Abstract
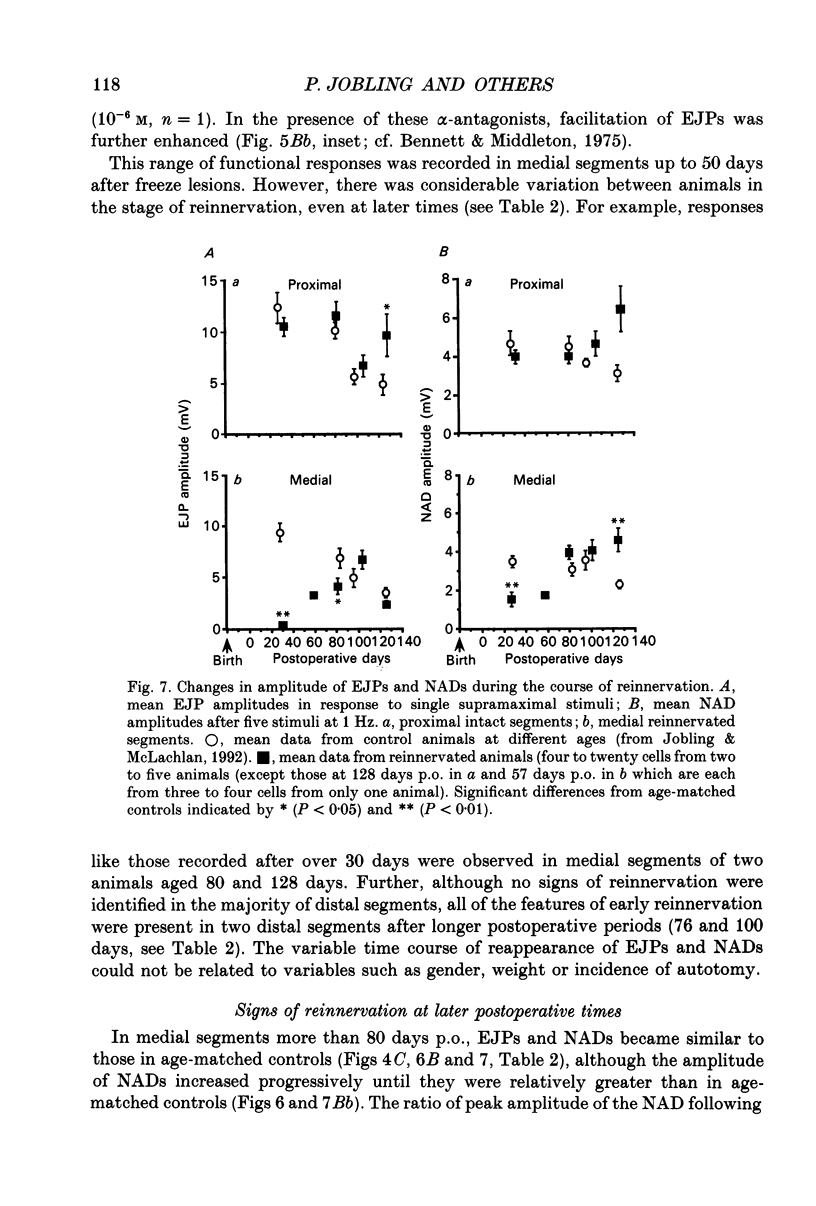
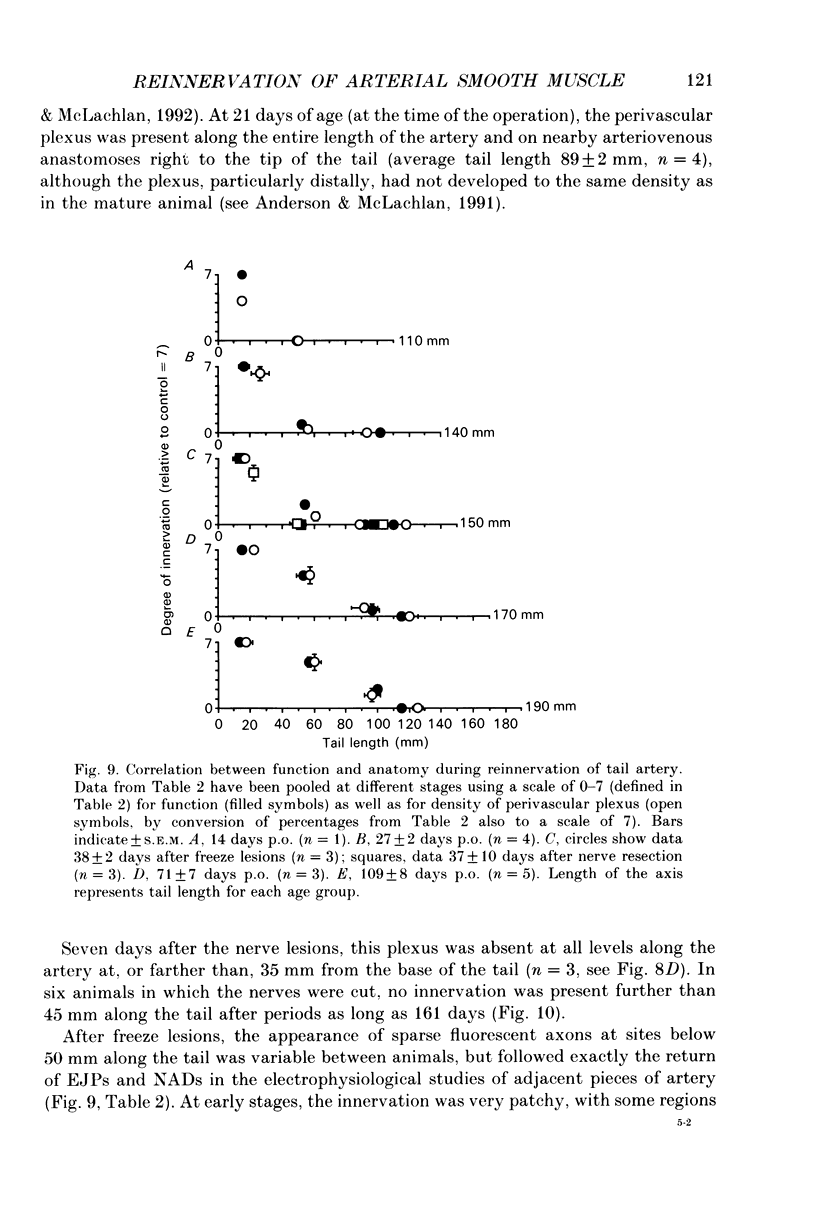
1. Responses to perivascular stimuli have been recorded with intracellular microelectrodes from the smooth muscle of isolated segments of the main caudal artery of rats at various times between 7 and 128 days after all four collector nerve trunks had been lesioned near the base of the tail at 21 days of age. 2. In proximal segments (< 40 mm distal to the lesions), excitatory junction potentials (EJPs) and neurogenic alpha-depolarizations (NADs) evoked by stimuli presented via a proximally located suction electrode were similar to those in the same segments of unoperated control animals of the same age. Supramaximal EJPs in these segments decreased in amplitude with age. 3. Stimuli just supramaximal for EJPs in innervated preparations failed to evoke responses in segments farther than 30-40 mm distal to the lesions at any time after the nerves had been cut and 1 cm excised. Higher voltages evoked slow depolarizing potentials (SDPs) which were of longer time course than