Abstract
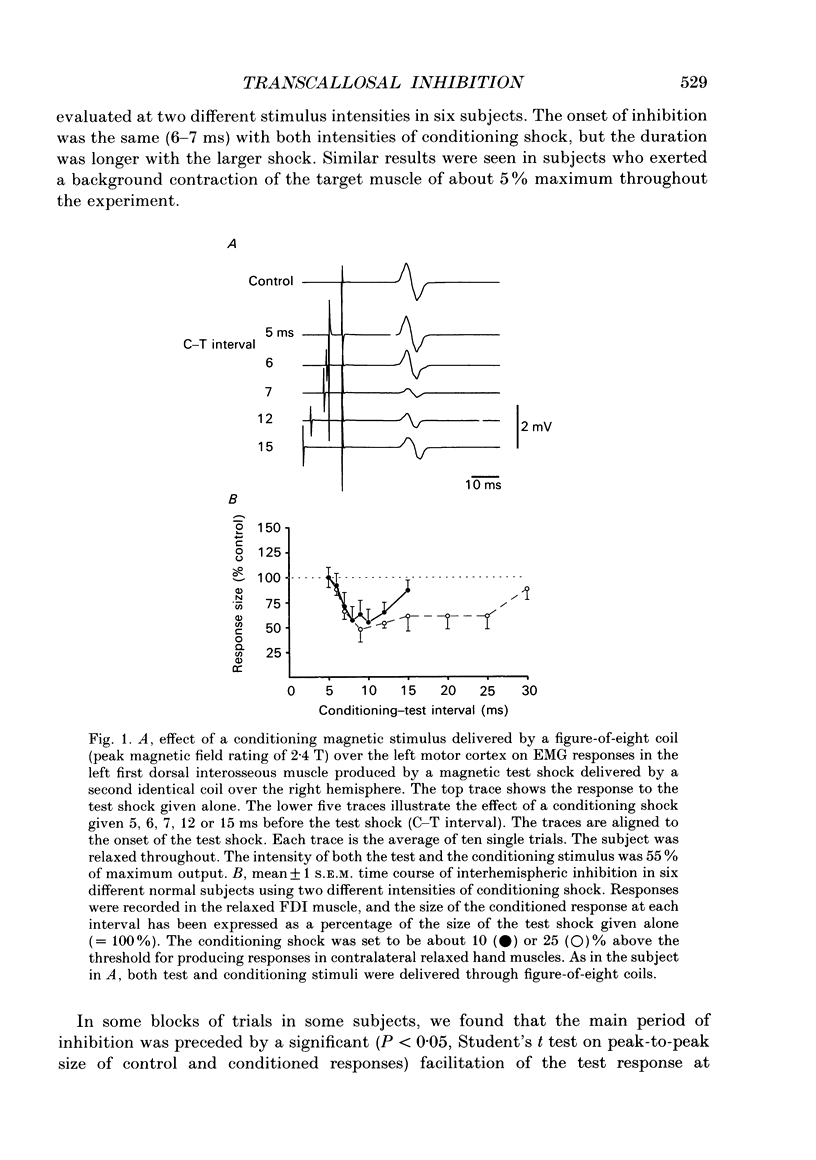
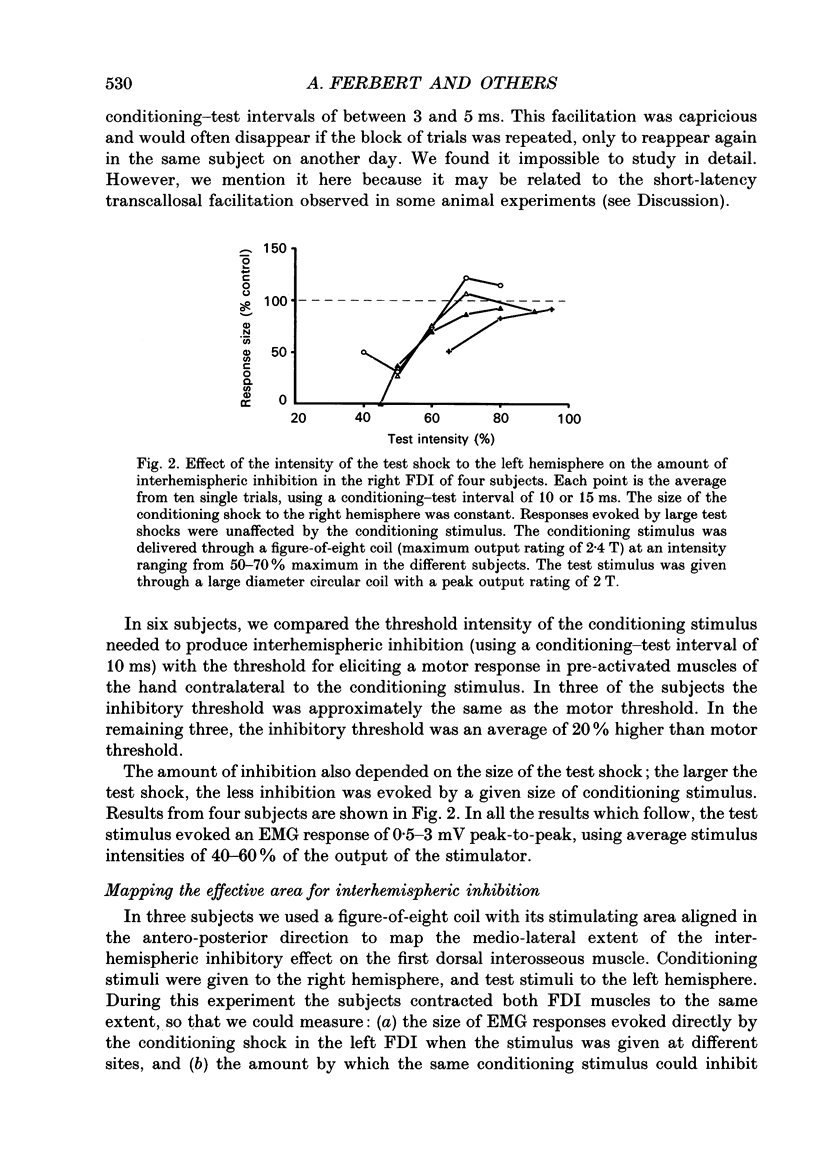
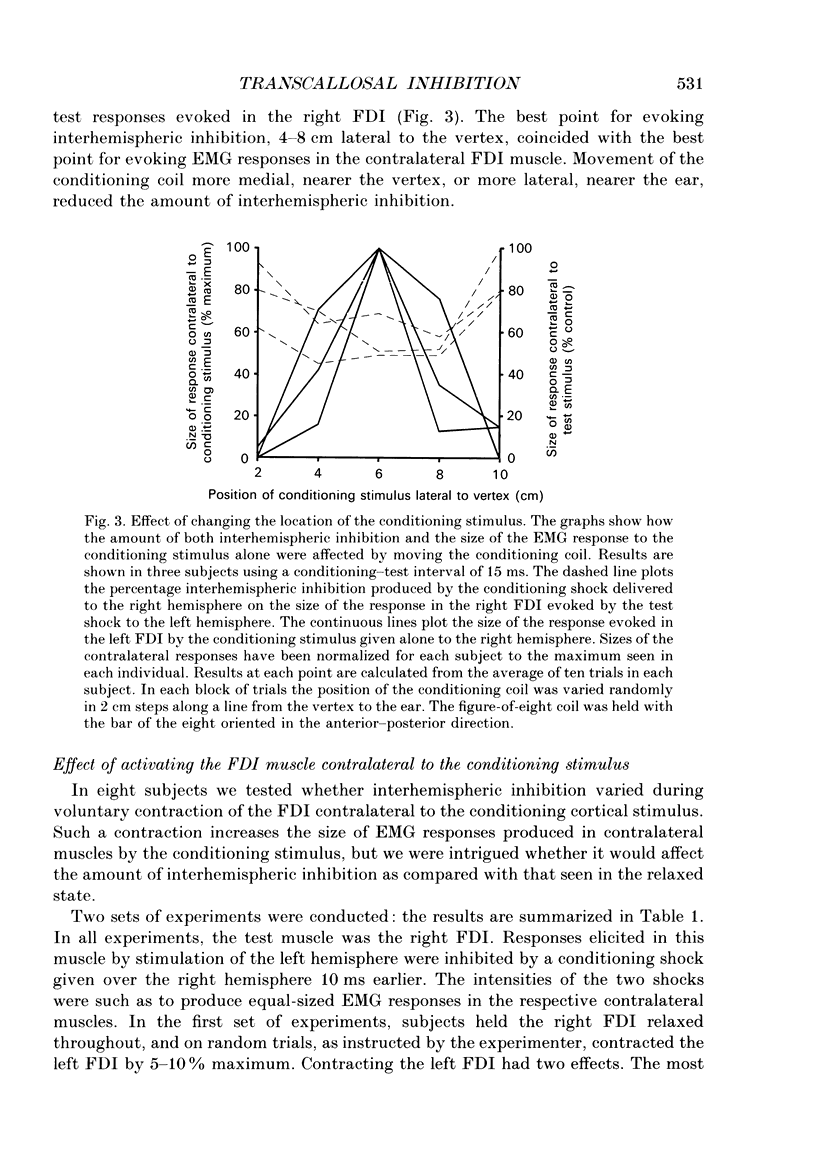
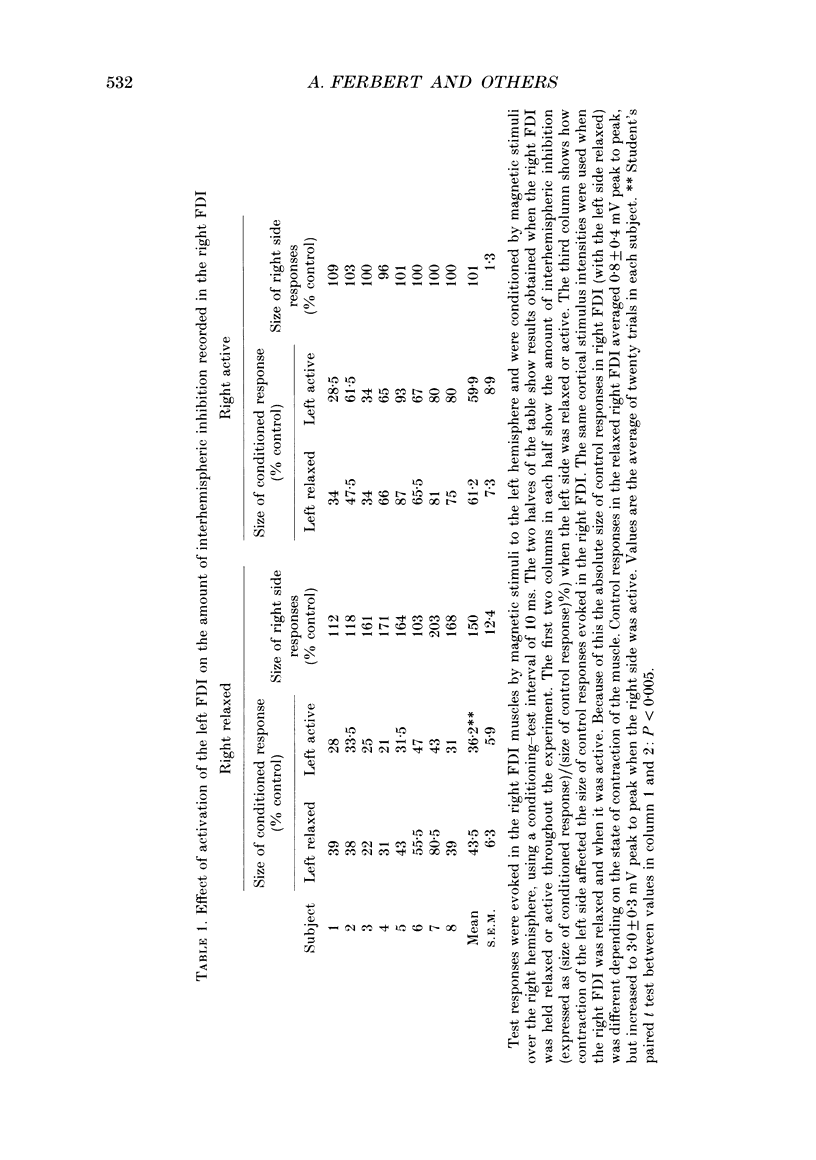
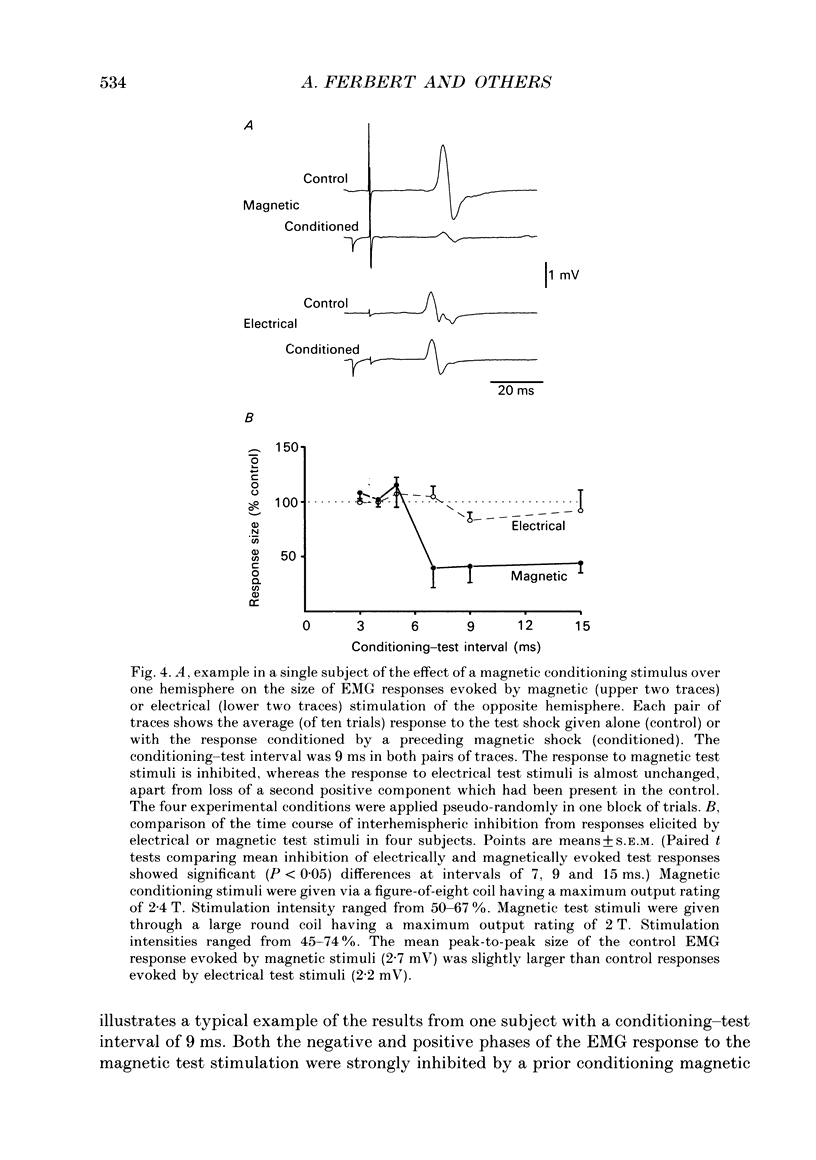
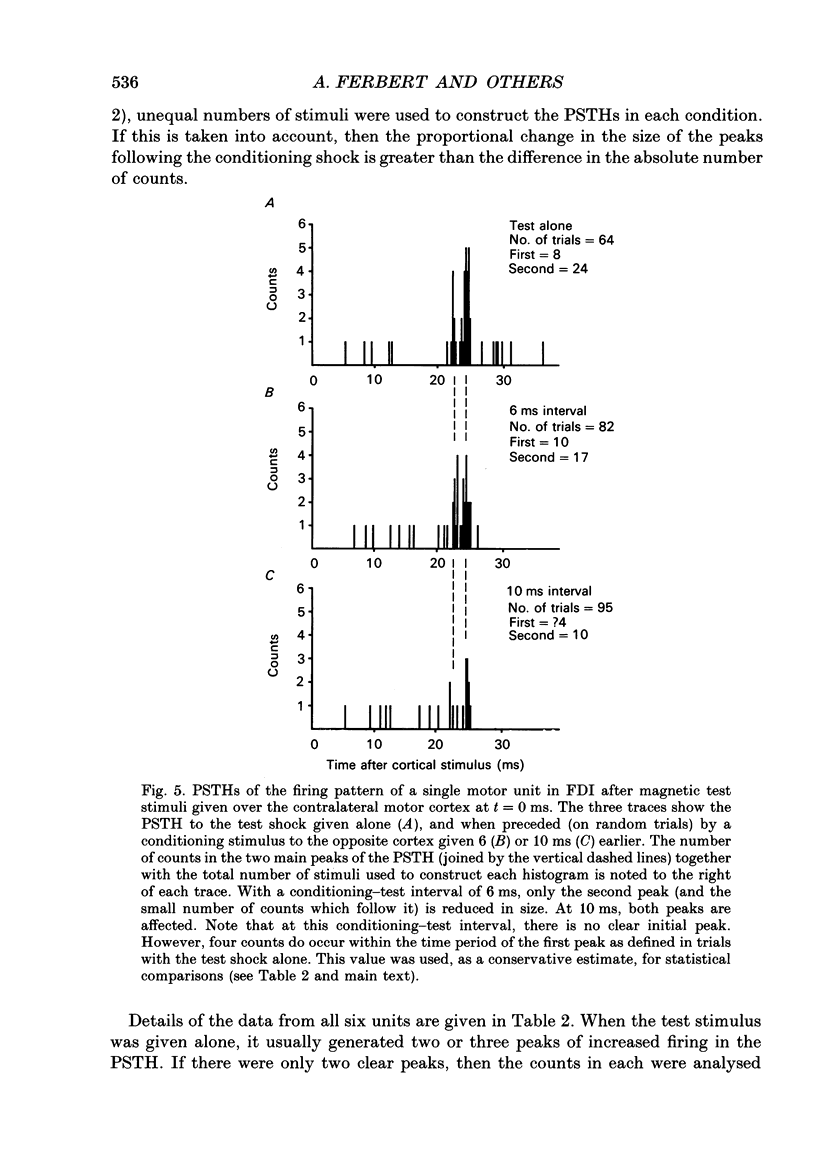
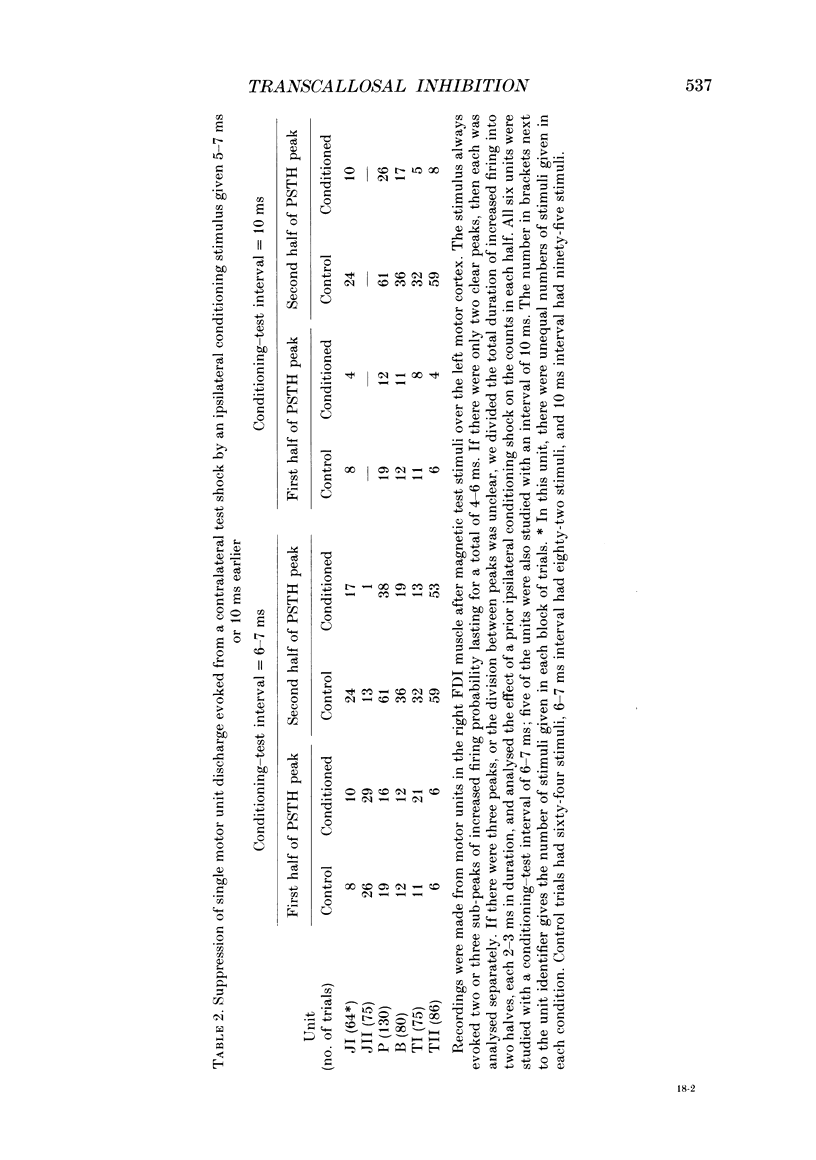
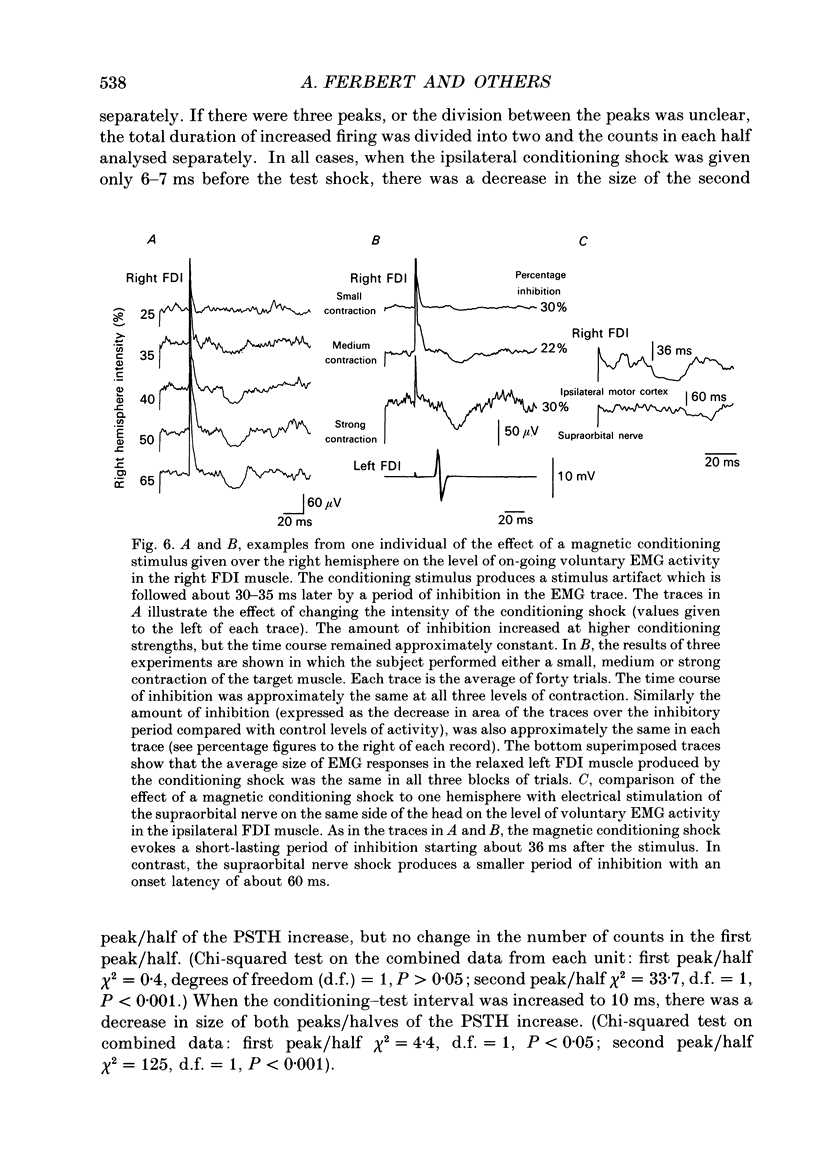
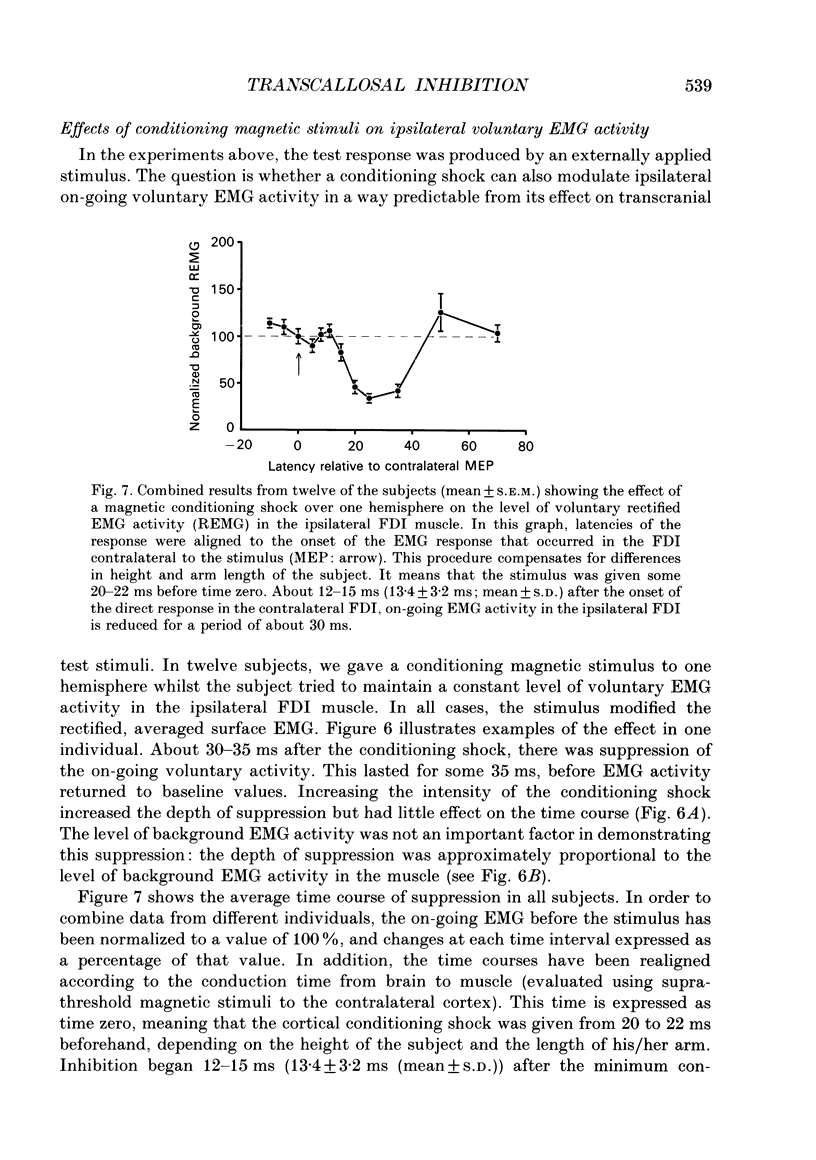
1. Using two magnetic stimulators, we investigated the effect of a conditioning magnetic stimulus over the motor cortex of one hemisphere on the size of EMG responses evoked in the first dorsal interosseous (FDI) muscle by a magnetic test stimulus given over the opposite hemisphere. 2. A single conditioning shock to one hemisphere produced inhibition of the test response evoked from the opposite hemisphere when the conditioning-test interval was 5-6 ms or longer. We shall refer to this as interhemispheric inhibition. However, the minimum latency of inhibition observed using surface EMG responses may have underestimated the true interhemispheric conduction time. Single motor unit studies suggested values 4-7 ms longer than the minimum interval