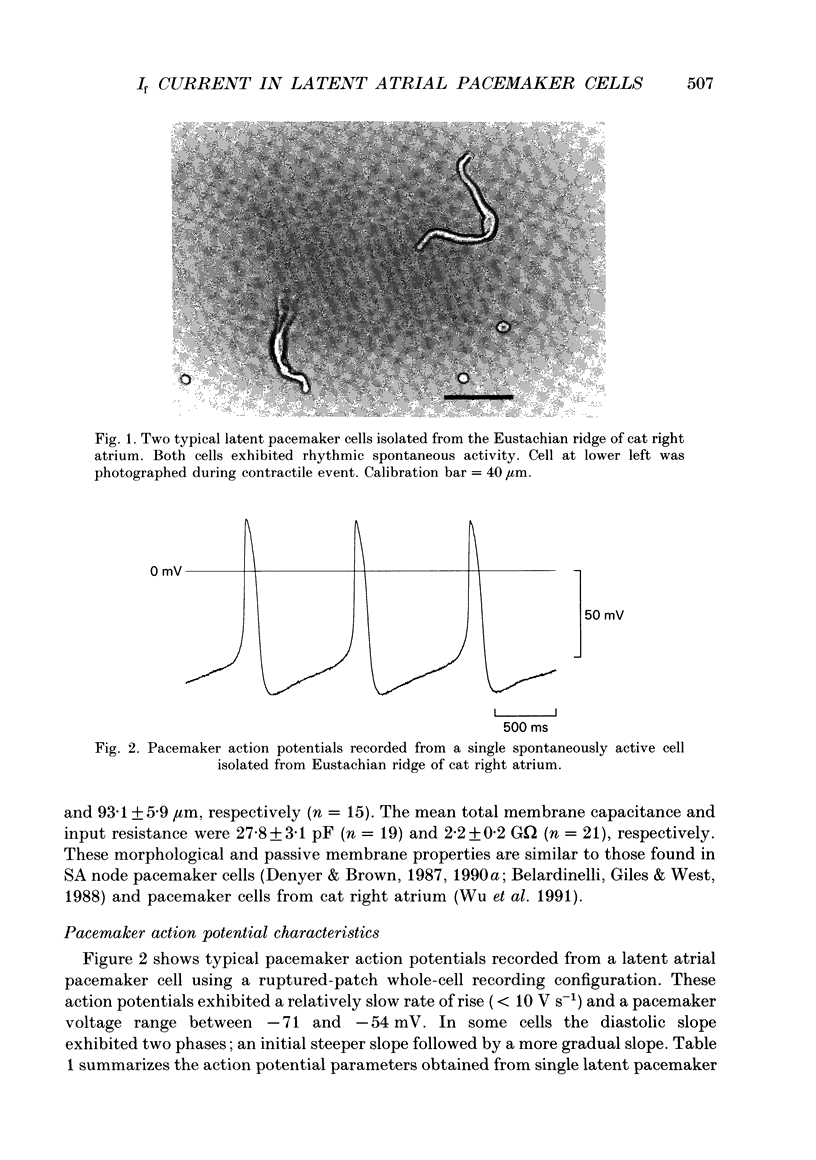
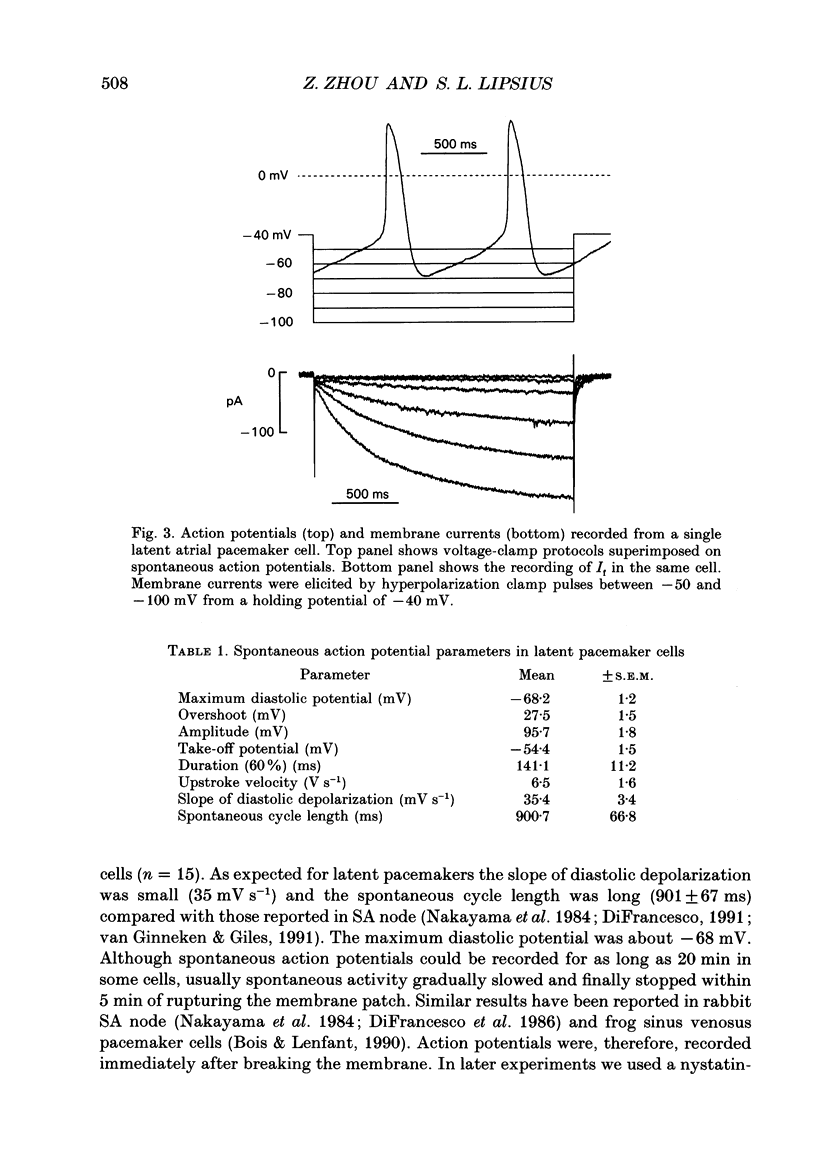
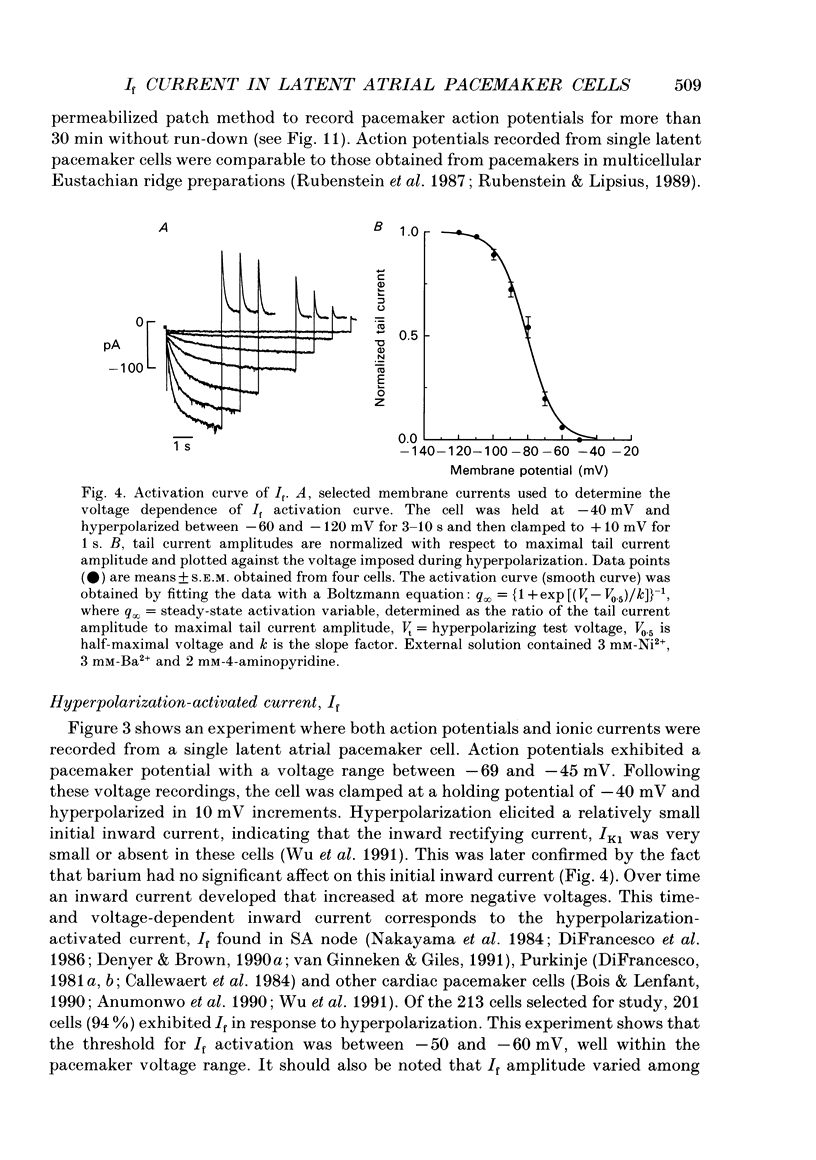
Abstract
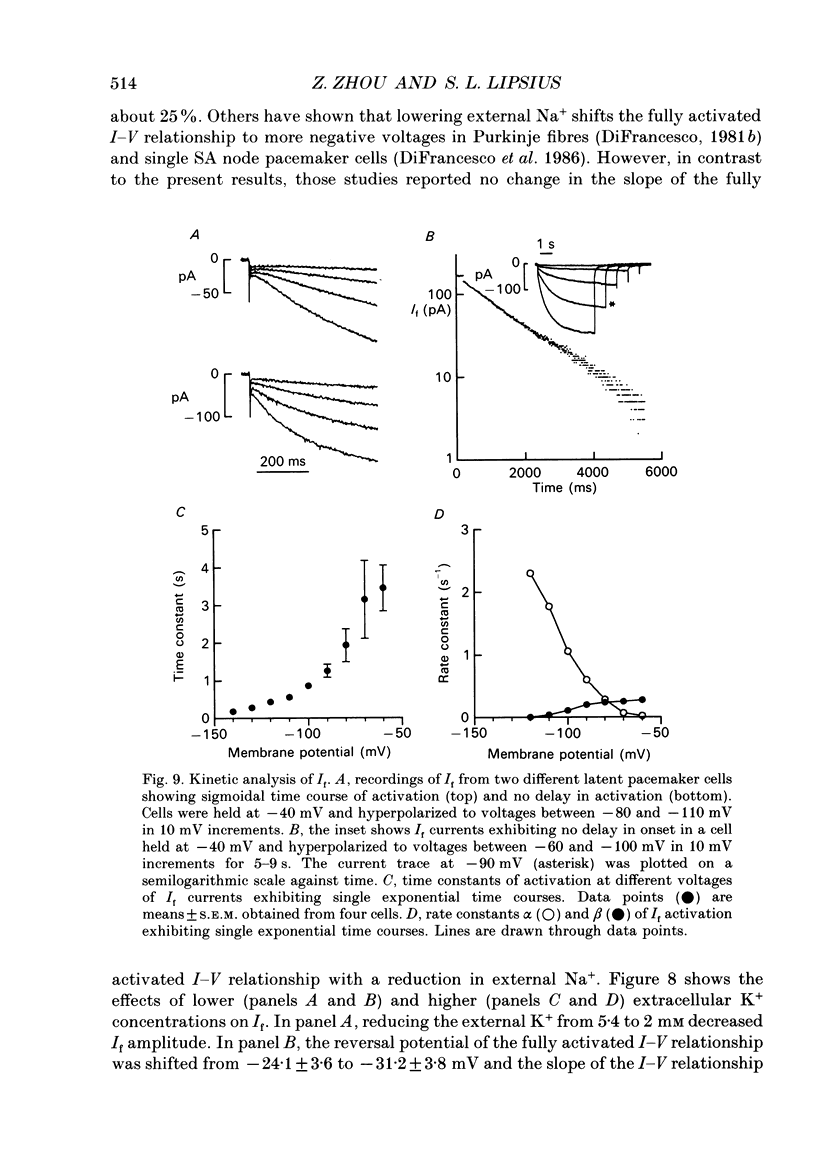
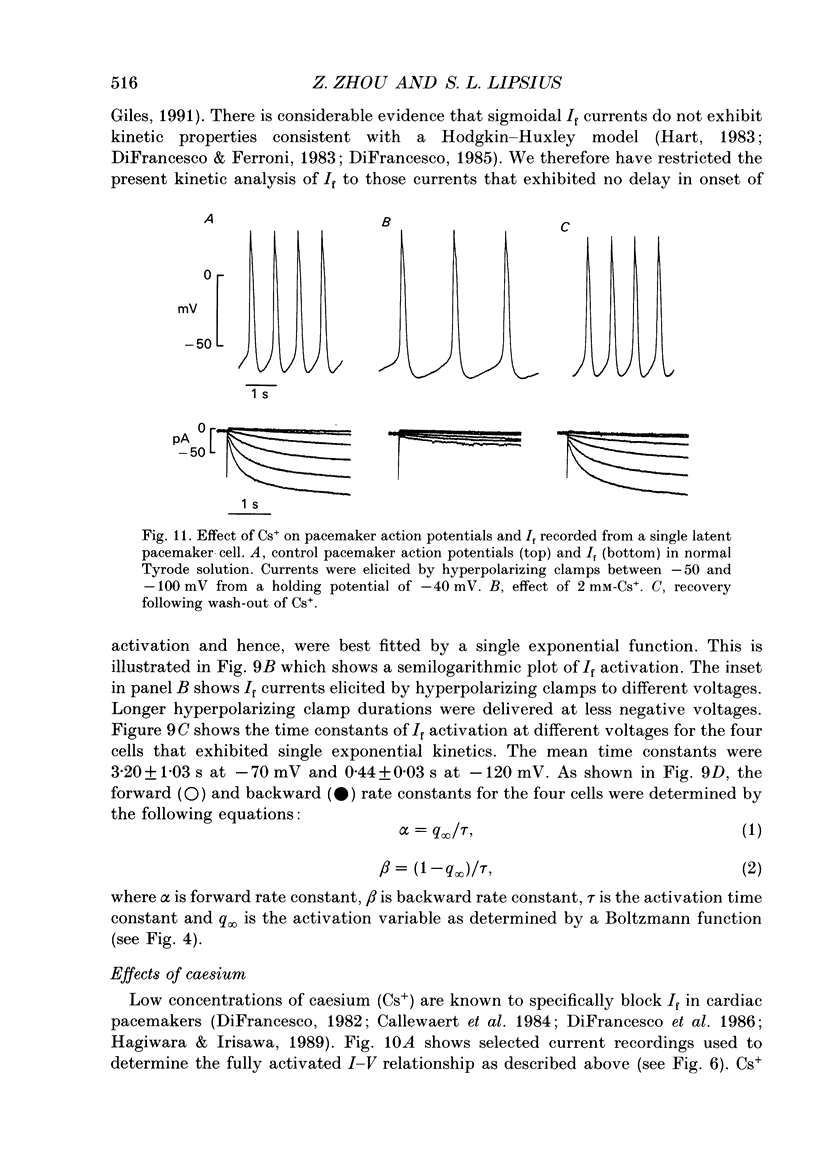
1. Single latent pacemaker cells were isolated from the Eustachian ridge of cat right atrium using Langendorff perfusion and enzyme dispersion techniques. Whole-cell patch-clamp techniques were used to study the hyperpolarization-activated inward current (I(f)). 2. All cells studied beat rhythmically. Pacemaker activity was recorded in the voltage range -68 +/- 1 to -54 +/- 2 mV and its cycle length was 901 +/- 67 ms (72 +/- 5 beats min-1) at 34-36 degrees C. Cells were elongated with tapered ends, and appeared bent or crinkled without obvious striations. Mean cell diameter and length were 7.4 +/- 0.5 microns and 93.1 +/- 5.9 microns, respectively (n = 15). Input resistance and total membrane capacitance were 2.2 +/- 0.2 G omega and 27.8 +/- 3.1 pF, respectively. 3. Hyperpolarizing clamp steps more negative than -50 mV elicited a time-dependent increasing inward current that was maximally activated at -120 mV. Activation of I(f) was well within the pacemaker voltage range. Half-maximal activation voltage and slope factor were calculated, using a Boltzmann function, to be -80.5 mV and 8.4, respectively. 