Abstract
Objectives
To investigate the impact of COVID-19 pandemic measures on hospitalizations and the alterations and persistence of the epidemiological patterns of 12 common respiratory pathogens in children during the COVID-19 pandemic and after the cessation of the “zero-COVID-19” policy in southern China.
Methods
Respiratory specimens were collected from hospitalized children with acute respiratory infections at Shenzhen Children’s Hospital from January 2020 to June 2024. Twelve common respiratory pathogens were detected using multiplex PCR. Data on demographic characteristics, pathogen detection rates, epidemiological patterns, co-infections, and ICU admission rates were compared between the ‘during COVID-19’ period (Phase 1: January 2020 to December 2022) and the ‘post COVID-19’ period (Phase 2: January 2023 to June 2024).
Results
In Phase 2, there was a significant increase in average annual cases, with a higher median age of affected children, higher pathogen detection rates, and increased co-infection rates compared to Phase 1. The epidemiological patterns of most pathogens were altered by the COVID-19 pandemic. Human Parainfluenza Virus, Human Metapneumovirus, Human Bocavirus (HBOV), and Human Coronavirus remained active during Phase 1, while Mycoplasma pneumoniae (Mp) and Adenovirus (ADV) were low, and Respiratory Syncytial Virus (RSV) lacked a seasonal peak in 2022. In Phase 2, Mp, ADV, and RSV experienced outbreaks, with Mp’s high prevalence continuing into 2024. RSV showed out-of-season epidemics for two consecutive years. Influenza A (H1N1), Influenza A (H3N2), and InfB lost their seasonal patterns during Phase 1 but reemerged and regained their seasonal characteristics in 2023–2024. ICU admission rates did not significantly differ between the two phases, except for HBOV, which had higher rates in Phase 2.
Conclusion
The epidemiological patterns of various respiratory pathogens were affected by the COVID-19 pandemic to varying degrees. Pathogens suppressed during the pandemic experienced outbreaks or out-of-season epidemics after the lifting of non-pharmaceutical interventions, with Mp and RSV continuing into the second year and HBOV associated ICU admission rates increasing in the post-pandemic era. Continuous monitoring of these patterns is essential to understand the duration of these effects and to inform effective response strategies.
Keywords: COVID-19, Non-pharmaceutical interventions, Pathogens, Epidemiology, Children, Acute respiratory infection
Background
Respiratory infections are major cause of pediatric hospitalization and pose a significant threat to global child health. Respiratory viruses and Mycoplasma pneumoniae (Mp) are particularly prevalent in children with community-acquired pneumonia (CAP), frequently leading to severe illness and, in some cases, mortality [1]. The COVID-19 pandemic has presented unprecedented challenges to global public health systems and has concurrently reshaped the epidemiological patterns of other respiratory pathogens [2].
During the pandemic, a suite of non-pharmaceutical interventions (NPI) measures were implemented in China and globally to mitigate the spread of SARS-CoV-2. These strategies, including mask-wearing, hand hygiene, social distancing, and lockdowns, profoundly influenced not only the trajectory of COVID-19 but also, serendipitously, the prevalence of other respiratory pathogens [3–5]. This broad-spectrum reduction in pathogen transmission has been associated with the emergence of “immunity debt,” a concept describing the diminished protective immunity due to prolonged reduced exposure to pathogens, thereby increasing the vulnerability of the population to a range of diseases [6]. The relaxation of NPI measures has been correlated with observed resurgences and the occurrence of out-of-season or unusually severe respiratory infections, as documented in recent literature [4, 7–9]. The altered epidemiological patterns of multiple respiratory pathogens in the post-pandemic era and their ongoing impact have garnered significant attention, underscoring the importance of sustained surveillance.
