Abstract
Background
Conflicting data exist regarding sex-specific outcomes after cardiac arrest. This study investigates sex disparities in the provision of critical care and outcomes of in-hospital (IHCA) and out-of-hospital cardiac arrest (OHCA) patients.
Methods
Analysis of adult cardiac arrest patients admitted to certified Swiss intensive care units (ICUs) (01/2008–12/2022) using the nationwide prospective ICU registry. The primary outcome was ICU mortality, with secondary outcomes including ICU admission probability and advanced treatment provision.
Results
Among 41,733 individuals (34.9% women), 21,692 patients (30.6% women) were admitted to ICUs (16,571 OHCA patients/5121 IHCA patients). Women were less likely to be admitted to the ICU than men (incidence rate ratio 0.82 [95% CI 0.80–0.85] and had a higher ICU mortality (41.8% vs 36.2%; p < 0.001). Mortality differences were more pronounced in OHCA patients (unadjusted HR: 1.35 [95% CI 1.28–1.43]; adjusted HR: 1.19 [95% CI 1.12–1.25]). In IHCA patients, mortality differences were less pronounced (unadjusted HR: 1.14 [95% CI 1.04–1.25]) and vanished after adjustment for confounders: adjusted HR: 1.03 [95% CI 0.94–1.13]). Women after cardiac arrest were older, more severely ill, and received fewer interventions before (44.7% vs 54.0%; p < 0.001) and during ICU stay. A subgroup analysis of 11,202 patients revealed that treatment limitations were more frequent in women (46.7% vs 38.7%; p < 0.001). However, these limitations were associated with an increased risk of death in both sexes.
Conclusions
This study highlights sex disparities in short-term mortality and ICU resource allocation among cardiac arrest patients, with women potentially facing disadvantages, in particular after OHCA. The limitations of ICU registry data, particularly the lack of detailed cardiac arrest-specific and comorbidity information, restrict definitive conclusions. Future research should prioritize prospective studies with more granular data to better understand and address these disparities.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-025-05262-5.
Keywords: Sex differences, Sex disparities, Gender differences, Cardiac arrest, ICU admission rate, Resuscitation, CPR
Introduction
Cardiac arrest is a leading cause of death worldwide despite many advances in cardiopulmonary resuscitation and intensive care [1–3]. Survivors frequently suffer from long-term disabilities and post-intensive care syndrome [4–7]. Sex and gender-related factors may impact out-of-hospital cardiac arrest (OHCA) outcomes, as factors associated with favourable outcomes, such as shockable initial rhythm or provision of bystander cardiopulmonary resuscitation (CPR) and defibrillation, are less frequently observed in women than in men [8–10]. Several recent observational studies provide an increasing body of evidence for worse short and long-term survival rates, neurological status, and quality of life in women successfully resuscitated from cardiac arrest [3, 7, 10–12]. In contrast, two recent systematic reviews with meta-analyses found no sex differences in survival to hospital discharge after adjusting for available confounders. However, these results must be interpreted cautiously due to the significant heterogeneity of included studies indicating potential sociocultural and geographical differences [13, 14]. For in-hospital cardiac arrest (IHCA) patients, data on sex differences in the provision of intensive care resources and survival is scarce [3, 15].
Sex and gender differences in admission to intensive care units (ICUs) and the provision of invasive and non-invasive treatments before and during intensive care (e.g., percutaneous coronary interventions (PCI), targeted temperature management) have been identified as potential mediators of the survival discrepancies observed in cardiac arrest patients [10, 16]. The disparity in outcomes might not be solely related to the provision of treatment but also to how decisions about end-of-life care are made, as women after cardiac arrest are more likely to undergo withdrawal from life-sustaining therapies [17]. Women are also more likely to have treatment limitations in place, such as do-not-resuscitate (DNR) orders, which may reflect different preferences for care or biases in medical decision-making [18]. Most studies regarding sex- and gender differences in cardiac arrest outcomes, provision of intensive care, and end-of-life care were conducted in North America, Asia, Australia, and Northern Europe, with only little data from Western Europe [13]. The present study aims to assess sex-specific differences and temporal trends in ICU mortality, ICU admission rates, critical care, and treatment limitations in short-term cardiac arrest survivors in a large nationwide ICU registry from Western Europe.
Methods
Databases and study population
The study used ICU data from the prospective Swiss ICU registry (MDSi-Minimal Dataset) of the Swiss Society of Intensive Care Medicine (SSICM). The registry constitutes a mandatory continuous data repository, including a minimal dataset for every patient admitted to officially accredited ICUs in Switzerland (81 to 86 ICUs over the 15-years study period). After validation and completeness check, the data is anonymized and incorporated into a centralized database, as outlined previously [19, 20]. Additionally, data on overall hospital admissions of adult patients with cardiac arrest admitted to any Swiss hospital during the study period were requested from the Swiss Federal Statistical Office (FSO). All patients aged ≥ 18 years who were admitted to a Swiss ICU between January 1, 2008, and December 31, 2022, with the primary diagnosis of cardiac arrest according to the MDSi dataset were included. The overall number of patients aged ≥ 18 years with a diagnosis of cardiac arrest admitted to any Swiss hospital was electronically identified and extracted from the FSO dataset. Supplementary Fig. 1 depicts patient selection from both datasets. Details regarding study reporting, assessed variables, and definitions are described in the Supplementary Material.
Outcome measures
The primary outcome was ICU mortality. The incidence of ICU admission after cardiac arrest and the provision of advanced treatments were assessed as key secondary outcome measures. Advanced treatments included interventions before ICU admission and treatments during the ICU stay. Further secondary outcomes included ICU length of stay and discharge destination. ICU mortality and the incidence of ICU admission were additionally assessed over time to gain insights into temporal trends.
