Abstract
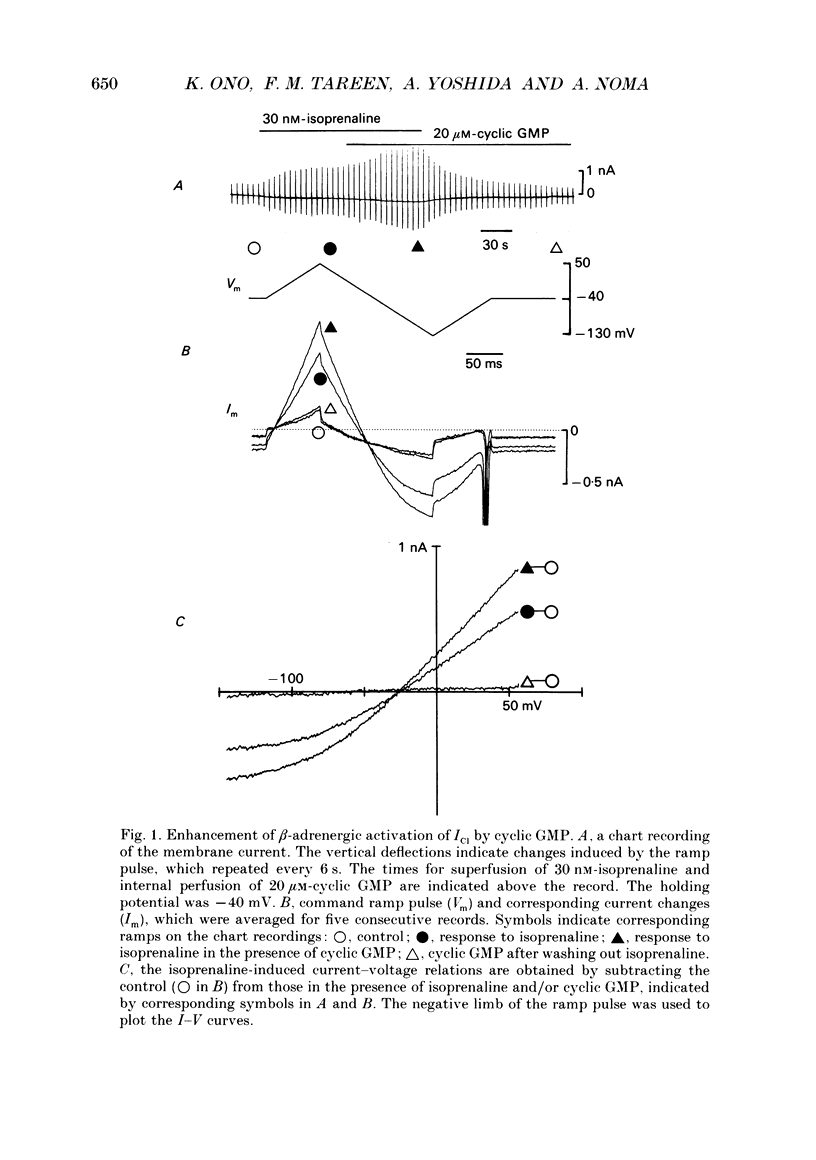
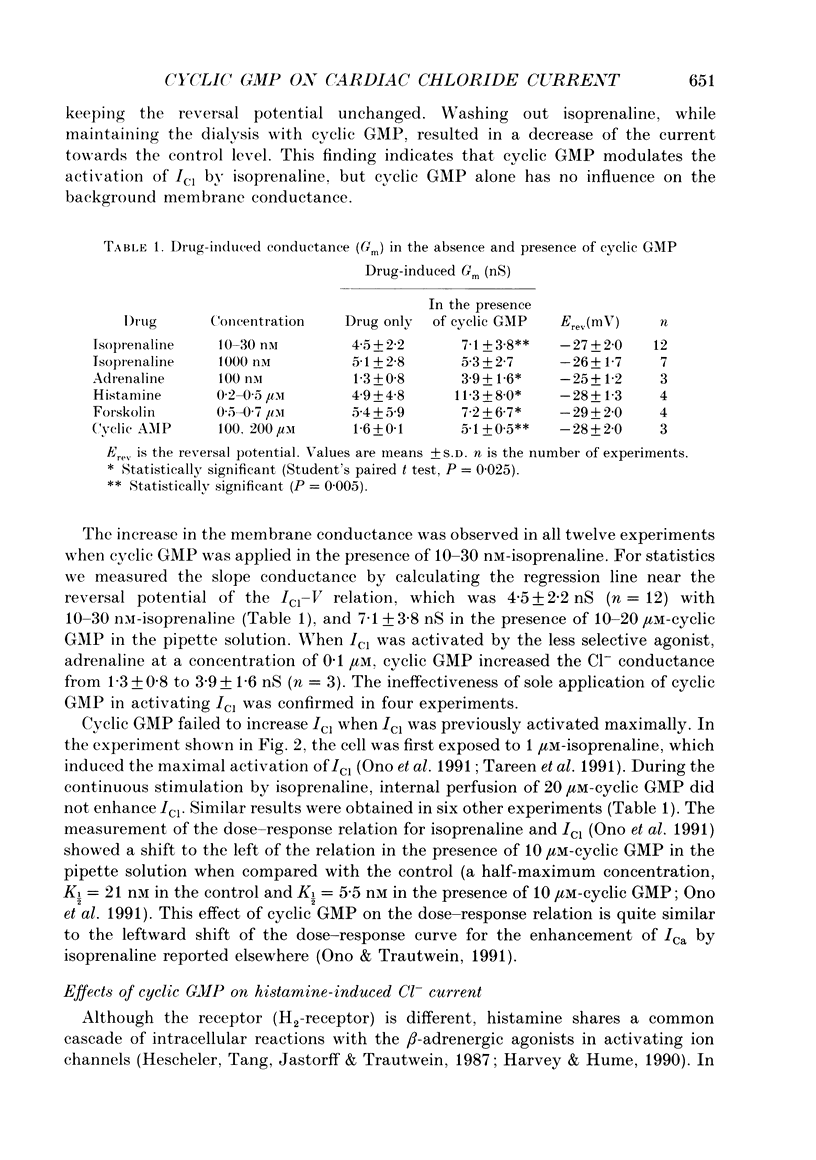
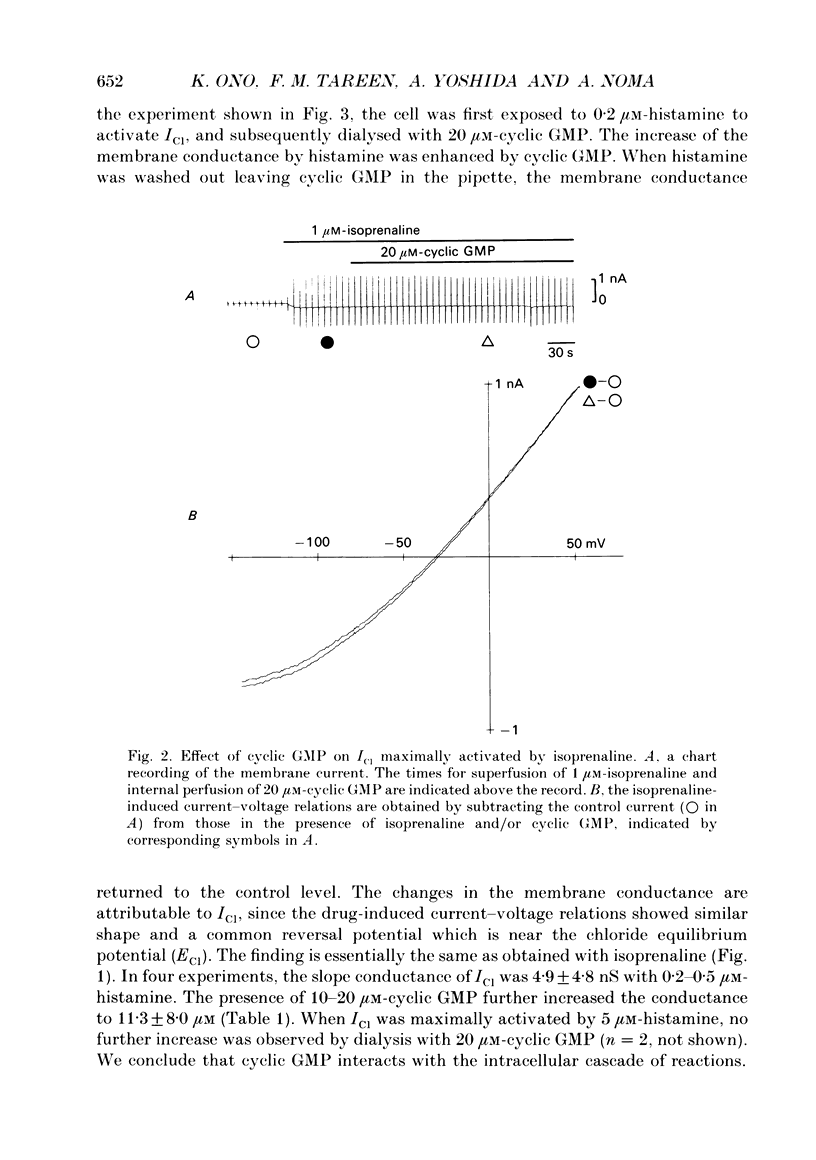
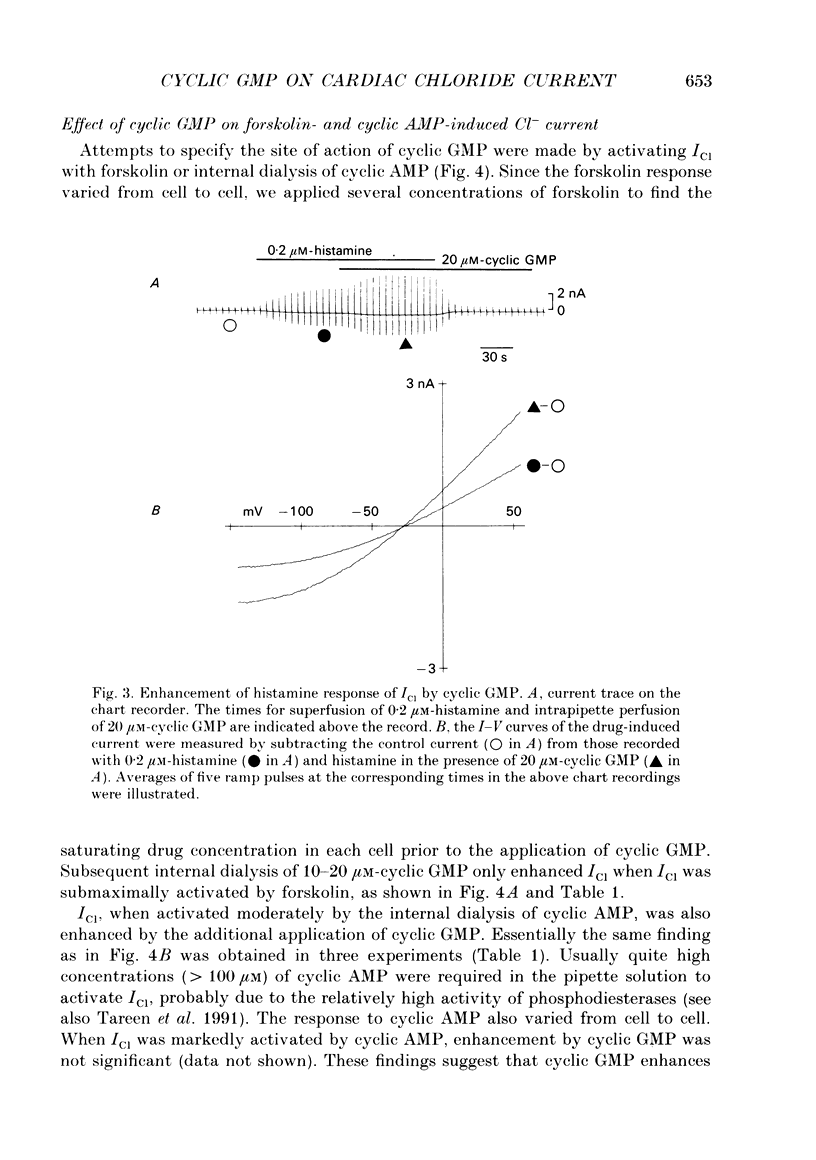
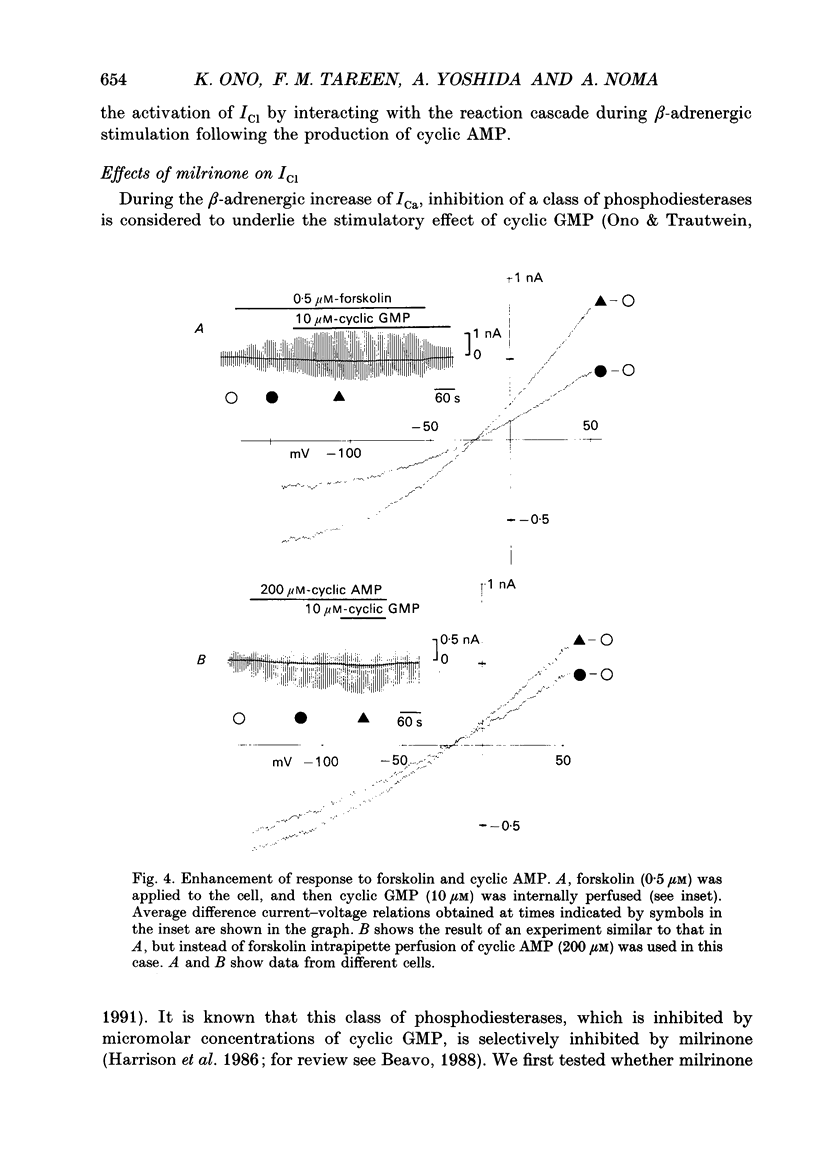
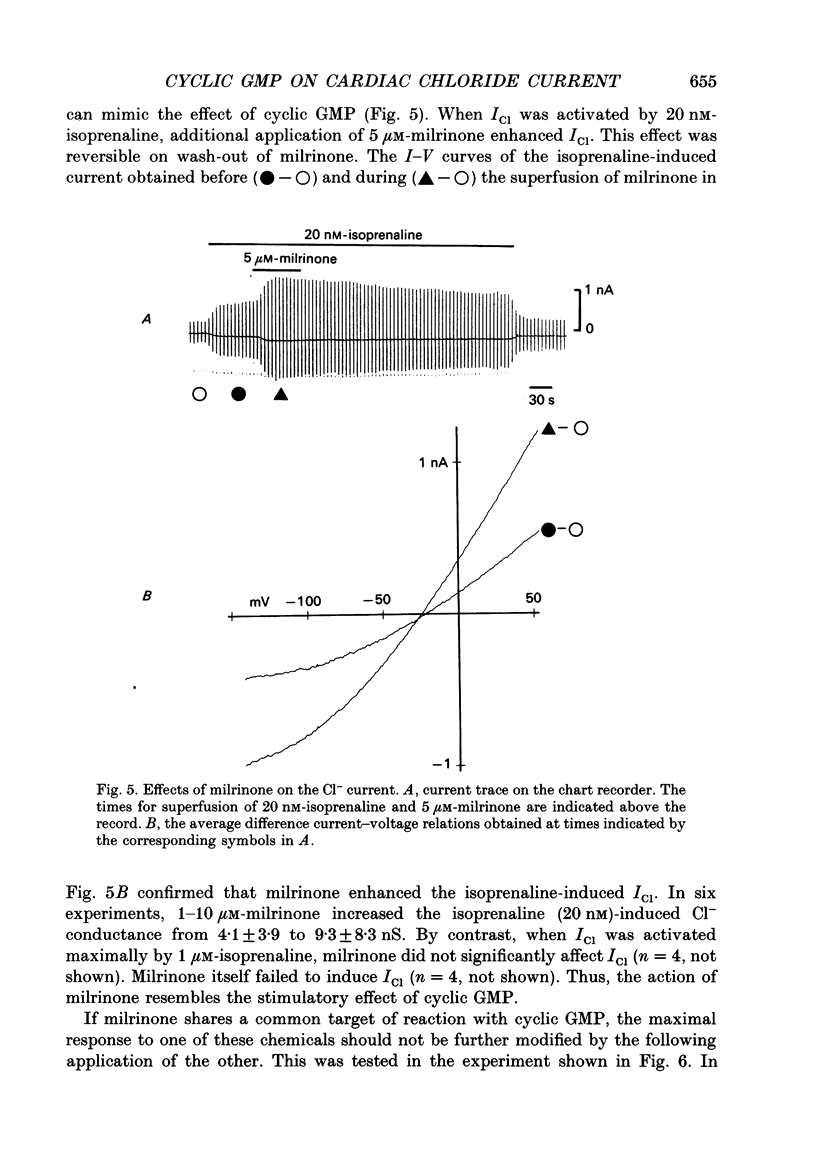
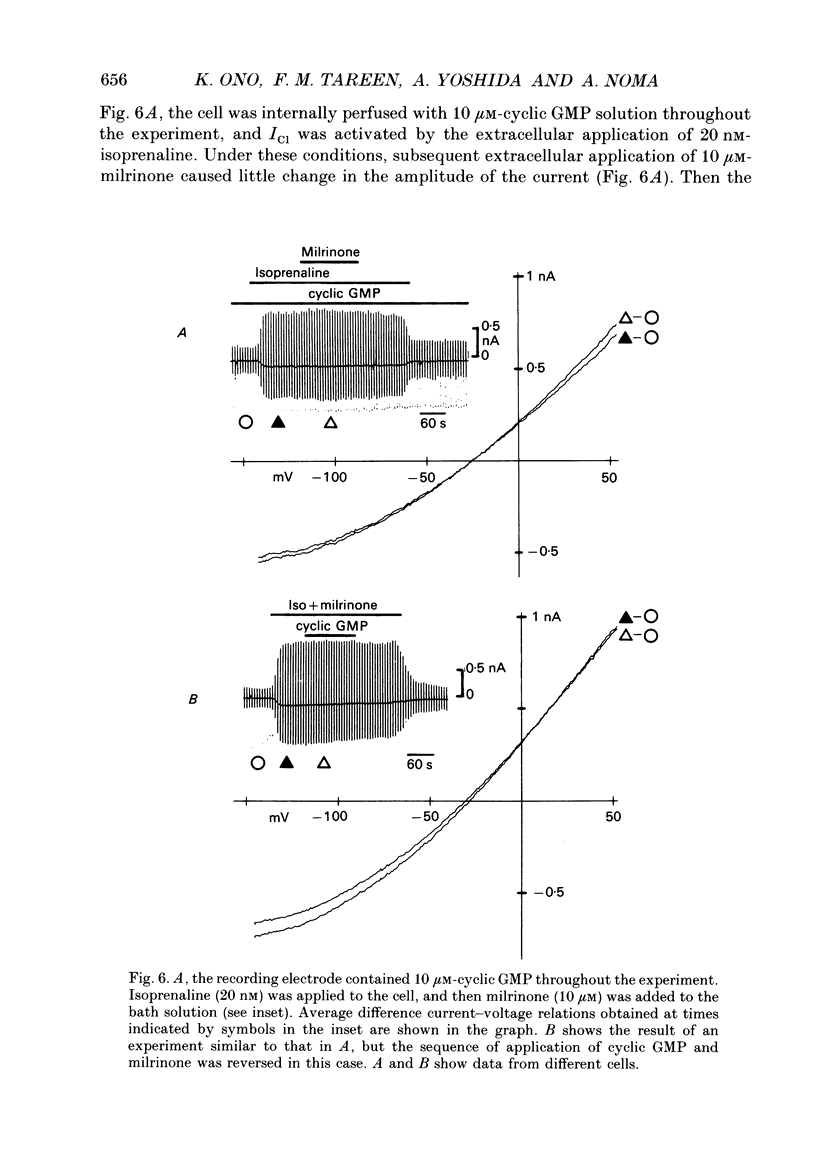
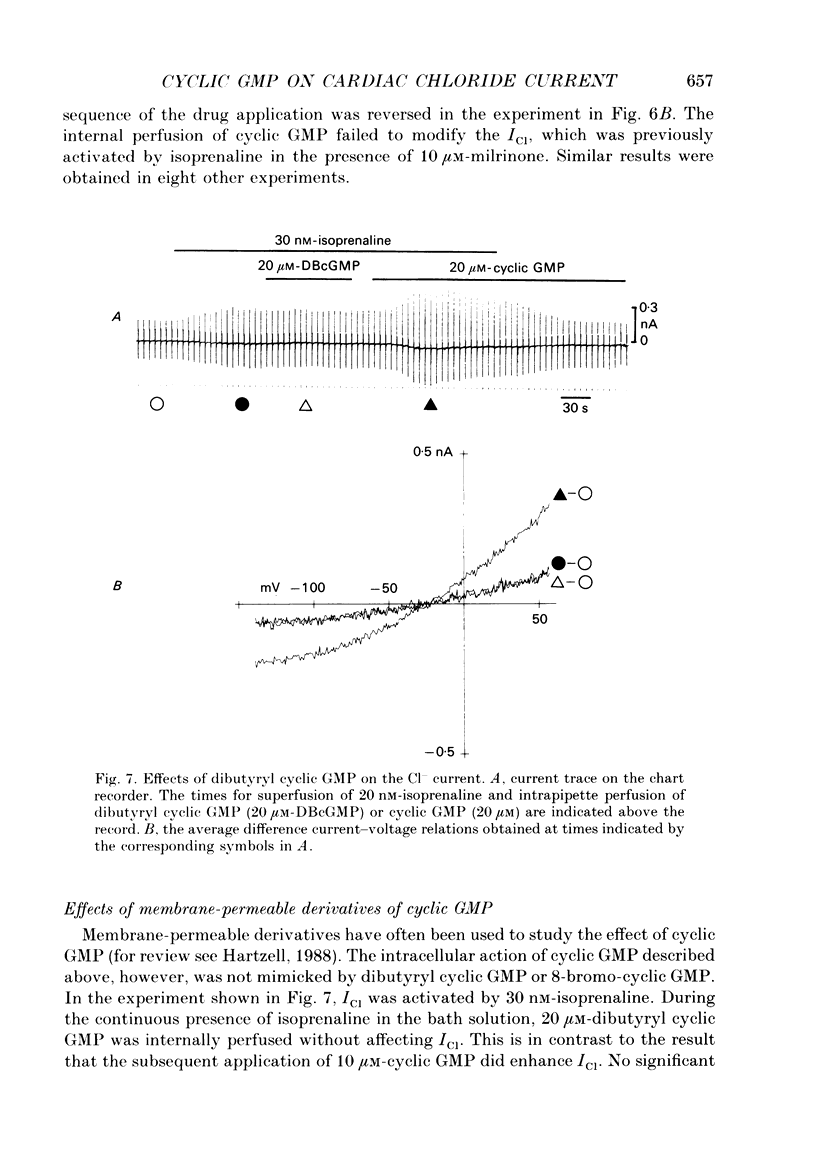
1. Effects of cyclic GMP on the catecholamine-induced chloride current (ICl) were studied using the whole-cell patch-clamp technique combined with internal perfusion in single ventricular myocytes dispersed from guinea-pig heart. 