observed with surface EMG. 3. Interhemispheric inhibition was seen when the test muscle was active or relaxed. Increasing the intensity of the conditioning stimulus increased the duration of inhibition: increasing the intensity of the test stimulus reduced the depth of inhibition. 4. The conditioning coil had to be placed on the appropriate area of scalp for inhibition to occur. The effect of the conditioning stimulus was maximal when it was applied over the hand area of motor cortex, and decreased when the stimulus was moved medial or lateral to that point. 5. The inhibitory effect on the test stimulus probably occurred at the level of the cerebral cortex. In contrast to the inhibition of test responses evoked by magnetic test stimuli, test responses evoked in active FDI by a small anodal electric shock were not significantly inhibited by a contralateral magnetic conditioning stimulus. Similarly, H reflexes in relaxed forearm flexor muscles were unaffected by conditioning stimuli to the ipsilateral hemisphere. However, inhibition was observed if the experiment was repeated with the muscles active.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANUMA H., OKAMOTO K. Unitary study on evoked activity of callosal neurons and its effect on pyramidal tract cell activity on cats. Jpn J Physiol. 1959 Dec 15;9:473–483. doi: 10.2170/jjphysiol.9.473. [DOI] [PubMed] [Google Scholar]
- ASANUMA H., OKUDA O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962 Mar;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Brinkman J., Kuypers H. G. Splitbrain monkeys: cerebral control of ipsilateral and contralateral arm, hand, and finger movements. Science. 1972 May 5;176(4034):536–539. doi: 10.1126/science.176.4034.536. [DOI] [PubMed] [Google Scholar]
- Brown P., Day B. L., Rothwell J. C., Thompson P. D., Marsden C. D. Intrahemispheric and interhemispheric spread of cerebral cortical myoclonic activity and its relevance to epilepsy. Brain. 1991 Oct;114(Pt 5):2333–2351. doi: 10.1093/brain/114.5.2333. [DOI] [PubMed] [Google Scholar]
- Catsman-Berrevoets C. E., Lemon R. N., Verburgh C. A., Bentivoglio M., Kuypers H. G. Absence of callosal collaterals derived from rat corticospinal neurons. A study using fluorescent retrograde tracing and electrophysiological techniques. Exp Brain Res. 1980;39(4):433–440. doi: 10.1007/BF00239308. [DOI] [PubMed] [Google Scholar]