EJPs. Similar responses occurred in segments over 60 mm distal to the lesions at 20-50 days after the nerves had been frozen, and in all segments sampled over 100 mm distal to nerve lesions. 4. Spontaneous transient depolarizations (STDs) were recorded at all depths of the media in denervated segments. These occurred at frequencies similar to those of spontaneous events (including attenuated spontaneous EJPs) in innervated segments. 5. The earliest signs of reinnervation (24-42 days after freeze lesions) consisted of very small amplitude EJPs of normal time course which facilitated markedly during a short train of stimuli (5-10 Hz); these were followed by NADs which were large relative to the amplitudes of the EJPs. Less commonly, small focal EJPs of brief time course (resembling spontaneous EJPs in superficial cells of innervated arteries) were evoked in very restricted regions of the vessel wall. 6. At later times (57-128 days postoperative), six of eight segments located 40-70 mm distal to freeze lesions showed EJPs of nearly control amplitude, but NADs that were larger than in equivalent segments from control animals. In the remaining two cases, reinnervation at this level was similar to that seen at the earliest postoperative times. High stimulus voltages prolonged the decay of EJPs in both control and reinnervated arteries. 7. Sensitivity to exogenous noradrenaline, assessed in terms of membrane depolarization, was increased in both denervated and reinnervated segments. 8. Catecholamine fluorescence disappeared from the arteries at a distance greater than 30-40 mm distal to the site of the nerve lesions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
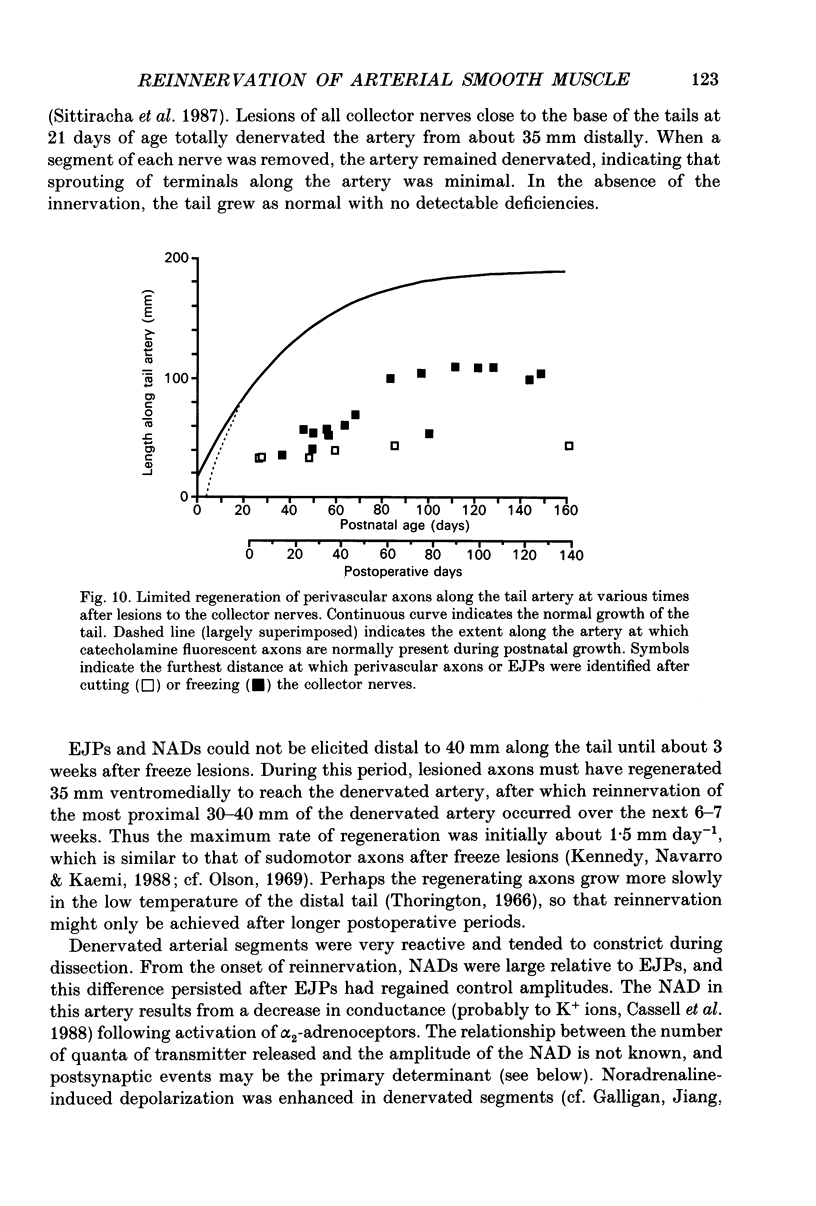
PDF


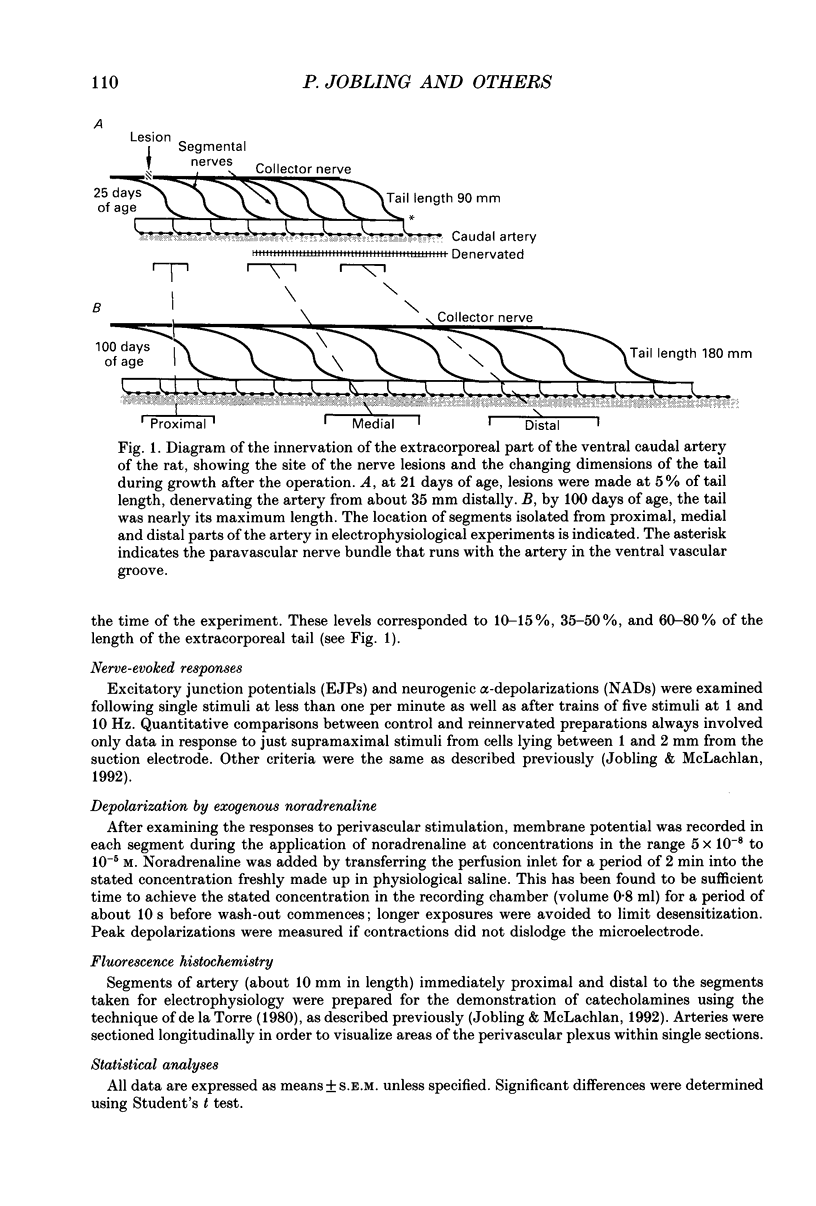
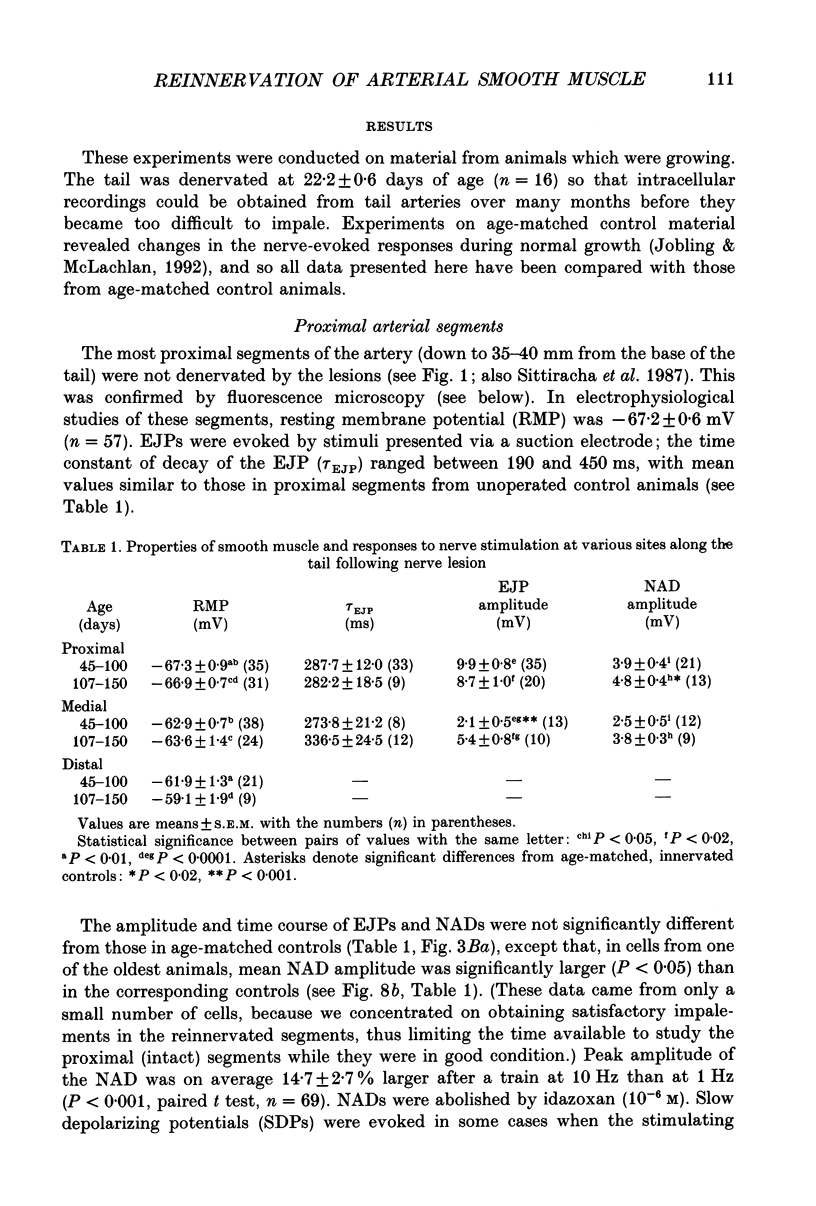
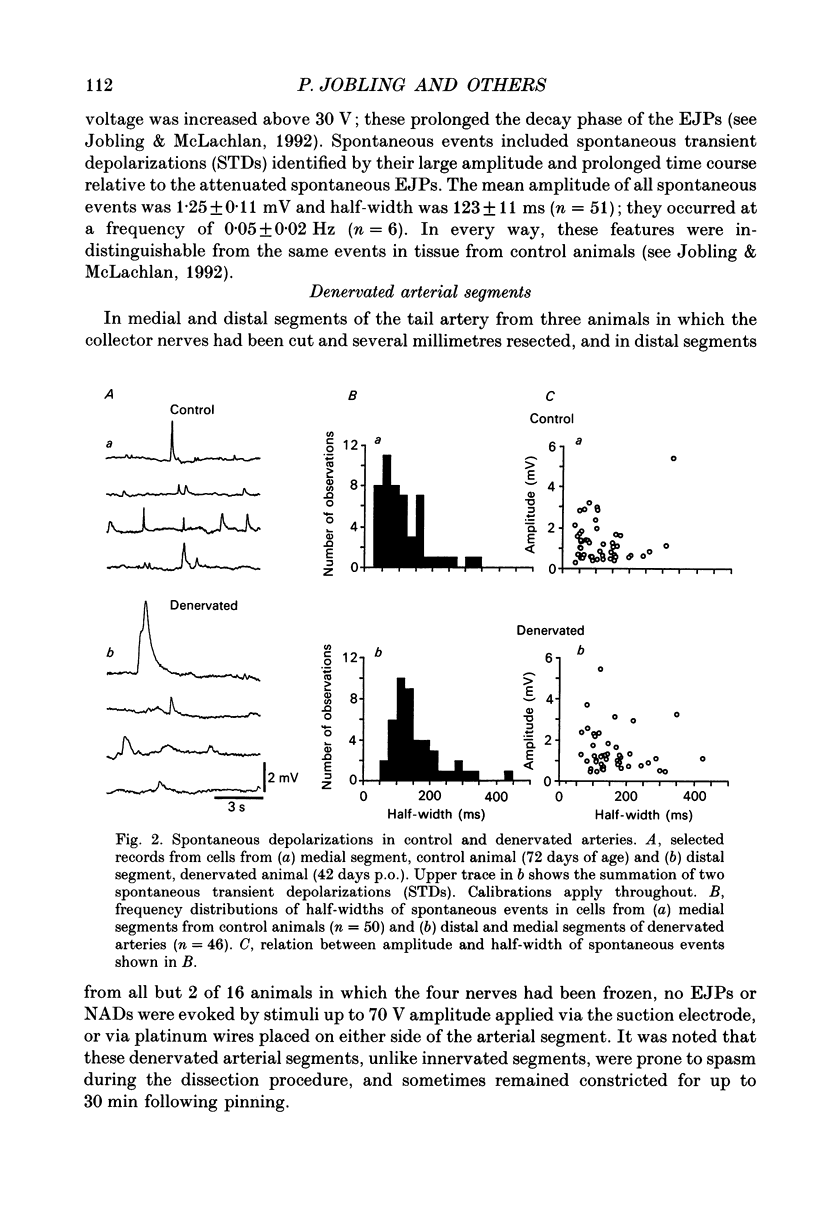








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., McLachlan E. M. The time course of the development of the sympathetic innervation of the vasculature of the rat tail. J Auton Nerv Syst. 1991 Aug;35(2):117–132. doi: 10.1016/0165-1838(91)90055-8. [DOI] [PubMed] [Google Scholar]