4. The fully activated current-voltage (I-V) relationship was approximately linear at voltages more negative than -30 mV and showed outward rectification at more positive voltages. The reversal potential of I(f) was -26 mV and the fully activated conductance was 1.75 +/- 0.14 nS (n = 21). Caesium (2 mM) blocked I(f) at voltages more negative than the reversal potential. Reducing extracellular Na+ or K+ shifted the reversal potential more negative, and increasing extracellular K+ exerted the opposite effect. Reducing extracellular Na+ decreased I(f) amplitude and the slope of the fully activated I-V relationship, and elevated extracellular K+ increased I(f) amplitude and the slope of the fully activated I-V relationship. 5. Some pacemaker cells exhibited a short delay in the onset of I(f) activation whereas other pacemaker cells exhibited little, if any, delay in activation. I(f) currents exhibiting no delay in activation were best fitted by a single exponential function with a mean time constant of 3.20 +/- 1.03 s at -70 mV (n = 4). 6. A nystatin-permeabilized patch recording method was used to record spontaneous pacemaker action potentials and I(f) from the same pacemaker cell. Caesium (2 mM) inhibited I(f) by more than 90% (at -70 mV), and decreased the slope of diastolic depolarization, resulting in a 48 +/- 5% decrease in spontaneous rate.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



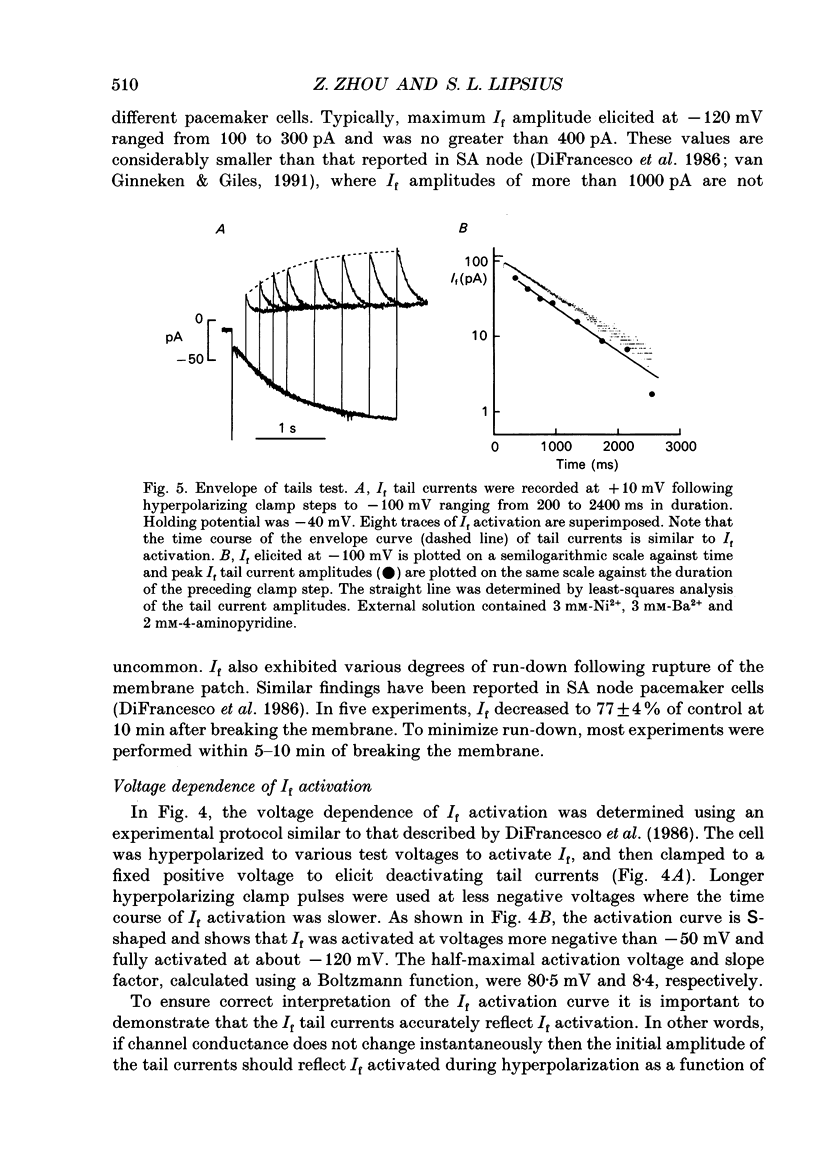
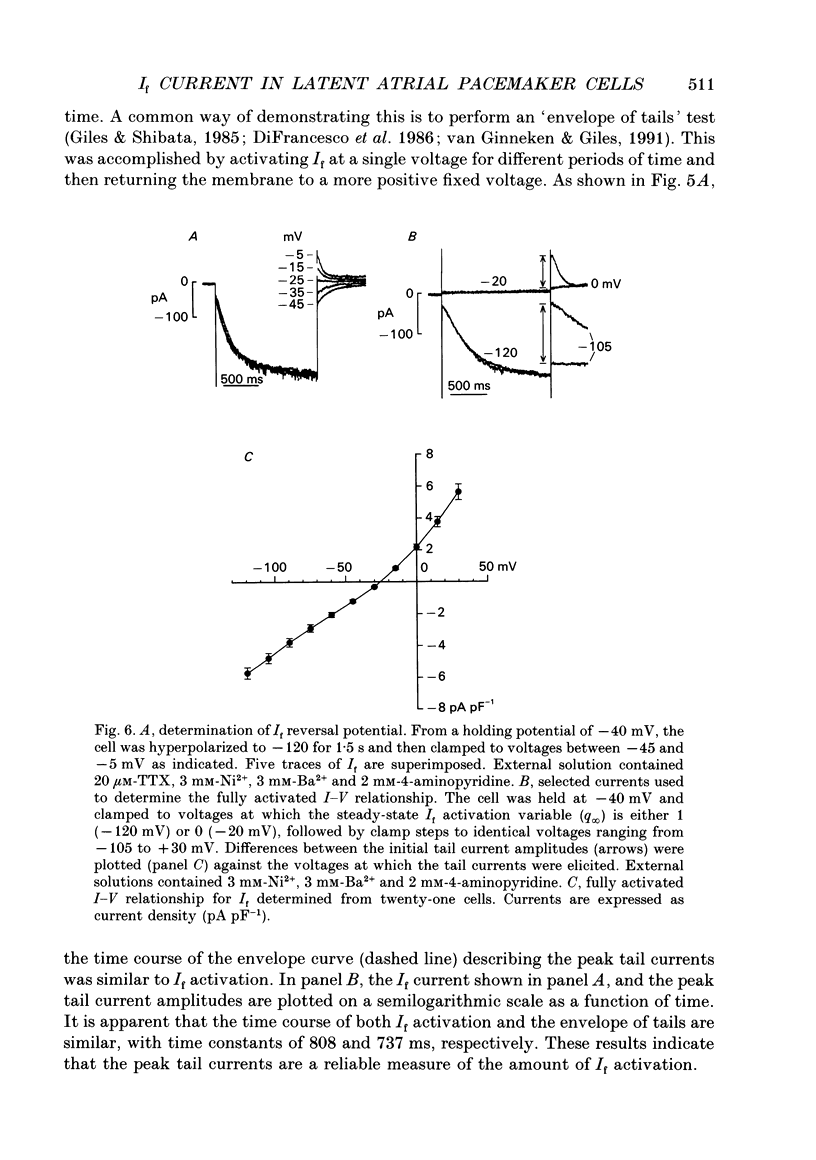
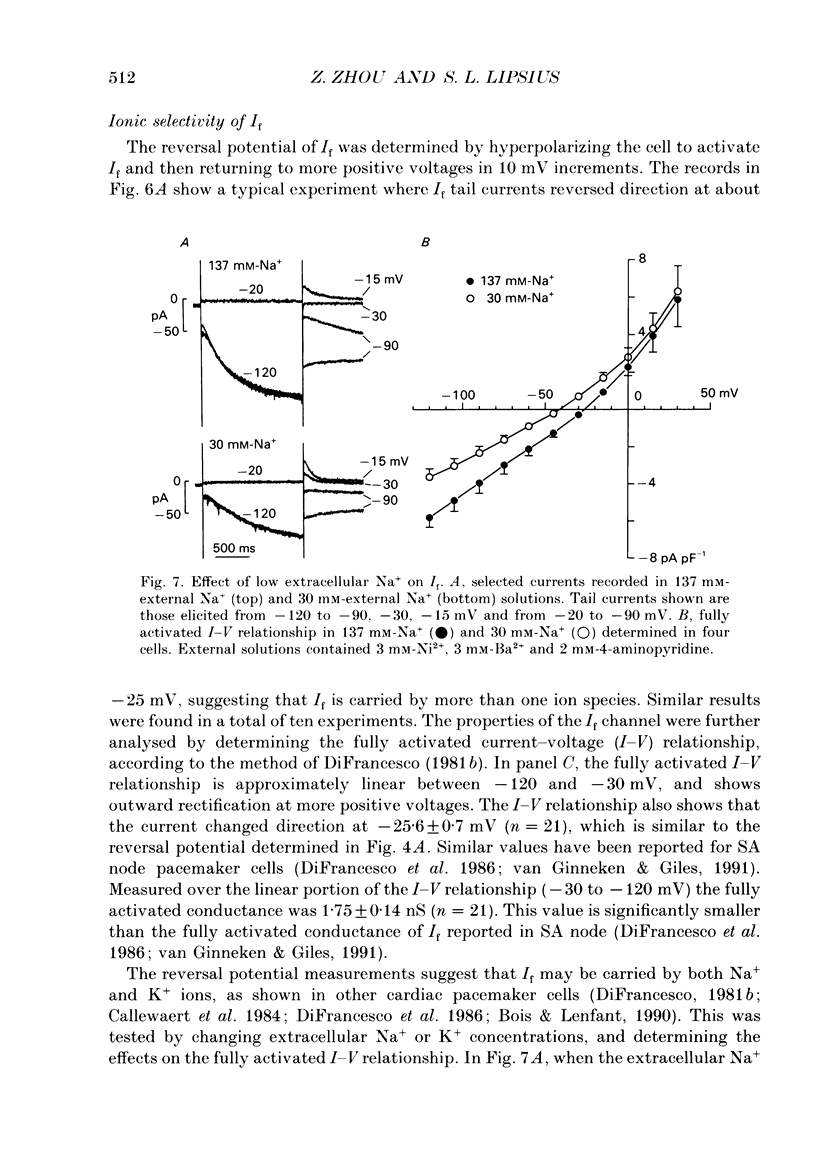
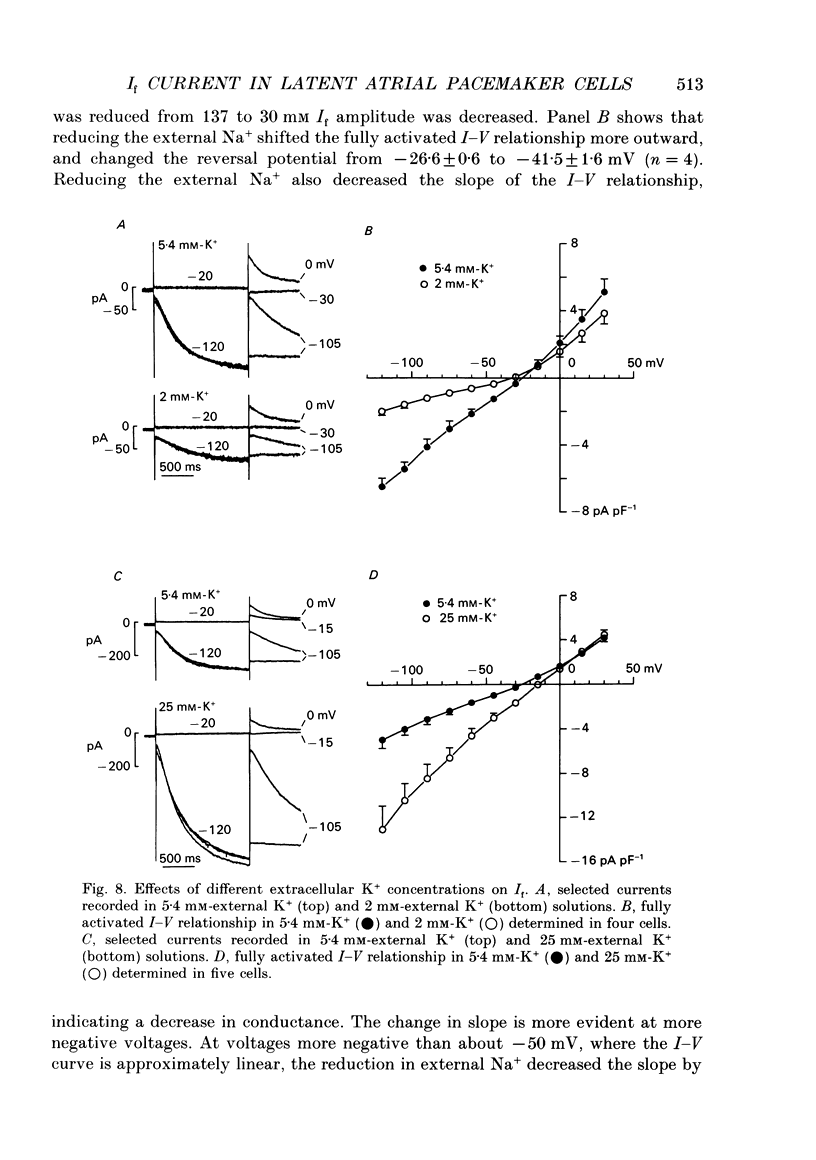
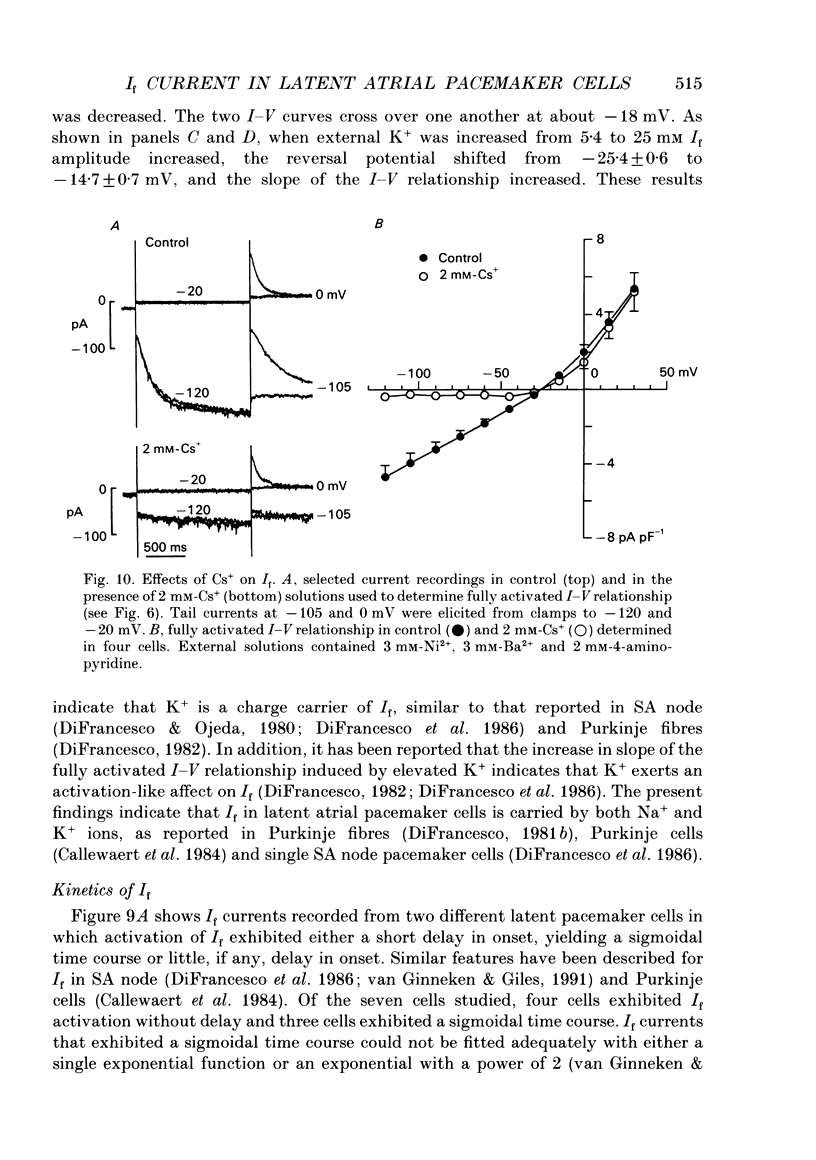







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumonwo J. M., Delmar M., Jalife J. Electrophysiology of single heart cells from the rabbit tricuspid valve. J Physiol. 1990 Jun;425:145–167. doi: 10.1113/jphysiol.1990.sp018097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Giles W. R., West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988 Nov;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker W. K., Mackaay A. J., Masson-Pévet M., Bouman L. N., Becker A. E. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980 Jan;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Boineau J. P., Schuessler R. B., Hackel D. B., Miller C. B., Brockus C. W., Wylds A. C. Widespread distribution and rate differentiation of the atrial pacemaker complex. Am J Physiol. 1980 Sep;239(3):H406–H415. doi: 10.1152/ajpheart.1980.239.3.H406. [DOI] [PubMed] [Google Scholar]
- Bois P., Lenfant J. Isolated cells of the frog sinus venosus: properties of the inward current activated during hyperpolarization. Pflugers Arch. 1990 May;416(3):339–346. doi: 10.1007/BF00392071. [DOI] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990 Oct;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J Physiol. 1990 Sep;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J., Brown H. A method for isolating rabbit sinoatrial node cells which maintains their natural shape. Jpn J Physiol. 1987;37(5):963–965. doi: 10.2170/jjphysiol.37.963. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A. Delayed activation of the cardiac pacemaker current and its dependence on conditioning pre-hyperpolarizations. Pflugers Arch. 1983 Mar 1;396(3):265–267. doi: 10.1007/BF00587866. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The contribution of the 'pacemaker' current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991 Mar;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler D. E., Jones S. B., Gunnar W. P., Loeb J. M., Murdock D. K., Randall W. C. Cardiac arrhythmias in the conscious dog after excision of the sinoatrial node and crista terminalis. Circulation. 1979 Mar;59(3):468–475. doi: 10.1161/01.cir.59.3.468. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Shibata E. F. Voltage clamp of bull-frog cardiac pace-maker cells: a quantitative analysis of potassium currents. J Physiol. 1985 Nov;368:265–292. doi: 10.1113/jphysiol.1985.sp015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H., Schumacher T., Verdonck F. An identification of the K activated Na pump current in sheep Purkinje fibres. Pflugers Arch. 1982 Sep;394(3):256–263. doi: 10.1007/BF00589101. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hart G. The kinetics and temperature dependence of the pace-maker current if in sheep Purkinje fibres. J Physiol. 1983 Apr;337:401–416. doi: 10.1113/jphysiol.1983.sp014631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. N., Sherf L., Fine G., Morales A. R. Comparative ultrastructure of the sinus node in man and dog. Circulation. 1966 Jul;34(1):139–163. doi: 10.1161/01.cir.34.1.139. [DOI] [PubMed] [Google Scholar]