2. When ICl was activated by submaximal doses of isoprenaline (0.01-0.1 microM), adrenaline (0.5-1 microM) and histamine (0.2-0.5 microM), intracellular dialysis with cyclic GMP (10-100 microM) induced an extra increase of ICl. No further increase of ICl was induced by cyclic GMP when ICl was maximally activated. In the absence of agonists, cyclic GMP failed to induce ICl. 3. The enhancement by cyclic GMP was also observed when ICl was activated by external application of 0.2-1.0 microM-forskolin or by internal dialysis with a pipette solution containing 50-200 microM-cyclic AMP. 4. In contrast to cyclic GMP, 10-1000 microM-dibutyryl cyclic GMP and 8-bromo-cyclic GMP were ineffective in modifying ICl. 5. Milrinone (1-10 microM), a specific inhibitor of a kind of phosphodiesterase which is inhibited by cyclic GMP, also enhanced ICl activated by submaximal doses of isoprenaline. Milrinone itself did not activate ICl. 6. When ICl was enhanced by 5 microM-milrinone, an additional application of cyclic GMP failed to increase ICl. In the presence of cyclic GMP, milrinone failed to enhance ICl. 7. The above findings on ICl are analogous to the enhancement by cyclic GMP of the beta-adrenergic stimulation of the Ca2+ current reported in the same preparation, and support the hypothesis that in mammalian cardiac cells cyclic GMP potentiates elevation of cyclic AMP induced by beta-adrenergic agents, and thereby increases the amplitudes of ionic currents.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Corbin J. D., Ogreid D., Miller J. P., Suva R. H., Jastorff B., Døskeland S. O. Studies of cGMP analog specificity and function of the two intrasubunit binding sites of cGMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):1208–1214. [PubMed] [Google Scholar]