Statistical analysis
Descriptive statistics are shown as mean ± Standard Deviation (SD) or median and interquartile range (IQR) for continuous variables, and numbers with proportions for categorical ones. Differences between sexes were assessed using the Chi-square test. Continuous variables were visually checked for normality. A multivariable Cox regression model was used to explore the association of sex differences with ICU mortality, adjusting for confounders such as age, in-hospital cardiac arrest, Nine Equivalents of Nursing Manpower Use Score (NEMS) score, pre-ICU interventions, ICU type, and level of care. ICU treatments (e.g., renal replacement therapy [RRT], invasive and non-invasive respiratory support, and vasoactive drugs were included as covariates to account for their impact on ICU mortality and potential differences in treatment allocation between sexes. Simplified Acute Physiology Score II [SAPS II] was excluded due to multicollinearity. Time to ICU death was the interval between ICU admission and death or discharge. Hazard ratios (HR) with 95% confidence intervals (95% CI) were reported. A second multivariable analysis (Model 2) included patients with information on treatment limitations available since 2016. Poisson regression was applied to estimate ICU admission and mortality trends, reporting incidence rate ratios (IRR) with 95% CIs to compare sexes. As less than 1% of data was missing and assumed random, no imputation was performed. All analyses were performed using Stata MP/18 (StataCorp, 2023).
Results
Baseline characteristics
During the 15-year study period, a total of 41,733 hospital admissions (14,558 [34.9%] women) with a diagnosis of cardiac arrest were identified, of whom 21,692 patients (6,626 [30.6%] women) were admitted to certified Swiss ICUs and included in the final analysis, Supplementary Fig. 1. The mean age was 65.8 ± 14.6) years, with women being older than men (67.0 years vs 65.2 years, p < 0.001). 16,571 (76.4%) patients were classified as OHCA (29.3% women) and 5,121 patients as IHCA (34.7% women). Mean overall illness severity at ICU admission estimated by the SAPS II score was higher in women (62.4 ± 23.1 vs 61.3 ± 23.1). The median overall NEMS was higher in women (103 [IQR 85–140] vs 100 [IQR 83–130]), Table 1. A subgroup analysis of IHCA and OHCA patients revealed higher SAPS II and NEMS scores in female OHCA patients only, Supplementary Table 1. Men were more frequently discharged to other ICUs or intermediate care (33.4% vs 28.9%, p < 0.001), while women were more often discharged to the regular ward (68.6% vs 63.9%, p < 0.001), Table 1.
Table 1.
Baseline characteristics and outcomes stratified by sex category
| Total | Men | Women | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | 21,692 | 15,066 | 6626 | ||||
| Age (years)—Mean (SD) | 65.7 (14.6) | 65.2 (14.2) | 67.0 (15.4) | < 0.001 | |||
| Age categories | |||||||
| Age < 45 | 1793 (8.3%) | 1191 (7.9%) | 602 (9.1%) | ||||
| Age 45–65 | 7889 (36.4%) | 5836 (38.7%) | 2053 (31.0%) | ||||
| Age > 65 | 12,010 (55.4%) | 8039 (53.4%) | 3971 (59.9%) | ||||
| Type of cardiac arrest | |||||||
| In-hospital cardiac arrest | 5121 (23.6%) | 3346 (22.2%) | 1775 (26.8%) | < 0.001 | |||
| Out-of-hospital cardiac arrest | 16,571 (76.4%) | 11,720 (77.8%) | 4851 (73.2%) | ||||
| Admission information | |||||||
| Patient’s pre-hospital origin | 0.22 | ||||||
| From home | 14,983 (69.1%) | 10,398 (69.0%) | 4585 (69.2%) | ||||
| Other hospital | 2903 (13.4%) | 2010 (13.3%) | 893 (13.5%) | ||||
| Nursing home | 43 (0.2%) | 24 (0.2%) | 19 (0.3%) | ||||
| Other | 3763 (17.4%) | 2634 (17.5%) | 1129 (17.0%) | ||||
| Patients’ in-hospital origin | < 0.001 | ||||||
| Emergency room | 9474 (43.7%) | 6503 (43.2%) | 2971 (44.9%) | ||||
| Operating room/post-interventional | 5891 (27.2%) | 4340 (28.8%) | 1551 (23.4%) | ||||
| Other ICU | 1206 (5.6%) | 877 (5.8%) | 329 (5.0%) | ||||
| IMC/PACU | 560 (2.6%) | 369 (2.5%) | 191 (2.9%) | ||||
| Ward | 2959 (13.6%) | 1888 (12.5%) | 1071 (16.2%) | ||||
| Other | 1602 (7.4%) | 1089 (7.2%) | 513 (7.7%) | ||||
| Illness severity and use of ICU resources | |||||||
| SAPS-II—Mean (SD) | 61.63 (23.11) | 61.30 (23.10) | 62.38 (23.11) | 0.001 | |||
| Sum NEMS of all nursing shifts—Median (IQR) | 101 (83–133) | 100 (83–130) | 103 (85–140) | < 0.001 | |||
| NEMS first nursing shift—Median (IQR) | 12.22 (5.78–29.33) | 11.49 (5.52–26.15) | 14.21 (6.48–36.04) | < 0.001 | |||