- Cracco R. Q., Amassian V. E., Maccabee P. J., Cracco J. B. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989 Nov-Dec;74(6):417–424. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Mazières L., Morin C., Nielsen J., Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81(1):35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Harrison L. M., Stephens J. A. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol. 1989 Nov;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Dressler D., Maertens de Noordhout A., Marsden C. D., Nakashima K., Rothwell J. C., Thompson P. D. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989 May;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Thompson P. D., Dick J. P., Nakashima K., Marsden C. D. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett. 1987 Mar 20;75(1):101–106. doi: 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- Di Stefano M., Morelli M., Marzi C. A., Berlucchi G. Hemispheric control of unilateral and bilateral movements of proximal and distal parts of the arm as inferred from simple reaction time to lateralized light stimuli in man. Exp Brain Res. 1980 Jan;38(2):197–204. doi: 10.1007/BF00236741. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Eyre J. A., Lemon R. N., Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990 Jun;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney D. M., Orem J. M. Influence of antidromic callosal volleys on single units in visual cortex. Exp Neurol. 1971 Nov;33(2):310–321. doi: 10.1016/0014-4886(71)90023-9. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. S. Cross-cuing mechanisms and ipsilateral eye-hand control in split-brain monkeys. Exp Neurol. 1969 Jan;23(1):11–17. doi: 10.1016/0014-4886(69)90029-6. [DOI] [PubMed] [Google Scholar]
- Gould H. J., 3rd, Cusick C. G., Pons T. P., Kaas J. H. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986 May 15;247(3):297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Hess C. W., Mills K. R., Murray N. M. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett. 1986 Nov 11;71(2):235–240. doi: 10.1016/0304-3940(86)90565-3. [DOI] [PubMed] [Google Scholar]
