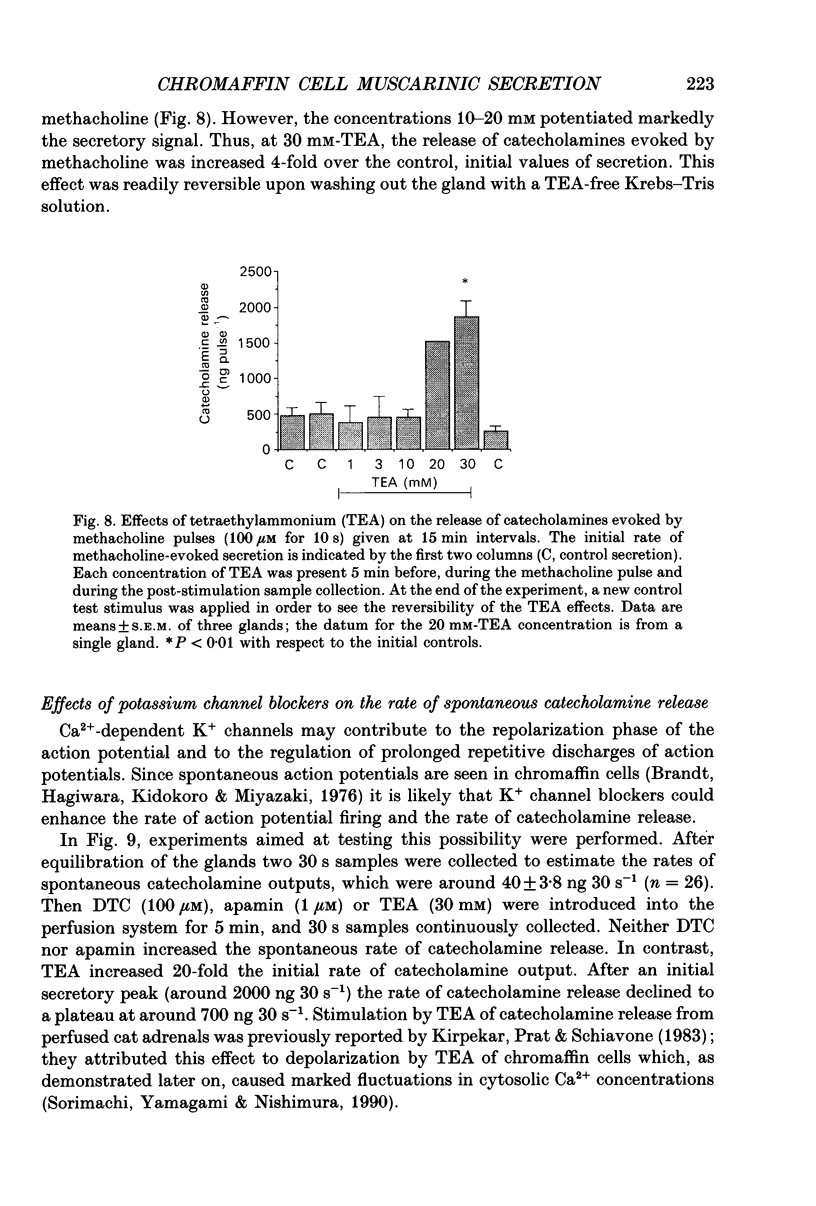
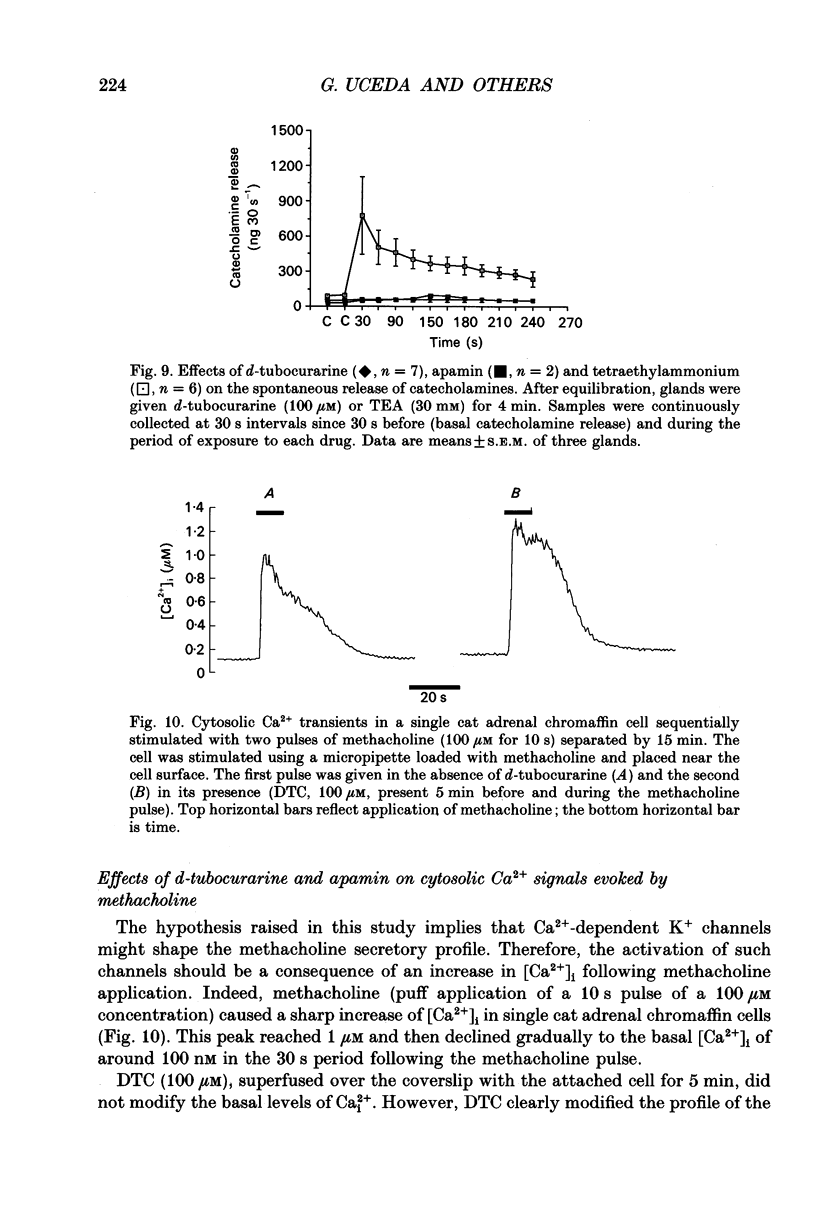
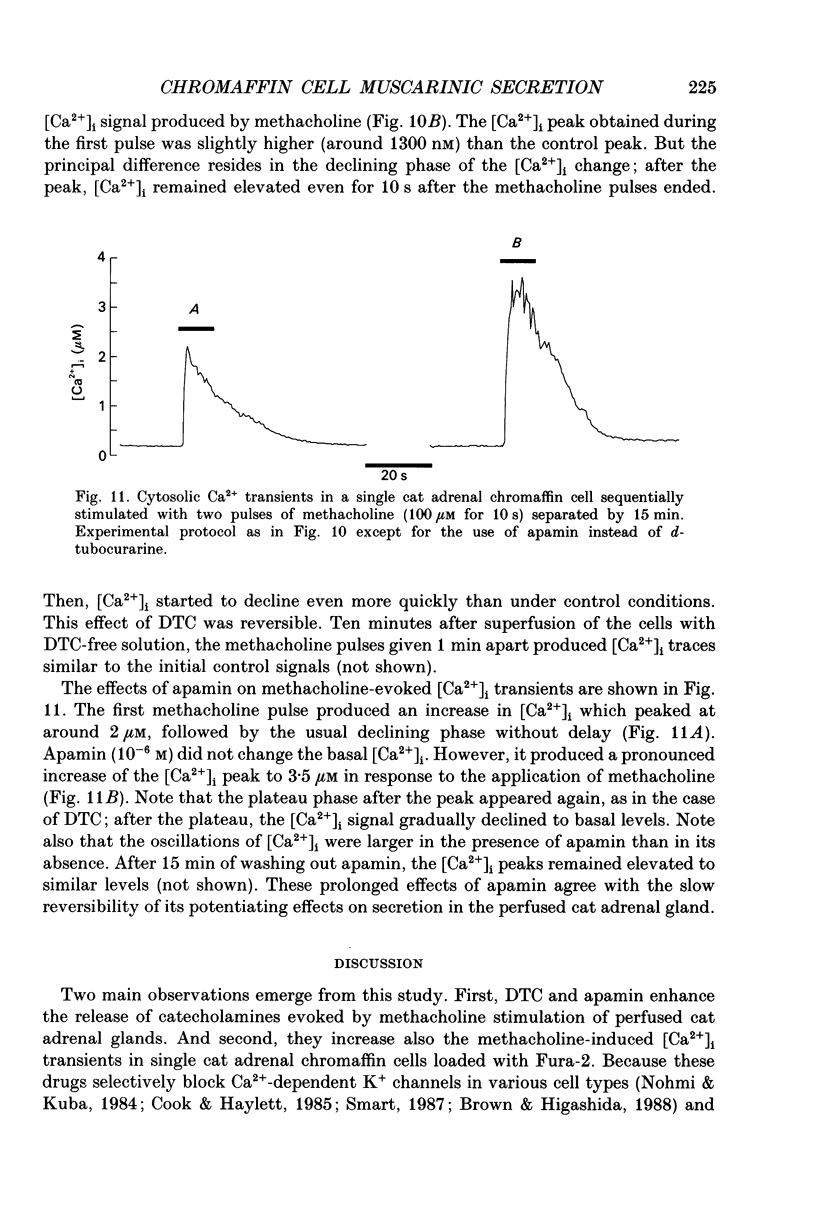
Abstract
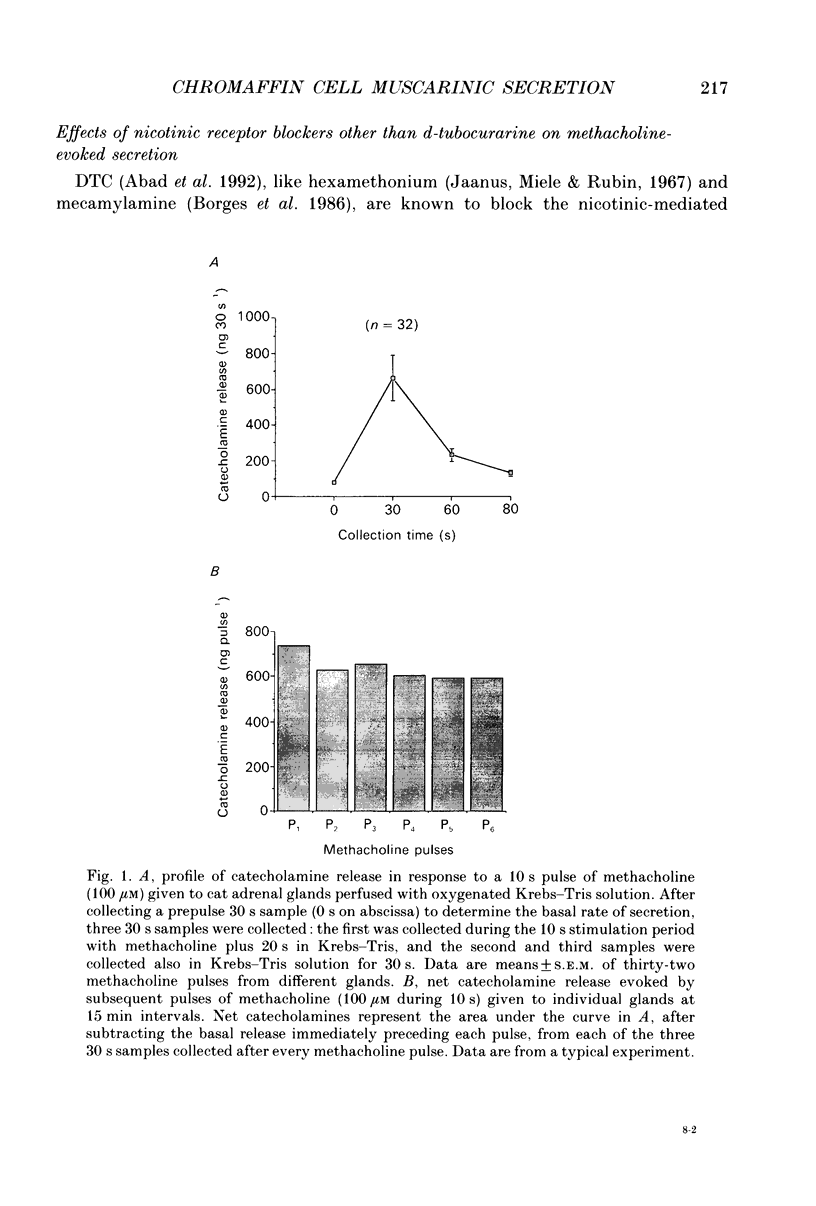
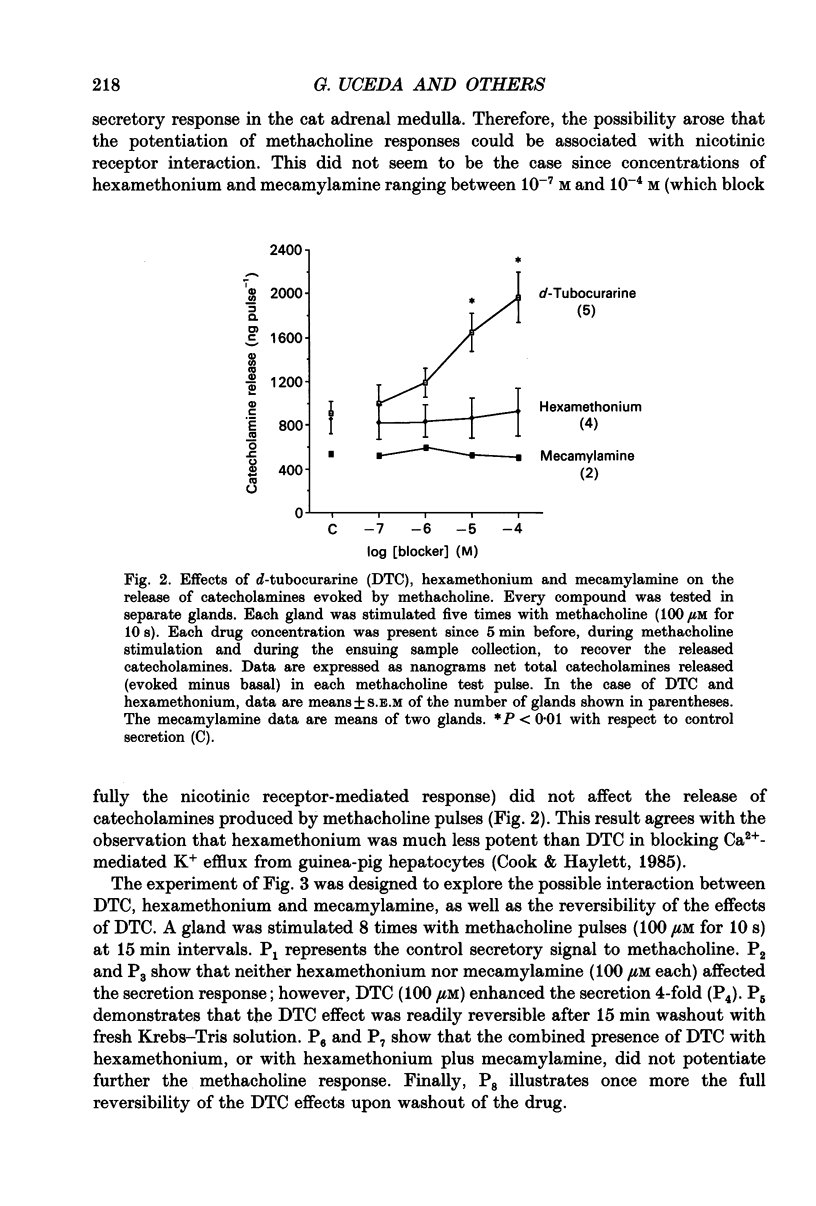
1. This study was aimed at testing the hypothesis that Ca(2+)-dependent K+ channels regulate the release of catecholamines mediated by muscarinic stimulation of cat adrenal chromaffin cells. Two parameters were measured: the secretory response to brief pulses of methacholine (100 microM for 10 s) in intact cat adrenal glands perfused at a high rate with oxygenated Krebs solution; and the changes in cytosolic Ca2+ concentrations, [Ca2+]i, produced by puff applications of methacholine pulses (also 100 microM for 10 s) in isolated single cat adrenal chromaffin cells loaded with Fura-2. 