| NEMS last nursing shift—Median (IQR) | 8.82 (3.56–23.94) | 8.18 (3.30–21.60) | 10.29 (4.21–30.00) | < 0.001 | |||
| SAS > 4 points | 6804 (31.4%) | 5119 (34.0%) | 1685 (25.4%) | < 0.001 | |||
| Interventions and/or surgeries before ICU admission | |||||||
| No intervention | 10,597 (48.9%) | 6933 (46.0%) | 3664 (55.3%) | < 0.001 | |||
| Percutaneous coronary intervention | 6459 (29.8%) | 5126 (34.0%) | 1333 (20.1%) | ||||
| Cardiac surgery/other cardiovascular interventions | 1879 (8.7%) | 1282 (8.5%) | 597 (9.0%) | ||||
| Non-cardiac surgery | 2757 (12.7%) | 1725 (11.5%) | 1032 (15.6%) | ||||
| Treatments during ICU stay | |||||||
| Intravenous medications | 21,423 (98.8%) | 14,888 (98.8%) | 6535 (98.6%) | 0.24 | |||
| Mechanical ventilation | 17,973 (82.9%) | 12,494 (82.9%) | 5479 (82.7%) | 0.67 | |||
| Additional respiratory support | 12,695 (58.5%) | 9052 (60.1%) | 3643 (55.0%) | < 0.001 | |||
| 1 Vasoactive medication | 15,340 (70.9%) | 10,759 (71.6%) | 4581 (69.3%) | < 0.001 | |||
| > 1 Vasoactive medication | 8234 (38.1%) | 5849 (38.9%) | 2385 (36.1%) | < 0.001 | |||
| Renal replacement therapy | 1666 (7.7%) | 1213 (8.1%) | 453 (6.8%) | 0.002 | |||
| Advanced Intervention in the ICU | 11,227 (51.8%) | 7910 (52.5%) | 3317 (50.1%) | < 0.001 | |||
| Advanced Intervention outside the ICU | 10,044 (46.5%) | 7129 (47.5%) | 2915 (44.3%) | < 0.001 | |||
| ICU type | |||||||
| Medical ICU | 3191 (14.7%) | 2317 (15.4%) | 874 (13.2%) | < 0.001 | |||
| Surgical ICU | 1116 (5.1%) | 749 (5.0%) | 367 (5.5%) | ||||
| Interdisciplinary ICU | 17,197 (79.3%) | 11,849 (78.7%) | 5348 (80.7%) | ||||
| Other | 188 (0.9%) | 151 (1.0%) | 37 (0.6%) | ||||
| Level of care | |||||||
| Tertiary university hospital | 8371 (38.6%) | 6000 (39.8%) | 2371 (35.8%) | < 0.001 | |||
| Large hospital | 5222 (24.1%) | 3717 (24.7%) | 1505 (22.7%) | ||||
| Regional hospital/no categorization | 8099 (37.3%) | 5349 (35.5%) | 2750 (41.5%) | ||||
| Discharge information and outcomes | |||||||
| Patient’s discharge destination (patients discharged alive) | |||||||
| Ward | 8790 (65.29%) | 6145 (63.94%) | 2645 (68.63%) | < 0.001 | |||
| Other ICU or IMC | 4320 (32.09%) | 3,208 (33.38%) | 1112 (28.85%) | ||||
| Discharge to rehabilitation or home | 105 (0.78%) | 79 (0.82%) | 26 (0.67%) | ||||
| Other | 249 (1.85%) | 178 (1.85%) | 71 (1.84%) | ||||
| ICU length of stay—Median (IQR) | 2 (1–5) | 3 (1–6) | 2 (1–5) | < 0.001 | |||
| ICU mortality | 8228 (37.9%) | 5456 (36.2%) | 2772 (41.8%) | < 0.001 | |||
Descriptive statistics are presented using mean and standard deviation (SD) or median and interquartile range (IQR: 25th–75th percentile) for continuous variables and numbers and proportions for categorical variables
ICU Intensive care unit, IMC Intermediate care unit, IQR Interquartile range, NEMS Nine equivalents of nursing manpower use score, PACU Post-anesthesia care unit SAS Sedation-agitation scale, SAPS-II Simplified Acute Physiology Score II, SD Standard deviation
Primary outcome—ICU mortality
Sex differences and outcomes (Model I)—overall
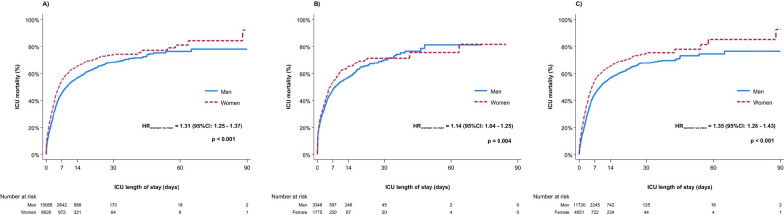
Overall, ICU death was reported in 8,228 (37.9%) of 21,692 patients and was higher in women compared to men (41.8% vs 36.2%, p < 0.001), Table 1. Women experienced a higher ICU mortality as early as day 1 after ICU admission (ICU mortality day 1 men 14.1% [95% CI 13.6–14.7] vs. women 18.9% [17.9–19.9]), Supplementary Table 2. Survival analysis revealed an increased hazard for ICU death in women compared to men (unadjusted HR 1.31 [95% CI 1.25–1.37]) across the ICU stay, which was already evident in the first seven days after ICU admission (unadjusted HR 1.33 [95% CI 1.26–1.39]), Figs. 1 and 2, Supplementary Table 2. When estimating the incidence rate ratio (IRR) stratified for age and sex, women aged 50–69 years had a higher incidence rate for ICU death (IRR between 1.15 and 1.42) compared to men. In contrast, no sex differences were observed in the age groups < 50 years and ≥ 70 years, Fig. 4, Supplementary Table 3.
Fig. 1.
Kaplan–Meier estimate of ICU mortality until 90 days after ICU admission. A Overall, B In-hospital cardiac arrest, C Out-of-hospital cardiac arrest. Abbreviations: CI Confidence interval; HR Hazard ratio; ICU Intensive care unit
Fig. 2.
Kaplan–Meier estimate of ICU mortality during the first 7 days after ICU admission. A Overall, B In-hospital cardiac arrest, C Out-of-hospital cardiac arrest. Abbreviations: CI Confidence interval; HR Hazard ratio; ICU Intensive care unit
Fig. 4.