Shenzhen, situated in the southern part of China, has a population exceeding seventeen million. The Shenzhen Children’s Hospital is the region’s only pediatric medical facility. Since late 2019, multiplex polymerase chain reaction (PCR) testing for 12 respiratory pathogens has been conducted on all children admitted with acute respiratory infections (ARI), yielding a robust dataset encompassing these pathogens. Previous research conducted by our department by January 2022 has documented that the stringent lockdown measures instituted during the COVID-19 pandemic had a profound impact on the epidemiology of respiratory pathogens, with a notable decrease in the incidence of MP, influenza A, and adenovirus (ADV) infections. Conversely, infections with respiratory syncytial virus (RSV) and human parainfluenza virus (HPIV) increased [10, 11]. The COVID-19 pandemic lasted until the end of 2022, when the “zero-COVID-19” policy ended and restrictions were fully lifted as of January 2023. This study aims to delineate the epidemiological profiles of these 12 respiratory pathogens among hospitalized children with ARI during the COVID-19 pandemic (2020–2022), the first year after the lifting of COVID-19 restrictions (2023), and the second year from Jan to Jun (2024). Insights into the fluctuations in respiratory pathogen prevalence will contribute to the formulation of subsequent public health initiatives and prophylactic strategies.
Methods
Study subjects and ethics statement
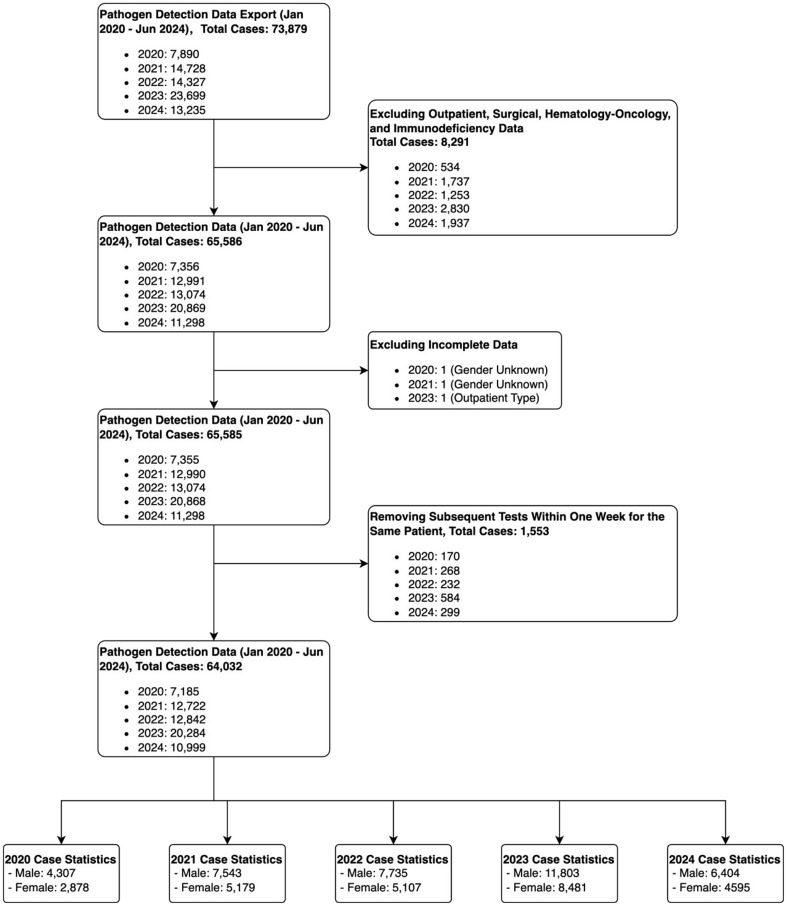
This study enrolled patients diagnosed with ARI and admitted to the pediatric wards of Shenzhen Children’s Hospital from January 2020 to June 2024. Inclusion criteria encompassed individuals presenting with one or more respiratory symptoms affecting the respiratory tract, including rhinorrhea, sore throat, cough, wheezing, dyspnea, tachypnea, with or without fever. Exclusion criteria were applied as follows: (1) hematological malignancy, or a history of hematopoietic stem cell transplantation; (2) repeat testing for the same pathogen within a one-week period; and (3) incomplete age or gender information (Fig. 1). Laboratory and demographic data for the enrolled patients were extracted from electronic medical records. The study protocol was reviewed and granted approval with a waiver of informed consent by the Shenzhen Children’s Hospital Ethical Committee. The research was conducted in compliance with pertinent ethical guidelines and regulatory standards.