2. A pulse of methacholine released 805 +/- 164 ng of catecholamines (mean of thirty-two pulses). d-Tubocurarine (DTC) increased the secretory response in a concentration-dependent manner. The maximum increase (around 1000 ng catecholamines over control values) was reached at 100 microM-DTC and the EC50 was around 10 microM. 3. The secretory responses to methacholine alone, or to the combination of methacholine plus DTC, were strongly dependent on the extracellular Ca2+ concentration, [Ca2+]o. Thus Ca2+o removal from the perfusing solution for 5-10 min abolished catecholamine release. 4. At 0.1 microM, isradipine (an L-type Ca2+ channel blocker) inhibited by 71% the secretory response to DTC plus methacholine. At 1 microM, Bay K 8644 (an L-type Ca2+ channel activator) increased 2-fold the secretory response to DTC plus methacholine (2746 ng of catecholamines). 5. Apamin (1 microM) increased 3.5-fold the secretory response to methacholine pulses (from 500 to 1800 ng of catecholamines). 6. Methacholine pulses enhanced [Ca2+]i from the resting level of 100 nM to a peak of 1000 nM which quickly declined to basal level. DTC (100 microM) enhanced by 20% the [Ca2+]i peak and substantially prolonged its duration. 7. Apamin (1 microM) increased by 60% the [Ca2+]i peak evoked by methacholine, and delayed the initiation of decline of the [Ca2+]i peak. 8. These results are compatible with the idea that muscarinic stimulation depolarizes the cat adrenal chromaffin cell through an unidentified mechanism. Depolarization is probably counteracted by activation of Ca2+i-dependent K+ channels. Therefore, inhibition of these channels enhances depolarization and firing of action potentials which activate voltage-dependent L-type Ca2+ channels to increase further the Ca2+i signal and the secretory response. Thus Ca2+i-dependent K+ channels, probably of the small-conductance type (SK), seem to be involved in the modulation of muscarinic-evoked catecholamine release responses in cat adrenal chromaffin cells.