Incidence rate ratios (IRR) of ICU admission and ICU death in women and men across age groups and overall number of cardiac arrest admissions. Abbreviations: ICU Intensive Care Unit
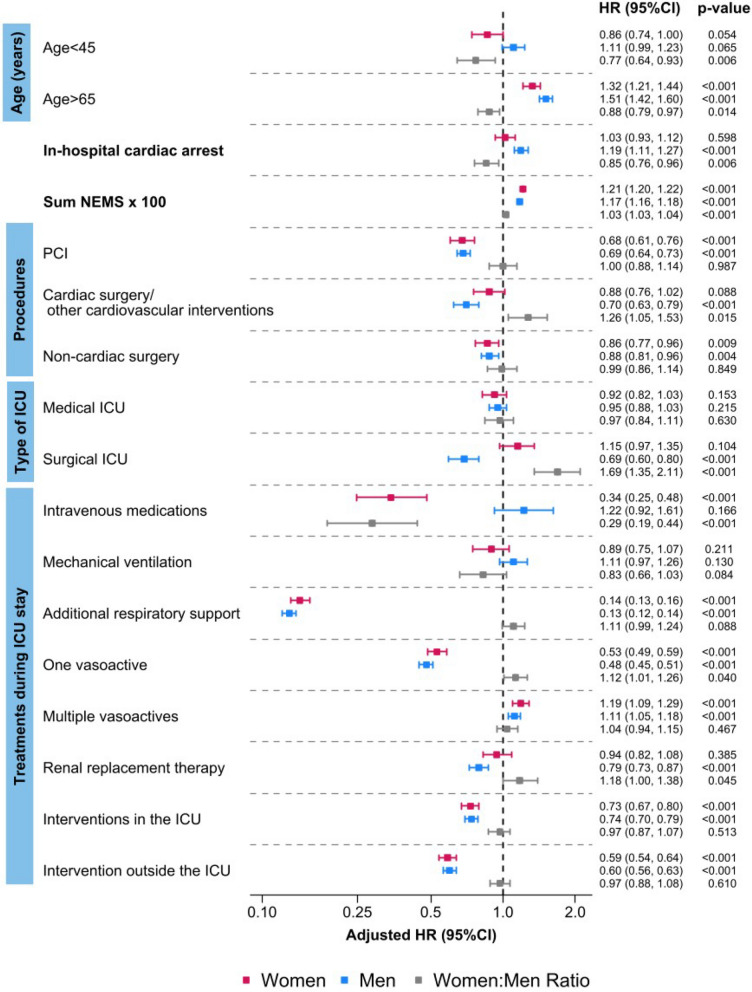
In the adjusted multivariable model, women exhibited a consistently higher risk of ICU death compared to men (adjusted HR [aHR] 1.14 [95% CI 1.09–1.20]), Supplementary Table 4. IHCA, in comparison to OHCA, was linked to an increased mortality risk only in men (aHR 1.19 [95% CI 1.11–1.27]). Age > 65 years was associated with an increased risk of dying in both sexes, which was more pronounced in men (ratio women:men aHR 0.88 [95% CI 0.79–0.97]), Fig. 3, Supplementary Table 4. All registered interventions before ICU admission were associated with a lower risk of ICU death in men and women, except for cardiac surgery/other cardiovascular interventions, which was not associated with a risk reduction for women (aHR 0.88 [95% CI 0.76–1.02], men: aHR 0.70 [95% CI 0.63–0.79]), Fig. 3, Supplementary Table 4.
Fig. 3.
Sex differences in ICU mortality risk from multivariable analysis. Abbreviations: HR Hazard ratio; ICU Intensive care unit; NEMS Nine equivalents of Nursing Manpower Use Score; PCI Percutaneous coronary intervention
Primary outcomes and sex differences stratified by in-hospital and out-of-hospital cardiac arrest
ICU death was reported in 2,063 of 5,121 (40.3%) IHCA patients and in 6,165 of 16,571 (37.2%) OHCA patients, Supplementary Table 1. In both groups, survival analysis revealed an increased hazard for ICU death in women compared to men across the ICU stay, which was more pronounced in OHCA patients: IHCA (unadjusted HR 1.14 [95% CI 1.04–1.25]); OHCA (unadjusted HR 1.35 [95% CI 1.28–1.43]), Fig. 1B, C.
The increased hazard for ICU death in women was already evident in the first seven days after admission and more prominent in OHCA patients: IHCA (unadjusted HR 1.15 (95% CI 1.04–1.26); OHCA (unadjusted HR 1.38 (95% CI 1.30–1.46), Fig. 2B, C. Along with that, women experienced a higher mortality at all time points in OHCA. Conversely, in IHCA patients, a significantly higher ICU mortality in women during the first 7 days was only observed on days 5 and 7, Supplementary Table 2.
In the multivariable analysis, female compared to male sex was linked to a higher risk of ICU death only in OHCA patients (aHR 1.19 [95% CI 1.12–1.25], Supplementary Table 5.
PCI was associated with a mortality risk reduction in both sexes, while cardiac surgery was connected to a lower risk of ICU death only in men independent of cardiac arrest type: IHCA (aHR 0.64 [95% CI 0.50–0.82]); OHCA (aHR 0.81 [95% CI 0.71–0.91]). Non-cardiac procedures were associated with a risk reduction in women with IHCA, while only men experienced a risk reduction after OHCA. Please refer to the Supplementary material (Supplementary Figs. 2 & 3, Supplementary Table 5) for further details.
Primary outcome and sex differences in the subgroup of patients with treatment limitations (model II)
We performed multivariable analysis (Model II) in a subgroup of 11,202 patients (30.4% women) with information regarding treatment limitations. Women had significantly more treatment limitations (46.7% vs 38.7%, p < 0.001), but the risk of dying did not differ between sexes. More details are available in the Supplementary material (Supplementary Results, Supplementary Fig. 4, Supplementary Tables 6 & 7).
Secondary outcomes
Sex differences in ICU admission rates
The incidence of ICU admission in patients after cardiac arrest was higher in men than in women, with women being 18% less likely to be admitted to ICUs compared to men (IRR 0.82 (95% CI 0.80–0.85), Figs. 4 and 5, Supplementary Table 8. Regarding age distribution, ICU admission incidence rates were higher in men ≥ 45 years compared to women of the same age (45–65 years [IRR = 1.12, 95% CI 1.06–1.18] and above 65 years [IRR = 1.24, 95% CI 1.19–1.29)], Supplementary Table 8 and Fig. 4. Further detailed information regarding sex differences and temporal trends of ICU admission rates and mortality is provided in the Supplementary results and Supplementary Table 8.
Fig. 5.