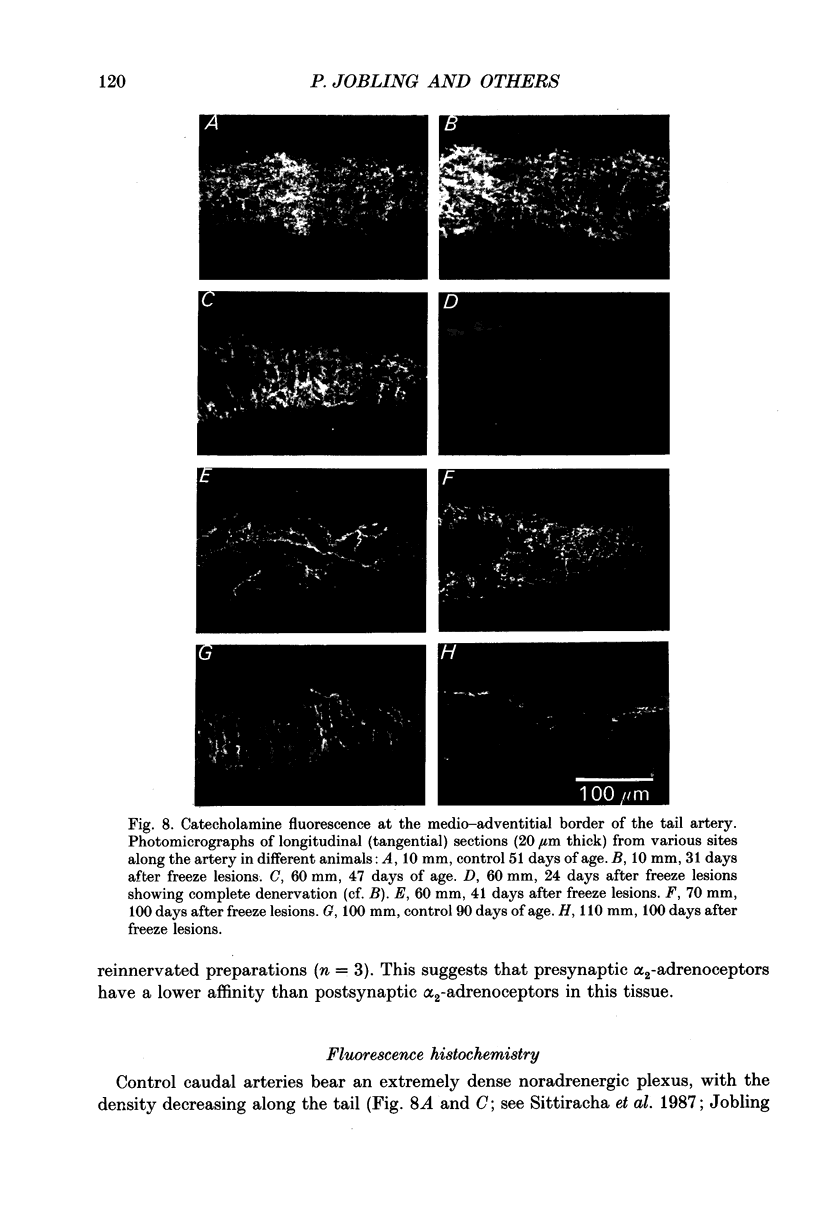
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Perry V. H., Lunn E. R., Gordon S., Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991 Mar;6(3):359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M., Sittiracha T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. J Physiol. 1988 Mar;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen T., MacCormick D. E., Toff W. D., Burnstock G., Lumley J. S. The effect of surgical procedures on blood vessel innervation. A fluorescence histochemical study of degeneration and regrowth of perivascular adrenergic nerves. Blood Vessels. 1982;19(2):65–78. [PubMed] [Google Scholar]
- Crews L. L., Wigston D. J. The dependence of motoneurons on their target muscle during postnatal development of the mouse. J Neurosci. 1990 May;10(5):1643–1653. doi: 10.1523/JNEUROSCI.10-05-01643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Torre J. C. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980 Oct;3(1):1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- Finch L., Haeusler G., Kuhn H., Thoenen H. Rapid recovery of vascular adrenergic nerves in the rat after chemical sympathectomy with 6-hydroxydopamine. Br J Pharmacol. 