- Jones S. B., Euler D. E., Hardie E., Randall W. C., Brynjolfsson G. Comparison of SA nodal and subsidiary atrial pacemaker function and location in the dog. Am J Physiol. 1978 Apr;234(4):H471–H476. doi: 10.1152/ajpheart.1978.234.4.H471. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kurachi Y., Noma A., Irisawa H. Action potential and membrane currents of single pacemaker cells of the rabbit heart. Pflugers Arch. 1984 Nov;402(3):248–257. doi: 10.1007/BF00585507. [DOI] [PubMed] [Google Scholar]
- Nathan R. D. Two electrophysiologically distinct types of cultured pacemaker cells from rabbit sinoatrial node. Am J Physiol. 1986 Feb;250(2 Pt 2):H325–H329. doi: 10.1152/ajpheart.1986.250.2.H325. [DOI] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Opthof T., de Jonge B., Masson-Pevet M., Jongsma H. J., Bouman L. N. Functional and morphological organization of the cat sinoatrial node. J Mol Cell Cardiol. 1986 Oct;18(10):1015–1031. doi: 10.1016/s0022-2828(86)80290-5. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Yang Q. F., Trautwein W. Effects of barium on the membrane currents in the rabbit S-A node. Pflugers Arch. 1982 Jul;394(1):78–84. doi: 10.1007/BF01108311. [DOI] [PubMed] [Google Scholar]