Temporal trend of incidence rate ratios (IRR) of ICU admission and ICU death in women and men; overall number of cardiac arrest admissions over time. Abbreviations: COVID-19 Coronavirus disease—2019; ICU Intensive Care Unit
Sex differences in treatments before and during ICU stay
Interventions before ICU admission were less frequently performed in women than in men (44.7% vs 54.0%, p < 0.001). PCI was more common in men, while women more often underwent rescue cardiac surgery/other cardiovascular procedures (except PCI), and non-cardiac surgery. This pattern was also observed in the IHCA and OHCA subgroups, Table 1 and Supplementary Table 1.
During the ICU stay, mechanical ventilation (MV) was equally provided to both sexes, but men more frequently received non-invasive respiratory support, vasoactives, RRT, and advanced interventions, Table 1. The sex differences were more evident in OHCA patients (OHCA: all treatments except MV and intravenous medication more frequent in men; IHCA: only MV, additional respiratory support, and RRT more frequent in men), Supplementary Table 1. Further detailed information regarding treatments is provided in Supplementary Table 1, Fig. 3, Supplementary Fig. 3 & 4.
Sex differences in ICU length of stay
Median ICU LOS was shorter in women (2 days [IQR 1–5] vs 3 days [IQR 1–6], p < 0.001) and was shorter in ICU non-survivors (2 days [IQR 0–4] vs 3 days [IQR 1–6], p < 0.001). Women had a significantly shorter ICU LOS regardless of survival status: ICU survivors (women 3 days [IQR 1–6] vs men 3 days [IQR 1–7], p < 0.001) and ICU non-survivors (women 1 day [IQR 0–3] vs men 2 days [IQR 0–4], p < 0.001). These observations remained constant in the subgroup analysis of OHCA and IHCA patients, except for IHCA non-survivors, where ICU LOS did not differ between men and women (p = 0.21), Supplementary Table 9.
Discussion
This nationwide study, including 41,733 hospitalized individuals (21,692 ICU patients), identified important sex differences in ICU mortality rates, ICU admission rates, and provision of advanced treatments after successfully resuscitated in- and out-of-hospital cardiac arrest. Women were less frequently admitted to ICUs, received fewer advanced treatments, and had a higher risk of ICU mortality when compared to men. When analyzing subgroups of OHCA and IHCA patients, mortality differences were particularly pronounced in OHCA patients. In addition, women after cardiac arrest showed significantly shorter median ICU stays independent from ICU survival. When stratified for age and sex, women died more often in the age groups 50–69 years. Treatment limitations were more frequent in women and were associated with an increased risk of ICU mortality in both sexes.
In this study, women had higher ICU mortality than men, consistent with lower survival rates in women across most studies, despite a higher likelihood of achieving return of spontaneous circulation [9, 12]. As observed in our study, women tend to be older and suffer from more comorbidities at the time of cardiac arrest, which may partly explain their greater illness severity at ICU admission and the higher risk of ICU death [21]. Interestingly, the higher illness severity at ICU admission in women was only observed in the subgroup of OHCA patients. Although our study lacked detailed information on comorbidities, prior studies have shown greater disease severity in women with cardiovascular conditions [21, 22]. The higher incidence of ICU death in women aged 50–69 compared to age-matched men may be linked to the biological effects of the perimenopausal period [23], as studies suggest that declining estrogen levels increase the risk of cardiovascular (CV) disease, ischemic heart disease, and cardiac arrest in women [24, 25]. However, these sex differences in CV outcomes seem to diminish in older age groups. Evidence indicates that while menopause accelerates CV risk factors in women, chronological aging may dominate over hormonal effects in the elderly [26].
In our study, in-hospital cardiac arrest (IHCA) was more common in women, while OHCA was more frequent in men. Women experienced a higher risk of ICU mortality overall, with a more pronounced difference in OHCA patients compared to IHCA patients. Along with that, adjusted hazard ratios demonstrated that female sex was independently associated with a higher risk of ICU death in OHCA but not in IHCA. These findings align with previous studies showing that women are less likely to receive bystander CPR and experience delays in resuscitation during OHCA, which may contribute to their higher ICU mortality risk and illness severity observed in our study [8–10]. In contrast, IHCA occurs in monitored hospital environments, where standardized protocols and rapid response systems might mitigate pre-resuscitation differences [15, 27].
While sex differences in pre-ICU and ICU care may have contributed to the higher mortality observed in women, early withdrawal of life-sustaining therapy (WLST) could also play a role, particularly in OHCA patients, where women consistently showed higher mortality rates from the first day of ICU admission onwards. The shorter ICU LOS observed in female OHCA non-survivors compared to male OHCA non-survivors may suggest earlier withdrawal of life-sustaining therapy (WLST) in women. Current guidelines recommend postponing prognostication and WLST for at least 72 h after cardiac arrest [28]. However, previous studies have highlighted that unjustified early WLST after cardiac arrest is common, with women being particularly at risk [12, 29]. In contrast, the absence of significant LOS differences in IHCA non-survivors and a more equalized risk of ICU mortality between sexes suggests a more standardized ICU care in this subgroup, potentially minimizing sex-related variability. While early WLST cannot be entirely excluded as a contributing factor to the higher early ICU mortality observed in women, the shorter ICU LOS in female survivors indicates that factors beyond early WLST, such as differences in ICU care pathways or treatment escalation decisions, may influence outcomes, particularly in OHCA patients. Indeed, sex differences in the provision of ICU treatments were more pronounced in the OHCA subgroup. Given the limitations in WLST timing granularity within our dataset, we caution against the overinterpretation of these findings and emphasize the need for prospective studies with detailed WLST timing and decision-making data to validate our observations.