1973 May;48(1):59–72. doi: 10.1111/j.1476-5381.1973.tb08222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan J. J., Jiang M. M., Shen K. Z., Surprenant A. Substance P mediates neurogenic vasodilatation in extrinsically denervated guinea-pig submucosal arterioles. J Physiol. 1990 Jan;420:267–280. doi: 10.1113/jphysiol.1990.sp017911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., Ngu M. C., van Helden D. F. Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurones of the ileum of the rat. J Auton Nerv Syst. 1985 Dec;14(4):317–334. doi: 10.1016/0165-1838(85)90079-7. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Högestätt E. D., Hammarström L. E., Andersson K. E., Holmin T. Contractile effects of various vasoactive agents in small rat portal veins and hepatic arteries and the influence of sympathetic denervation on the noradrenaline response. Acta Physiol Scand. 1986 Oct;128(2):309–315. doi: 10.1111/j.1748-1716.1986.tb07979.x. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kitamura K., Kuriyama H. Roles of extrajunctional receptors in the response of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J Physiol. 1983 Dec;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P., McLachlan E. M. An electrophysiological study of responses evoked in isolated segments of rat tail artery during growth and maturation. J Physiol. 1992 Aug;454:83–105. doi: 10.1113/jphysiol.1992.sp019255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Koltzenburg M. Plasticity of sympathetic reflex organization following cross-union of inappropriate nerves in the adult cat. J Physiol. 1991 May;436:309–323. doi: 10.1113/jphysiol.1991.sp018552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. On the fate of sympathetic and sensory neurons projecting into a neuroma of the superficial peroneal nerve in the cat. J Comp Neurol. 1984 May 10;225(2):302–311. doi: 10.1002/cne.902250213. [DOI] [PubMed] [Google Scholar]