Fig. 1.
The flowchart of case enrollment
The patients were divided into six age groups: (1) newborn(< 1 month), (2) infants (≥ 1 month, < 1 year), (3) toddlers (≥ 1 year, < 3 years), (4) pre-school children (≥ 3 years, < 6 years), (5) school-aged children (≥ 6 years, <14years) and 6)adolescents (≥ 14 years, < 18years).
Specimen detection
A SureX® 13 Respiratory Pathogen Multiplex Kit (Health Gene Technologies, Ningbo, China) was used to detect respiratory pathogens according to the recommended protocol, including RSV, ADV, HPIV, general influenza virus A (InfA), InfA(H1N1), InfA(H3N2), influenza B (InfB), human metapneumovirus (HMPV), human rhinovirus (HRV), human bocavirus (HBOV), human coronavirus (HCOV, including strains 229E, OC43, NL63, and HKU1), MP, and Chlamydia (Ch).
Statistical analyses
The “zero-COVID-19” policy was implemented at the beginning of 2020 and ended at the beginning of 2023 in China, thus the study periods were categorized as “During COVID-19” period (from January 2020 to December 2022) and “Post COVID-19” period (from January 2023 to June 2024). The detection rates and co-infection rates of these 12 prevalent respiratory pathogens were compared between these two periods. The epidemiological patterns of these pathogens were examined through the monthly distribution of detection rates. The differences in detection rates of these pathogens by gender and age, as well as the Intensive Care Unit (ICU) admission rates, were compared between these two phases.
Statistical analyses were conducted using SPSS 22.0 software (IBM, New York, United States). Categorical variables were expressed as numbers (%), while continuous variables were expressed as medians (interquartile range). Proportions for categorical variables (detection rates of pathogens, sex, and age) were compared using the chi-square test. Median ages were compared using the Mann-Whitney U test. All tests were two-tailed, and a value of P < 0.05 was considered statistically significant.
Results
Patient characteristics and detection rates during and post the COVID-19 periods
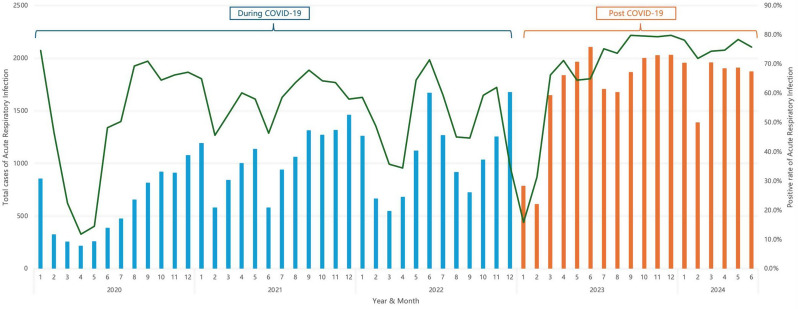
A total of 64,032 children with ARI were included in this study, with 32,749 cases from Jan 2020 to Dec 2022 (during COVID-19), and 31,283 cases from Jan 2023 to Jun 2024 (post COVID-19). The proportion of females and the median age increased in the post COVID-19 period. This trend culminated in a 91% increase in ARI hospital admissions yearly in the post COVID-19 period when compared to that during COVID-19. The pathogen detection rates and co-infection rates were significantly higher in the post COVID-19 period than during COVID-19. There was no difference in ICU admission rates between the COVID-19 and post COVID-19 periods (Table 1). Except for January and February 2023, when there was a nationwide surge in SARS-CoV-2 infections following the lifting of restrictions, there was a substantial rise in monthly ARI and pathogen detection rates throughout 2023 and into 2024 (Fig. 2).