Pre-ICU interventions like acute PCI or rescue cardiac surgery improve outcomes after cardiac arrest. However, important sex differences in pre-ICU interventions were observed in our study. Women with cardiovascular conditions, including acute myocardial infarction (AMI) and cardiogenic shock, often receive less aggressive treatments than men, leading to poorer outcomes [22, 30, 31]. This is mirrored by our study, revealing that women less frequently received interventions (incl. PCI) before ICU admission, independent of cardiac arrest type, while rescue cardiac surgery was more frequently performed in women. PCI and non-cardiac surgery were associated with a reduced ICU death risk in both sexes. In contrast, cardiac surgery/other cardiovascular interventions were only associated with a reduced ICU death risk in men. A more severe disease state at presentation in women may result in worse conditions requiring emergent surgical revascularization. Misperception of symptoms, under- or misdiagnosed cardiovascular illness, and sociocultural factors, such as women’s entrenchment in traditional gender roles and delayed healthcare-seeking behavior, have been linked to more advanced cardiovascular illness in women at presentation [32]. Additionally, older women may experience fewer witnessed OHCAs due to reduced social support or living alone, leading to a lower likelihood of receiving bystander CPR [33]. Delayed CPR and less frequent shockable initial rhythms are well-established mediators of higher rates of multiorgan failure and worse outcomes [9, 34]. These factors likely contribute to the higher mortality rate observed in female OHCA patients.
Most ICU treatments were more frequently applied to men, consistent with previous research [34]. While most organ support measures were associated with decreased mortality risk in both sexes, RRT was only associated with a lower mortality risk in men, independent of cardiac arrest entity. The more frequent use of RRT in men may stem from an underestimation of acute kidney injury (AKI) in women, leading to delayed or even withheld RRT [20]. In addition, biological factors such as a decline of estrogen and its renoprotective effects may impact AKI and outcomes in elderly women [36].
We found that women had an 18% lower likelihood of ICU admission compared to men, particularly in those aged over 55 years, a trend consistent over the 15-year study period. This aligns with prior studies showing lower ICU admission rates for women, especially in cardiovascular cases [22, 31, 37]. Female sex and older age have been linked to life-sustaining treatment limitations, potentially influencing ICU resource allocation if clinicians assume that women might prefer less aggressive care [12, 39]. Although we were unable to assess how treatment limitations impacted decision-making before ICU admission, our data revealed that women had a higher prevalence of treatment limitations upon ICU admission, more withdrawal of life-sustaining therapy, and more decisions taken by the medical caregivers during ICU stay. Despite this, the increased hazard of ICU death in patients with treatment limitations was similar between sexes, a finding consistent with previous data [12]. Therefore, we propose that unaccounted factors, including unconscious biases related to race, ethnicity, ability, age, and sex, may influence ICU admission and treatment decisions in a discriminatory manner, potentially leading to limited or even denied care [39].
Interestingly, we observed a significant decline in ICU admissions for cardiac arrest patients across both sexes during the COVID-19 pandemic (2020–2022), with the reduction being more pronounced in men. However, ICU mortality remained higher in women. During COVID-19, scarcity of critical care resources and prognosis-based triage may have led to limited ICU provision in cardiac arrest patients, which might also have reduced the over-admission of men compared to women [40]. In addition, increased healthcare strain and lockdowns were linked to fewer bystander-witnessed OHCAs and excess deaths at home or delayed hospital admissions during the pandemic [40, 41].
Limitations and strengths
There are important limitations to consider. First, the FSO and the MDSi datasets comprise limited variables and lack detailed information on patient demographics, comorbidities, cardiovascular risk factors, cardiac arrest-specific information, and sociocultural factors. Second, the definition of OHCA versus IHCA was solely based on the origin of the patients before ICU admission, with a risk of misclassification for some patients. However, considering the large sample size, it is unlikely to have changed our findings. Third, data on decision-making during pre-hospital, pre-ICU, and ICU care were limited or unavailable. Specifically, the lack of WLST timing granularity may have influenced our results. Fourth, due to the limitations of the MDSi dataset, we were unable to include information regarding witnessed/unwitnessed cardiac arrest, no-flow time, low-flow time, provision of bystander CPR, initial rhythm of cardiac arrest, detailed cardiac arrest etiology, provision of eCPR and provision of targeted temperature management into our analysis. Lastly, despite our large nationwide dataset, the restriction of our data to Switzerland and a mainly Caucasian population may limit the generalizability of our results.
The study has notable strengths. The database includes all patients from certified Swiss ICUs since 2008, offering a high internal and external validity with a large sample size and minimal missing data (< 1%). Additionally, detailed information on treatment limitations provides valuable insights into sex-specific ICU outcomes.
Conclusions
This nationwide study highlights sex disparities in short-term mortality and ICU resource allocation among cardiac arrest patients, with women potentially facing disadvantages, in particular after OHCA. While our findings suggest multifactorial causes, including biological differences, sociocultural factors, and potential biases, the limitations of ICU registry data, particularly the lack of detailed cardiac arrest-specific and comorbidity information, restrict definitive conclusions.
Future prospective studies are essential to investigate these dynamics further, supported by enhanced administrative databases that enable detailed analyses of sex- and gender-related differences in post-cardiac arrest care.
Supplementary Information
Acknowledgements
During the preparation of this work, the authors used the grammar check Software Grammarly (Grammarly Inc., San Francisco, USA) to proofread and increase readability and the large language model ChatGPT-4o (OpenAI, San Francisco, USA) to paraphrase and summarize some of the manuscript’s content. In addition, the large language model OpenEvidence (Cambridge, USA) to identify additional relevant literature in the field. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the publication's content. We thank Prof. Mark Kaufmann for providing expert advice about the data structure and for critically reviewing the manuscript. The authors thank the Federal Statistics Office for providing the hospital admission data and supporting data interpretation. They also thank the Swiss Society of Intensive Care Medicine for generously providing the MDSi—Minimal Dataset for Intensive Care Units, which formed the main body of the current analysis.
Author contributions
Simon A. Amacher and Caroline E. Gebhard planned and designed the study. Pimrapat Gebert performed the formal statistical analysis of the data provided by the Swiss Society of Intensive Care Medicine and the Federal Statistics Office. Simon A. Amacher, Pimrapat Gebert, and Caroline E. Gebhard interpreted the data. Caroline E. Gebhard and Simon A. Amacher wrote the inaugural draft of the manuscript. Simon A. Amacher, Tobias Zimmermann, Pimrapat Gebert, Pascale Grzonka, Sebastian Berger, Martin Lohri, Valentina Tröster, Ketina Arslani, Hamid Merdji, Catherine Gebhard, Sabina Hunziker, Raoul Sutter, Martin Siegemund and Caroline E. Gebhard interpreted the data, revised the final manuscript and substantially contributed to the inaugural draft. All authors approved the final submitted version of the manuscript.