Full text
PDF



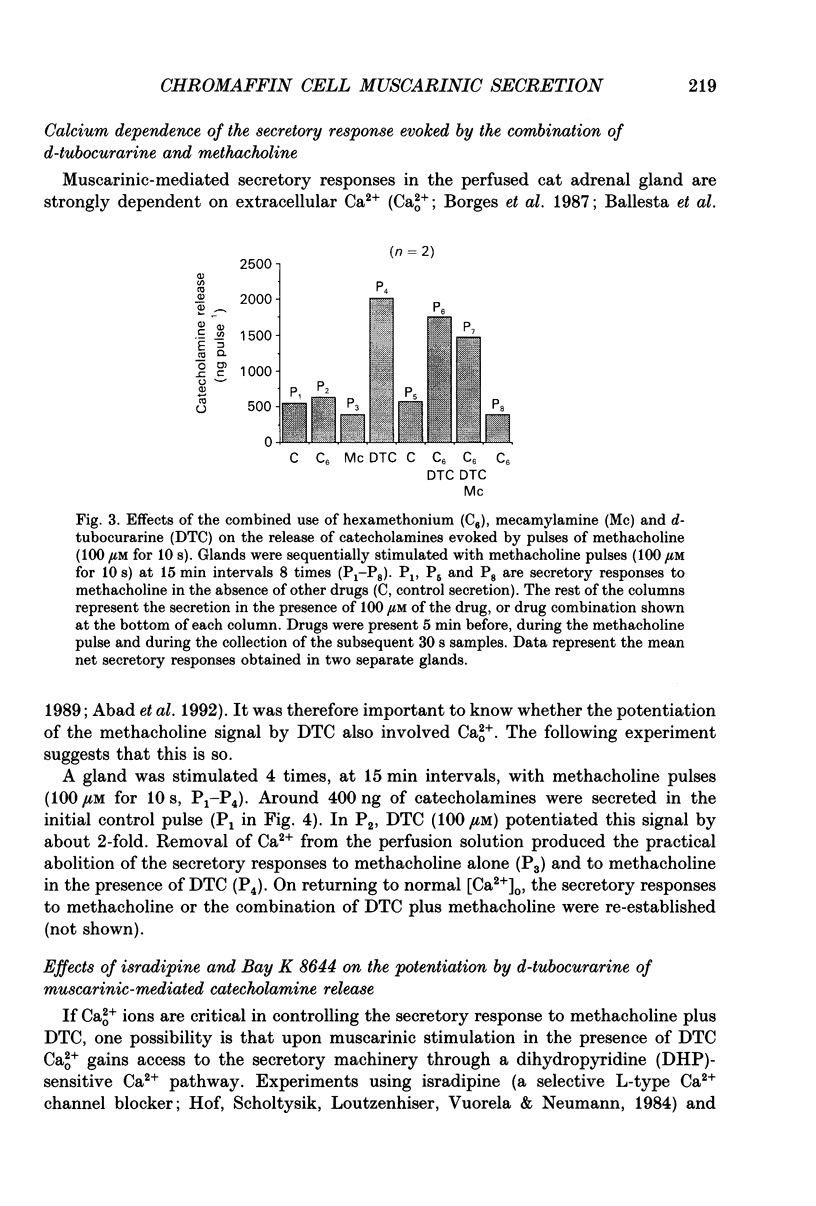
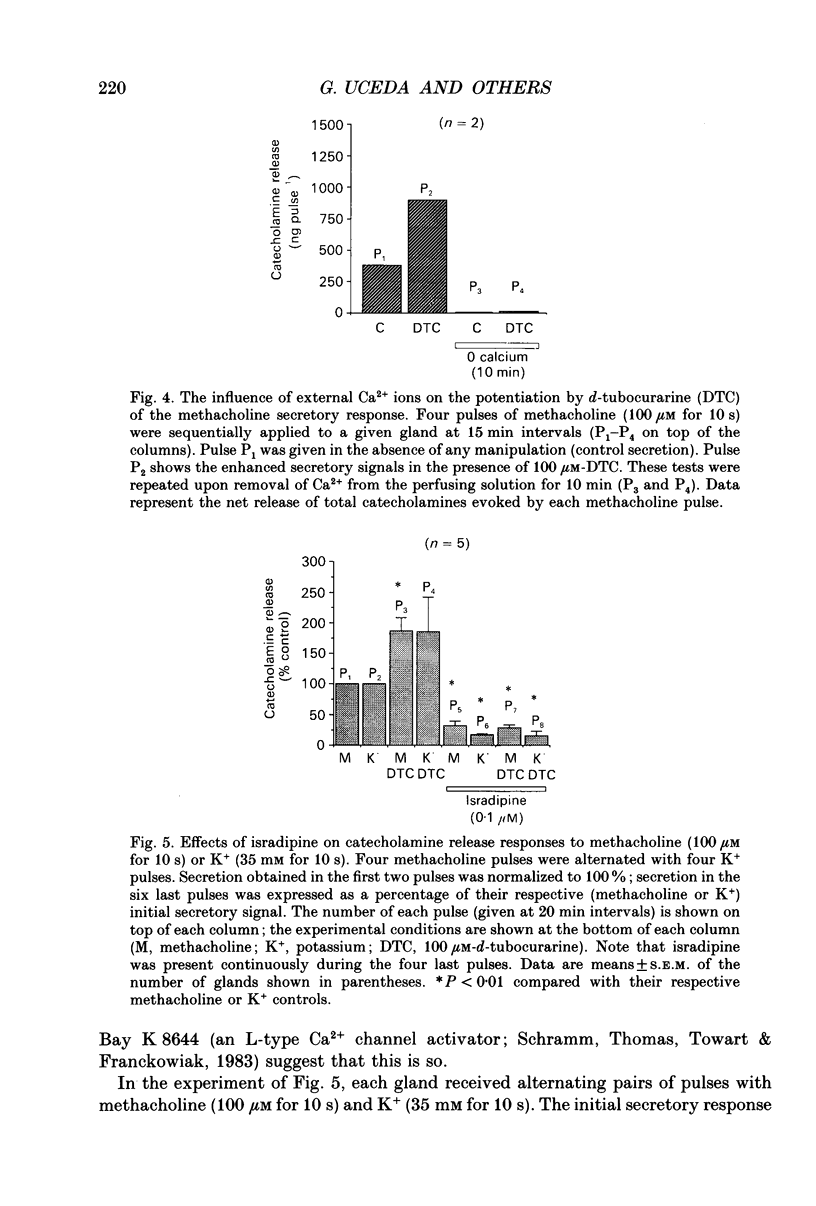
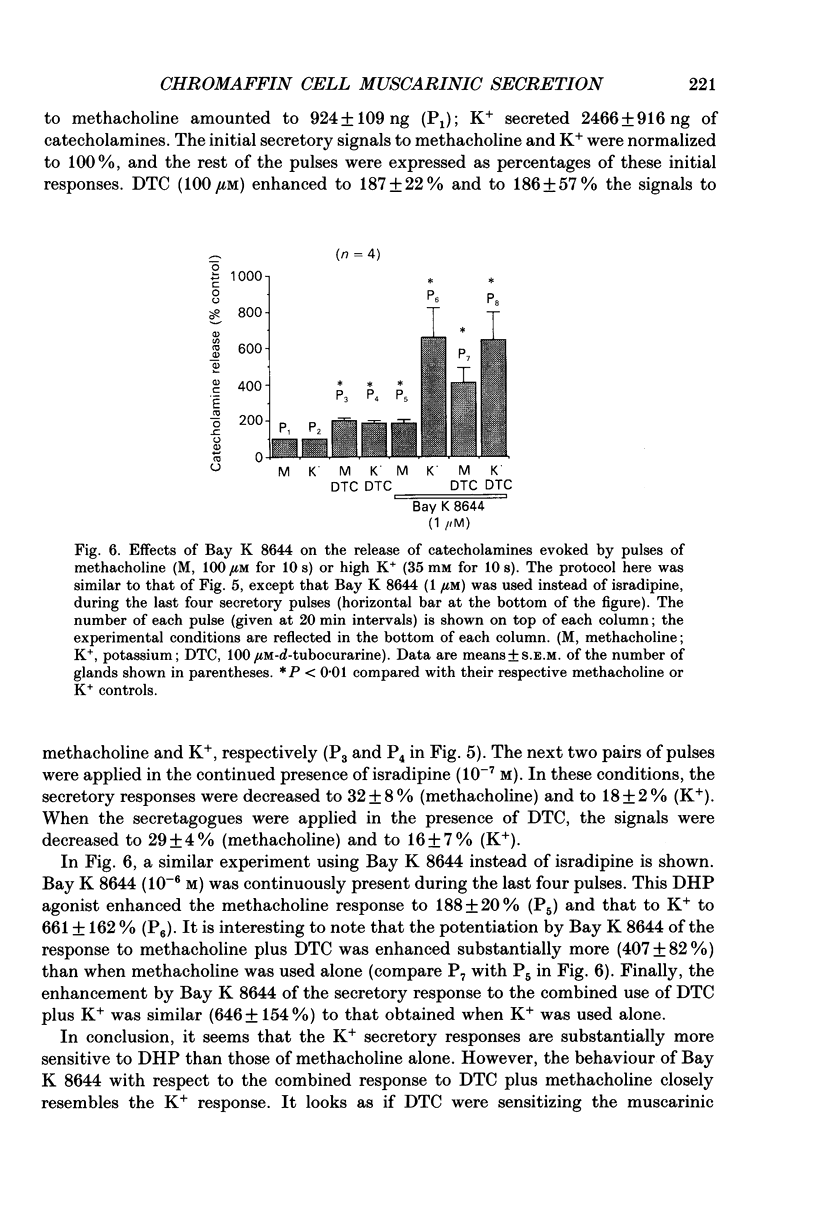
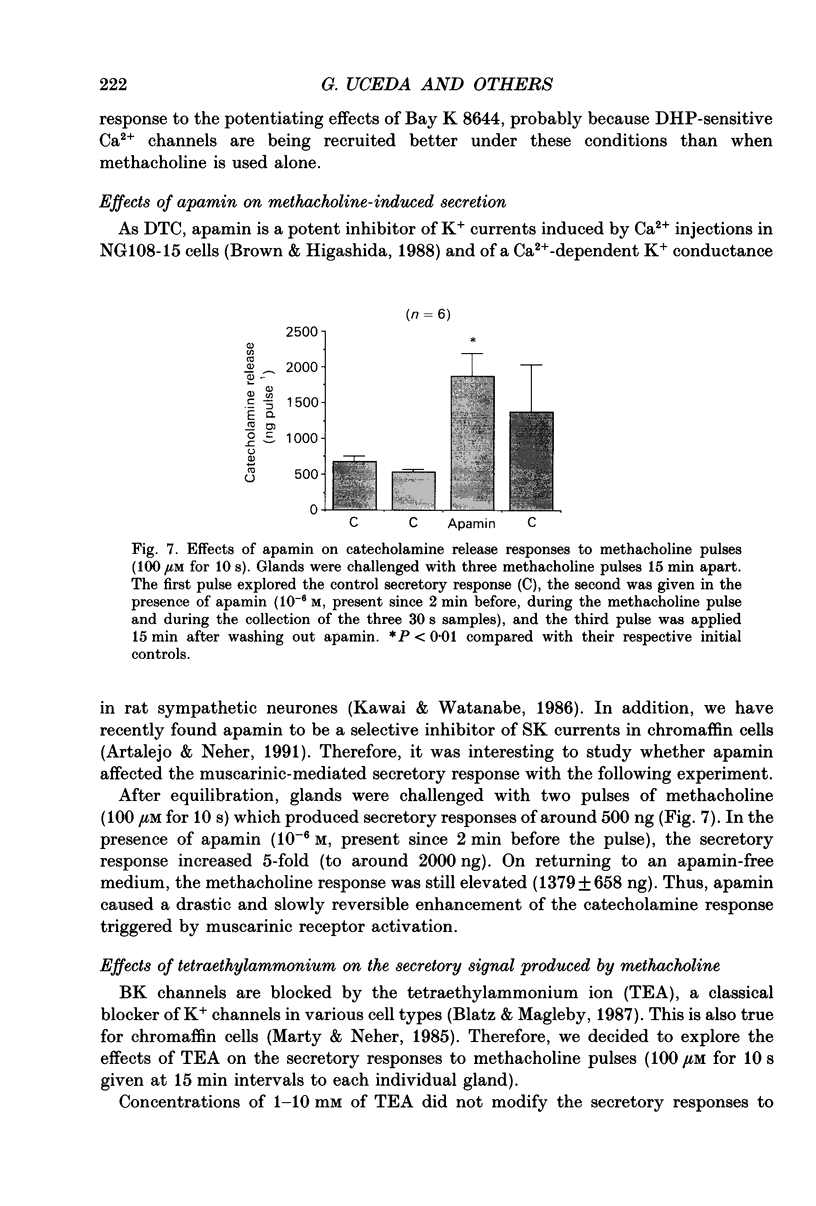





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad F., Garrido B., López M. G., García A. G. The source of calcium for muscarinic-mediated catecholamine release from cat adrenals. J Physiol. 1992 Jan;445:725–740. doi: 10.1113/jphysiol.1992.sp018947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike A., Mine Y., Sasa M., Takaori S. Voltage and current clamp studies of muscarinic and nicotinic excitation of the rat adrenal chromaffin cells. J Pharmacol Exp Ther. 1990 Oct;255(1):333–339. [PubMed] [Google Scholar]
- Albino A. P., Le Strange R., Oliff A. I., Furth M. E., Old L. J. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984 Mar 1;308(5954):69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Almazan G., Aunis D., García A. G., Montiel C., Nicolás G. P., Sánchez-García P. Effects of collagenase on the release of [3H]-noradrenaline from bovine cultured adrenal chromaffin cells. Br J Pharmacol. 1984 Apr;81(4):599–610. doi: 10.1111/j.1476-5381.1984.tb16124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta J. J., Borges R., García A. G., Hidalgo M. J. Secretory and radioligand binding studies on muscarinic receptors in bovine and feline chromaffin cells. J Physiol. 