- Randall W. C., Talano J., Kaye M. P., Euler D., Jones S., Brynjolfsson G. Cardiac pacemakers in absence of the SA node: responses to exercise and autonomic blockade. Am J Physiol. 1978 Apr;234(4):H465–H470. doi: 10.1152/ajpheart.1978.234.4.H465. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J., Lipsius S. L. Electrophysiology of functional subsidiary pacemakers in canine right atrium. Am J Physiol. 1985 Sep;249(3 Pt 2):H594–H603. doi: 10.1152/ajpheart.1985.249.3.H594. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J., Lipsius S. L., Randall W. C. Functional characteristics of sinoatrial and subsidiary pacemaker activity in the canine right atrium. Circulation. 1983 Jun;67(6):1378–1387. doi: 10.1161/01.cir.67.6.1378. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. S., Fox L. M., McNulty J. A., Lipsius S. L. Electrophysiology and ultrastructure of eustachian ridge from cat right atrium: a comparison with SA node. J Mol Cell Cardiol. 1987 Oct;19(10):965–976. doi: 10.1016/s0022-2828(87)80569-2. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. S., Lipsius S. L. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989 Apr;64(4):648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- Sealy W. C., Bache R. J., Seaber A. V., Bhattacharga S. K. The atrial pacemaking site after surgical exclusion of the sinoatrial node. J Thorac Cardiovasc Surg. 1973 Jun;65(6):841–850. [PubMed] [Google Scholar]
- Sherf L., James T. N. Fine structure of cells and their histologic organization within internodal pathways of the heart: clinical and electrocardiographic implications. Am J Cardiol. 1979 Aug;44(2):345–369. doi: 10.1016/0002-9149(79)90327-8. [DOI] [PubMed] [Google Scholar]
- Taylor J. J., D'Agrosa L. S., Burns E. M. The pacemaker cell of the sinoatrial node of the rabbit. Am J Physiol. 1978 Oct;235(4):H407–H412. doi: 10.1152/ajpheart.1978.235.4.H407. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Vereecke J., Carmeliet E., Lipsius S. L. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circ Res. 1991 Apr;68(4):1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- van Ginneken A. C., Giles W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J Physiol. 1991 Mar;434:57–83. doi: 10.1113/jphysiol.1991.sp018459. [DOI] [PMC free article] [PubMed] [Google Scholar]