Funding
Open access funding provided by University of Basel. None.
Availability of data and materials
This study is based on the MDSi dataset by the Swiss Society of Intensive Care Medicine (SSICM) and the Swiss Federal Statistical Office (FSO). Restrictions apply to the availability of this data and any requests must be made to the scientific committee of the SSICM (https://www.sgi-ssmi.ch) or/and the FSO (https://www.bfs.admin.ch/bfs/en/home.html).
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Northwestern and Central Switzerland (EKNZ UBE-15/47) and the scientific committee of the SSICM. It was carried out according to the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gräsner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, Bein B, Böttiger BW, Rosell-Ortiz F, Nolan JP, et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61–79. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerkeman M, Sultanian P, Lundgren P, Nielsen N, Helleryd E, Dworeck C, Omerovic E, Nordberg P, Rosengren A, Hollenberg J, et al. Trends in survival after cardiac arrest: a Swedish nationwide study over 30 years. Eur Heart J. 2022;43(46):4817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peskine A, Cariou A, Hajage D, Deye N, Guérot E, Dres M, Sonneville R, Lafourcade A, Navarro V, Robert H, et al. Long-term disabilities of survivors of out-of-hospital cardiac arrest: the Hanox study. Chest. 2021;159(2):699–711. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Beck K, Thommen E, Widmer M, Becker C, Loretz N, Gross S, Mueller J, Amacher SA, Bohren C, et al. Post-intensive care syndrome in out-of-hospital cardiac arrest patients: a prospective observational cohort study. PLoS ONE. 2022;17(10):e0276011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaow CYL, Teoh SE, Lim WS, Wang RSQ, Han MX, Pek PP, Tan BY, Ong MEH, Ng QX, Ho AFW. Prevalence of anxiety, depression, and post-traumatic stress disorder after cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2022;170:82–91. [DOI] [PubMed] [Google Scholar]
- 7.Amacher SA, Sahmer C, Becker C, Gross S, Arpagaus A, Urben T, Tisljar K, Emsden C, Sutter R, Marsch S, et al. Post-intensive care syndrome and health-related quality of life in long-term survivors of cardiac arrest: a prospective cohort study. Sci Rep. 2024;14(1):10533. 10.1038/s41598-024-61146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson V, Dankiewicz J, Nielsen N, Kern KB, Mooney MR, Riker RR, Rubertsson S, Seder DB, Stammet P, Sunde K, et al. Association of gender to outcome after out-of-hospital cardiac arrest–a report from the international cardiac arrest registry. Crit Care. 2015;19(1):182. 10.1186/s13054-015-0904-y.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom MT, Oving I, Berdowski J, van Valkengoed IGM, Bardai A, Tan HL. Women have lower chances than men to be resuscitated and survive out-of-hospital cardiac arrest. Eur Heart J. 2019;40(47):3824–34. 10.1093/eurheartj/ehz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böckler B, Preisner A, Bathe J, Rauch S, Ristau P, Wnent J, Gräsner JT, Seewald S, Lefering R, Fischer M. Gender-related differences in adults concerning frequency, survival and treatment quality after out-of-hospital cardiac arrest (OHCA): An observational cohort study from the German resuscitation registry. Resuscitation. 2024;194:110060. [DOI] [PubMed] [Google Scholar]
- 11.Morrison LJ, Schmicker RH, Weisfeldt ML, Bigham BL, Berg RA, Topjian AA, Abramson BL, Atkins DL, Egan D, Sopko G, et al. Effect of gender on outcome of out of hospital cardiac arrest in the Resuscitation Outcomes Consortium. Resuscitation. 2016;100:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mody P, Pandey A, Slutsky AS, Segar MW, Kiss A, Dorian P, Parsons J, Scales DC, Rac VE, Cheskes S, et al. Gender-based differences in outcomes among resuscitated patients with out-of-hospital cardiac arrest. Circulation. 2021;143(7):641–9. 10.1161/CIRCULATIONAHA.120.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakbar I, Ippolito M, Nassiri A, Delamarre L, Tadger P, Leone M, Einav S. Sex and out-of-hospital cardiac arrest survival: a systematic review. Ann Intens Care. 2022;12(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bijman LAE, Alotaibi R, Jackson CA, Clegg G, Halbesma N. Association between sex and survival after out-of-hospital cardiac arrest: a systematic review and meta-analysis. J Am Coll Emerg Phys Open. 2023;4(3):e12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernando SM, Tran A, Cheng W, Rochwerg B, Taljaard M, Vaillancourt C, Rowan KM, Harrison DA, Nolan JP, Kyeremanteng K, et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ. 2019;367:l6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng D, Li C, Yang X, Wang L. Gender differences and survival after an out-of-hospital cardiac arrest: a systematic review and meta-analysis. Intern Emerg Med. 2021;16(3):765–75. 10.1007/s11739-020-02552-4.pdf. [DOI] [PubMed] [Google Scholar]
- 17.Vogelsong MA, May T, Agarwal S, Cronberg T, Dankiewicz J, Dupont A, Friberg H, Hand R, McPherson J, Mlynash M, et al. Influence of sex on survival, neurologic outcomes, and neurodiagnostic testing after out-of-hospital cardiac arrest. Resuscitation. 2021;167:66–75. [DOI] [PubMed] [Google Scholar]
- 18.Perman SM, Siry BJ, Ginde AA, Grossestreuer AV, Abella BS, Daugherty SL, Havranek EP. Sex differences in do not attempt resuscitation” orders after out-of-hospital cardiac arrest and the relationship to critical hospital interventions. Clin Ther. 2019;41(6):1029–37. 10.1016/j.clinthera.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perren A, Cerutti B, Kaufmann M, Rothen HU, Swiss Society of Intensive Care M: A novel method to assess data quality in large medical registries and databases. Int J Qual Health Care 2019.https://www.ncbi.nlm.nih.gov/pubmed/30608577 [DOI] [PubMed]