- Jenny A. B. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979 Nov 1;188(1):137–145. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Wise S. P. Commissural columns in the sensory-motor cortex of monkeys. J Comp Neurol. 1979 Nov 1;188(1):113–135. doi: 10.1002/cne.901880110. [DOI] [PubMed] [Google Scholar]
- Katz R., Penicaud A., Rossi A. Reciprocal Ia inhibition between elbow flexors and extensors in the human. J Physiol. 1991 Jun;437:269–286. doi: 10.1113/jphysiol.1991.sp018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Chien-Ping W. U. Responses of the pyramidal tract to stimulation of the baboon's motor cortex. J Physiol. 1967 Aug;191(3):653–672. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami K., Hamada I. Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. J Neurophysiol. 1984 Oct;52(4):676–691. doi: 10.1152/jn.1984.52.4.676. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Naito H., Kurosaki T., Tamura Y. Effect of polarizing currents on transcallosal postsynaptic potentials of cat pyramidal tract cells. Brain Res. 1971 Dec 24;35(2):547–550. doi: 10.1016/0006-8993(71)90498-7. [DOI] [PubMed] [Google Scholar]
- Pappas C. L., Strick P. L. Anatomical demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol. 1981 Aug 20;200(4):491–500. doi: 10.1002/cne.902000404. [DOI] [PubMed] [Google Scholar]
- Rossini P. M., Caramia M. D., Zarola F. Mechanisms of nervous propagation along central motor pathways: noninvasive evaluation in healthy subjects and in patients with neurological disease. Neurosurgery. 1987 Jan;20(1):183–191. doi: 10.1097/00006123-198701000-00035. [DOI] [PubMed] [Google Scholar]
- Saron C. D., Davidson R. J. Visual evoked potential measures of interhemispheric transfer time in humans. Behav Neurosci. 1989 Oct;103(5):1115–1138. doi: 10.1037//0735-7044.103.5.1115. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Musazzi M., Nardone A., Seveso G. Interhemispheric transfer of voluntary motor commands in man. Electroencephalogr Clin Neurophysiol. 1984 May;57(5):441–447. doi: 10.1016/0013-4694(84)90074-9. [DOI] [PubMed] [Google Scholar]