1989 Nov;418:411–426. doi: 10.1113/jphysiol.1989.sp017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges R., Ballesta J. J., García A. G. M2 muscarinoceptor-associated ionophore at the cat adrenal medulla. Biochem Biophys Res Commun. 1987 Apr 29;144(2):965–972. doi: 10.1016/s0006-291x(87)80058-x. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Effect of activation of muscarinic receptors on intracellular free calcium and secretion in bovine adrenal chromaffin cells. Biochim Biophys Acta. 1985 Jul 30;846(1):167–173. doi: 10.1016/0167-4889(85)90122-3. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989 Apr 24;247(2):429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Minz B., Tsudzimura H. The mechanism of the nervous discharge of adrenaline. J Physiol. 1934 Jun 9;81(3):286–304. doi: 10.1113/jphysiol.1934.sp003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Holz R. W., Agranoff B. W. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981 Aug;37(2):491–497. doi: 10.1111/j.1471-4159.1981.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Gandía L., López M. G., Fonteríz R. I., Artalejo C. R., García A. G. Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett. 1987 Jun 26;77(3):333–338. doi: 10.1016/0304-3940(87)90523-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hof R. P., Scholtysik G., Loutzenhiser R., Vuorela H. J., Neumann P. PN 200-110, a new calcium antagonist: electrophysiological, inotropic, and chronotropic effects on guinea pig myocardial tissue and effects on contraction and calcium uptake of rabbit aorta. J Cardiovasc Pharmacol. 1984 May-Jun;6(3):399–406. [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Muscarinic receptor is coupled with a cation channel through a GTP-binding protein in guinea-pig chromaffin cells. J Physiol. 1991 May;436:511–529. doi: 10.1113/jphysiol.1991.sp018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Schiavone M. T. Effect of muscarine on release of catecholamines from the perfused adrenal gland of the cat. Br J Pharmacol. 1982 Nov;77(3):455–460. doi: 10.1111/j.1476-5381.1982.tb09318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Schiavone M. T. Modification of potassium-evoked release of noradrenaline by various ions and agents. Br J Pharmacol. 1983 Feb;78(2):277–285. doi: 10.1111/j.1476-5381.1983.tb09392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladona M. G., Aunis D., Gandía L., García A. G. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 1987 Feb;48(2):483–490. doi: 10.1111/j.1471-4159.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Lee F. L., Trendelenburg U. Muscarinic transmission of preganglionic impulses to the adrenal medulla of the cat. J Pharmacol Exp Ther. 1967 Oct;158(1):73–79. [PubMed] [Google Scholar]
- Livett B. G., Boksa P. Receptors and receptor modulation in cultured chromaffin cells. Can J Physiol Pharmacol. 1984 Apr;62(4):467–476. doi: 10.1139/y84-076. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. (+)-Tubocurarine blocks the Ca2+-dependent K+-channel of the bullfrog sympathetic ganglion cell. Brain Res. 1984 May 28;301(1):146–148. doi: 10.1016/0006-8993(84)90412-8. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Burgoyne R. D. A comparison of bradykinin, angiotensin II and muscarinic stimulation of cultured bovine adrenal chromaffin cells. Biosci Rep. 1989 Apr;9(2):243–252. doi: 10.1007/BF01116001. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi M., Yamagami K., Nishimura S. Tetraethylammonium stimulates adrenomedullary secretion by causing fluctuations in a cytosolic free Ca concentration. Brain Res. 1990 Jan 22;507(2):347–350. doi: 10.1016/0006-8993(90)90296-n. [DOI] [PubMed] [Google Scholar]