- 20.Zimmermann T, Kaufmann P, Amacher SA, Sutter R, Loosen G, Merdji H, Helms J, Todorov A, Gebert P, Regitz-Zagrosek V, et al. Sex differences in the SOFA score of ICU patients with sepsis or septic shock: a nationwide analysis. Crit Care. 2024;28(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith E, Tamis-Holland JE. Sex differences in the presentation and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock: a critical review of contemporary data and a look towards future directions. Curr Opin Crit Care. 2024;30(4):344–53. [DOI] [PubMed] [Google Scholar]
- 22.Todorov A, Kaufmann F, Arslani K, Haider A, Bengs S, Goliasch G, Zellweger N, Tontsch J, Sutter R, Buddeberg B et al: Gender differences in the provision of intensive care: a Bayesian approach. Intens Care Med 2021.https://www.ncbi.nlm.nih.gov/pubmed/33884452 [DOI] [PMC free article] [PubMed]
- 23.Dratva J, Gómez Real F, Schindler C, Ackermann-Liebrich U, Gerbase MW, Probst-Hensch NM, Svanes C, Omenaas ER, Neukirch F, Wjst M, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause. 2009;16(2):385–94. [DOI] [PubMed] [Google Scholar]
- 24.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation. 2020;142(25):e506–32. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS, Mieres JH. Sex differences in ischemic heart disease. Circul Cardiovasc Qual Outcomes. 2018;11(2):e004437. 10.1161/CIRCOUTCOMES.117.004437. [DOI] [PubMed] [Google Scholar]
- 26.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation. 2020;142(25):e506–32. 10.1161/CIR.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 27.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA. 2019;321(12):1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, et al. European resuscitation council and European society of intensive care medicine guidelines 2021: post-resuscitation care. Intens Care Med. 2021;47(4):369–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale CM, Sinuff T, Morrison LJ, Golan E, Scales DC. Understanding early decisions to withdraw life-sustaining therapy in cardiac arrest survivors: a qualitative investigation. Ann Am Thorac Soc. 2016;13(7):1115–22. [DOI] [PubMed] [Google Scholar]
- 30.Rob D, Kavalkova P, Smalcova J, Franek O, Smid O, Komarek A, Pisinger M, Belohlavek J. Gender differences and survival after out of hospital cardiac arrest. Am J Emerg Med. 2022;55:27–31. [DOI] [PubMed] [Google Scholar]
- 31.Arslani K, Tontsch J, Todorov A, Gysi B, Kaufmann M, Kaufmann F, Hollinger A, Wildi K, Merdji H, Helms J, et al. Temporal trends in mortality and provision of intensive care in younger women and men with acute myocardial infarction or stroke. Crit Care. 2023;27(1):14. 10.1186/s13054-022-04299-0.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regitz-Zagrosek V, Gebhard C. Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol. 2023;20(4):236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blewer AL, Starks MA, Malta-Hansen C, Sasson C, Ong MEH, Al-Araji R, McNally BF, Viera AJ. Sex differences in receipt of bystander cardiopulmonary resuscitation considering neighborhood racial and ethnic composition. J Am Heart Assoc. 2024;13(5):e031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei H, Hu J, Liu L, Xu D. Sex differences in survival after out-of-hospital cardiac arrest: a meta-analysis. Crit Care. 2020;24(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modra LJ, Higgins AM, Abeygunawardana VS, Vithanage RN, Bailey MJ, Bellomo R. Sex differences in treatment of adult intensive care patients: a systematic review and meta-analys. Crit Care Med. 2022;50(6):913–23. [DOI] [PubMed] [Google Scholar]
- 36.Darvishzadeh Mahani F, Khaksari M, Raji-amirhasani A. Renoprotective effects of estrogen on acute kidney injury: the role of SIRT1. Int Urol Nephrol. 2021;53(11):2299–310. 10.1007/s11255-020-02761-y. [DOI] [PubMed] [Google Scholar]
- 37.Modra L, Pilcher D, Bailey M, Bellomo R. Sex differences in intensive care unit admissions in Australia and New Zealand. Crit Care Resuscit. 2021;23(1):86–93. 10.3316/informit.693854602339304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris NA, Mazzeffi M, McArdle P, May TL, Burke JF, Bradley SM, Agarwal S, Badjatia N, Perman SM. Women receive less targeted temperature management than men following out-of-hospital cardiac arrest due to early care limitations—a study from the CARES Investigators. Resuscitation. 2021;169:97–104. [DOI] [PubMed] [Google Scholar]
- 39.FitzGerald C, Hurst S. : Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ennis JS, Riggan KA, Nguyen NV, Kramer DB, Smith AK, Sulmasy DP, Tilburt JC, Wolf SM, DeMartino ES. Triage procedures for critical care resource allocation during Scarcity. JAMA Netw Open. 2023;6(8):e2329688–e2329688. 10.1001/jamanetworkopen.2023.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scquizzato T, Landoni G, Paoli A, Lembo R, Fominskiy E, Kuzovlev A, Likhvantsev V, Zangrillo A. : Effects of COVID-19 pandemic on out-of-hospital cardiac arrests: a systematic revi. Resuscitation. 2020;157:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marijon E, Karam N, Jost D, Perrot D, Frattini B, Derkenne C, Sharifzadehgan A, Waldmann V, Beganton F, Narayanan K, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health. 2020;5(8):e437–43. 10.1016/S2468-2667(20)30117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is based on the MDSi dataset by the Swiss Society of Intensive Care Medicine (SSICM) and the Swiss Federal Statistical Office (FSO). Restrictions apply to the availability of this data and any requests must be made to the scientific committee of the SSICM (https://www.sgi-ssmi.ch) or/and the FSO (https://www.bfs.admin.ch/bfs/en/home.html).