- Schriefer T. N., Mills K. R., Murray N. M., Hess C. W. Evaluation of proximal facial nerve conduction by transcranial magnetic stimulation. J Neurol Neurosurg Psychiatry. 1988 Jan;51(1):60–66. doi: 10.1136/jnnp.51.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H., Yamashita Y., Kuroiwa Y. Electroencephalographic studies myoclonus. Brain. 1978 Sep;101(3):447–460. doi: 10.1093/brain/101.3.447. [DOI] [PubMed] [Google Scholar]
- Spidalieri G., Guandalini P., Franchi G. Motor responses mediated by orthodromic and antidromic activation of the rostral portion of the cat corpus callosum. Exp Brain Res. 1986;64(1):133–142. doi: 10.1007/BF00238209. [DOI] [PubMed] [Google Scholar]
- TOMASCH J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec. 1954 May;119(1):119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- Ugawa Y., Day B. L., Rothwell J. C., Thompson P. D., Merton P. A., Marsden C. D. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991 Sep;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins D. E., Hallett M., Berardelli A., Walshe T., Alvarez N. Physiologic analysis of the myoclonus of Alzheimer's disease. Neurology. 1984 Jul;34(7):898–903. doi: 10.1212/wnl.34.7.898. [DOI] [PubMed] [Google Scholar]