Table 1.
The clinical characteristics and pathogen detection rates during and post the COVID-19 periods
| During COVID-19 | Post COVID-19 | χ²/U | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | Overall | 2023 | 2024(from Jan to Jun) | Overall | |||
| ARI cases | 7185 | 12,722 | 12,842 | 32,749 | 20,284 | 10,999 | 31,283 | ||
| Positive cases | 4306 | 7651 | 6893 | 18,850 | 14,199 | 8333 | 22,532 | ||
| Positive rate | 59.9% | 60.1% | 53.7% | 57.6% | 70.0% | 75.8% | 72.0% | 1464.875 | < 0.001 |
| Co-infection cases | 542 | 801 | 707 | 2050 | 2828 | 1910 | 4738 | ||
| Co-infection rate | 7.5% | 6.3% | 5.5% | 6.3% | 13.9% | 17.4% | 15.1% | 771.488 | < 0.001 |
| Gender | |||||||||
| Male | 4307 | 7543 | 7735 | 19,585(59.9%) | 11,803 | 6404 | 18,207(58.1%) | ||
| Female | 2878 | 5179 | 5107 | 13,164(40.1%) | 8481 | 4595 | 13,076(41.9%) | ||
| Male: Female | 1:0.67 | 1:0.72 | 16.986 | < 0.001 | |||||
| Age | 1.75(0.67, 3.75) | 2.17(0.75, 4.25) | 2.58(0.90, 4.83) | 2.17(0.75, 4.33) | 3.67(1.25, 6.67) | 3.50(1.00, 6.42) | 3.67(1.17, 6.58) | 407,937,370 | <0.0001 |
| ICU case | 330 | 502 | 537 | 1369 | 799 | 425 | 1224 | ||
| ICU admission rate | 4.6% | 3.9% | 4.2% | 4.2% | 3.9% | 3.9% | 3.9% | 2.949 | 0.086 |
Fig. 2.
ARI cases (column) and Positive rate(line) of ARI monthly from 2020 to 2024(from Jan to Jun)
Prevalence of respiratory pathogens during and post the COVID-19 periods
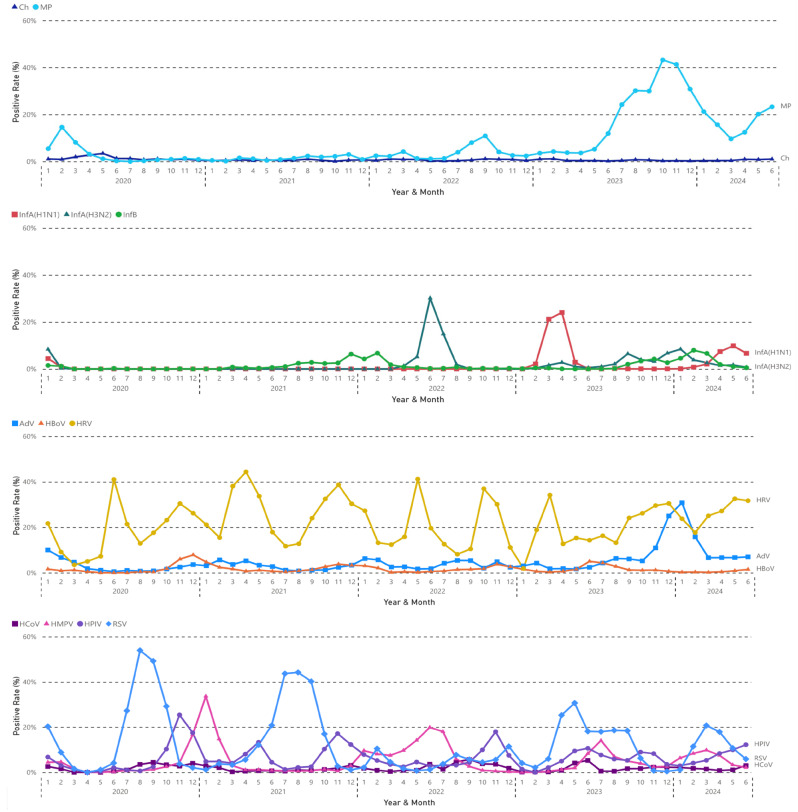
Figure 3 shows the epidemiological pattern of each pathogen. Except for Ch, which maintained a consistently low prevalence, all other pathogens experienced an increase in detection rates within a few months after the lifting of restrictions in 2023.