- Cramb G., Banks R., Rugg E. L., Aiton J. F. Actions of atrial natriuretic peptide (ANP) on cyclic nucleotide concentrations and phosphatidylinositol turnover in ventricular myocytes. Biochem Biophys Res Commun. 1987 Nov 13;148(3):962–970. doi: 10.1016/s0006-291x(87)80226-7. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic AMP phosphodiesterases and Ca2+ current regulation in cardiac cells. Life Sci. 1991;48(25):2365–2376. doi: 10.1016/0024-3205(91)90369-m. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Tang M., Jastorff B., Trautwein W. On the mechanism of histamine induced enhancement of the cardiac Ca2+ current. Pflugers Arch. 1987 Sep;410(1-2):23–29. doi: 10.1007/BF00581891. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Trautwein W. Potentiation by cyclic GMP of beta-adrenergic effect on Ca2+ current in guinea-pig ventricular cells. J Physiol. 1991 Nov;443:387–404. doi: 10.1113/jphysiol.1991.sp018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. Regulation of calcium slow channels of cardiac muscle by cyclic nucleotides and phosphorylation. J Mol Cell Cardiol. 1988 Mar;20 (Suppl 2):75–105. doi: 10.1016/0022-2828(88)90334-3. [DOI] [PubMed] [Google Scholar]
- Tareen F. M., Ono K., Noma A., Ehara T. Beta-adrenergic and muscarinic regulation of the chloride current in guinea-pig ventricular cells. J Physiol. 1991;440:225–241. doi: 10.1113/jphysiol.1991.sp018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Kaplan H. R. Subclasses of cyclic AMP phosphodiesterase in cardiac muscle. J Mol Cell Cardiol. 1987 Oct;19(10):1025–1036. doi: 10.1016/s0022-2828(87)80574-6. [DOI] [PubMed] [Google Scholar]