- Karanth S. S., Dhital S., Springall D. R., Polak J. M. Reinnervation and neuropeptides in mouse skin flaps. J Auton Nerv Syst. 1990 Nov;31(2):127–134. doi: 10.1016/0165-1838(90)90069-u. [DOI] [PubMed] [Google Scholar]
- Kennedy W. R., Navarro X., Kamei H. Reinnervation of sweat glands in the mouse: axonal regeneration versus collateral sprouting. Muscle Nerve. 1988 Jun;11(6):603–609. doi: 10.1002/mus.880110613. [DOI] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lorez H. P., Kuhn H., Bartholini G. Degeneration and regeneration of adrenergic nerves in mesenteric blood vessels, iris and atrium of the rat after 6-hydroxydopamine injection. J Neurocytol. 1975 Apr;4(2):157–176. doi: 10.1007/BF01098780. [DOI] [PubMed] [Google Scholar]
- Mira J. C. Maintien de la continuté de la lame basale des fibres nerveuses périphériques après "section" des axones par congélation localisée. C R Acad Sci Hebd Seances Acad Sci D. 1971 Nov 15;273(20):1836–1839. [PubMed] [Google Scholar]
- Mira J. C. Quantitative studies of the regeneration of rat myelinated nerve fibres: variations in the number and size of regenerating fibres after repeated localized freezings. J Anat. 1979 Aug;129(Pt 1):77–93. [PMC free article] [PubMed] [Google Scholar]
- Navarro X., Kennedy W. R. Sweat gland reinnervation by sudomotor regeneration after different types of lesions and graft repairs. Exp Neurol. 1989 Jun;104(3):229–234. doi: 10.1016/0014-4886(89)90034-4. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Olson L. Intact and regenerating sympathetic noradrenaline axons in the rat sciatic nerve. Histochemie. 1969;17(4):349–367. doi: 10.1007/BF00305459. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Schachner M., Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. J Cell Biol. 1986 Feb;102(2):420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittiracha T., McLachlan E. M., Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987 May;21(2):647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Higashi H., Nishi S. Noradrenaline mediates slow excitatory synaptic potentials in rat dorsal raphe neurons in vitro. Neurosci Lett. 1985 Nov 11;61(3):305–310. doi: 10.1016/0304-3940(85)90481-1. [DOI] [PubMed] [Google Scholar]