Fig. 3.
The epidemiological pattern of each respiratory pathogen monthly from 2020 to 2024(from Jan to Jun)
The prevalence of MP significantly decreased during COVID-19. However, a subsequent outbreak was observed after the lifting of restrictions in 2023, persisting into 2024. The detection rate of Ch remained consistently low throughout the study period.
All influenza viruses disappeared in 2020 during COVID-19 until the winter of 2021, when InfB re-emerged. An outbreak of InfA (H3N2) occurred from May to July 2022. An outbreak of InfA (H1N1) occurred from February to April 2023, soon after the lifting of restrictions. InfA (H1N1), InfA (H3N2), and InfB all re-emerged in winter 2023 and spring 2024.
HRV showed a regular and consistent prevalence pattern during COVID-19, with biannual peaks in April-June and October-November. An earlier peak was observed in March 2023, soon after the lifting of restrictions, and the detection rate remained high through 2024. HBOV also exhibited regular seasonal peaks, typically in November to December, with an atypical peak noted in June 2023 following the lifting of restrictions, but not in June 2024. The detection rates of ADV were low during COVID-19, increased since July 2023, and then a notable outbreak occurred in winter 2023.
RSV outbreaks occurred seasonally with a peak in August to September 2020 and July to September 2021, which was absent in 2022. An out-of-season RSV outbreak emerged in February 2023 following the lifting of restrictions. There was also an atypical out-of-season prevalence with a peak in March and April 2024. HPIV demonstrated a regular prevalence pattern with annual peaks in November, except for another peak in May 2021. An out-of-season peak was also observed soon after the lifting of restrictions in June 2023, as well as in 2024. The seasonal patterns of HBOV was regular with annual peaks in November, and an out-of-season peak was also observed in June 2023. HMPV and HCOV exhibited irregular prevalence patterns during COVID-19, yet both were active following the lifting of restrictions.
Co-infections during and post the COVID-19 periods
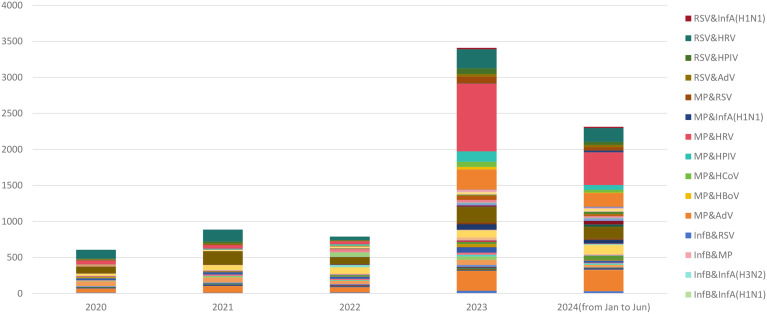
The prevalence rate of co-infections was 15.1% in the post-COVID-19 period, significantly higher than the 6.3% during COVID-19 (Table 1). In 2024, the prevalence rate of co-infections was 17.4%, even higher than the 13.9% observed in 2023 (χ²=28.536, P<0.001) (Table 1). The patterns and cases of co-infection are illustrated in Fig. 4. The leading five co-infection pathogen patterns are shown in Table 2, and these patterns were identical in both 2023 and 2024. HRV was the most frequently involved pathogen in co-infections, combining with RSV in 2020, with HPIV in 2021 and 2022, and with MP in 2023 and 2024 as the leading combinations (Table 2).
Fig. 4.
The co-infection pathogens and cases in 2020 to 2024(from Jan to Jun)
Table 2.
The leading five co-infection pathogens pattern in 2020 to 2024(from Jan to Jun)
| Respiratory pathogens | 2020 | N=(7185) | 2021 | (N = 12722) | 2022 | (N = 12824) | 2023 | (N = 20284) | 2024(from Jan to Jun) | (N = 10999) |
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| MP&HRV | 55 | (0.8%) | NA | NA | 44 | (0.3%) | 938 | (4.6%) | 453 | (4.1%) |
| AdV&HRV | 52 | (0.7%) | 88 | (0.7%) | 68 | (0.5%) | 274 | (1.4%) | 292 | (2.7%) |
| RSV&HRV | 122 | (1.7%) | 165 | (1.3%) | 49 | (0.4%) | 264 | (1.3%) | 191 | (1.7%) |
| HPIV&HRV | 92 | (1.3%) | 188 | (1.5%) | 98 | (0.8%) | 209 | (1.0%) | 156 | (1.4%) |
| MP&AdV | NA | NA | NA | NA | NA | NA | 281 | (1.4%) | 189 | (1.7%) |
| HMPV&HRV | NA | NA | 66 | (0.5%) | 90 | (0.7%) | NA | NA | NA | NA |
| HBoV&HRV | 65 | (0.9%) | 71 | (0.6%) | NA | NA | NA | NA | NA | NA |
| Others | 156 | (2.2%) | 223 | (1.8%) | 358 | (2.8%) | 862 | (4.2%) | 629 | (5.7%) |
| All | 542 | (7.5%) | 801 | (6.3%) | 707 | (5.5%) | 2828 | (13.9%) | 1910 | (17.4%) |
NA: not available
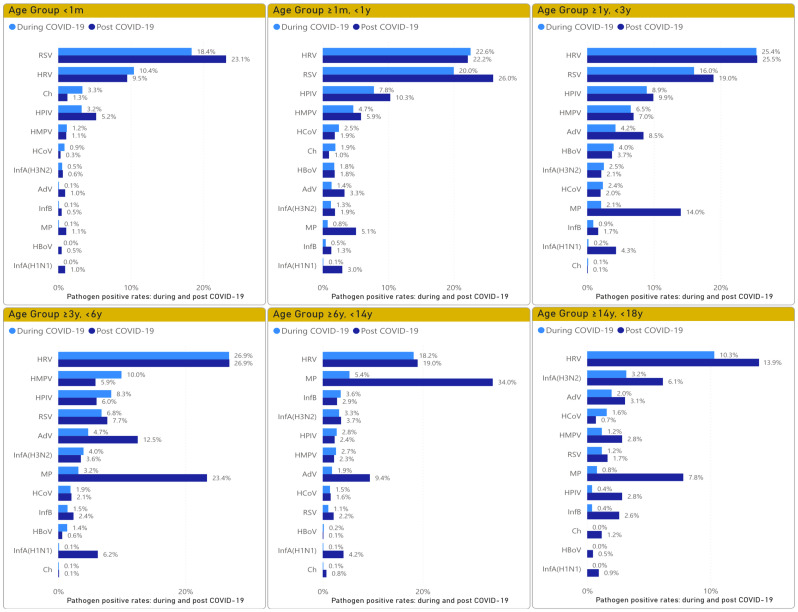
Prevalence of pathogens by gender and different age groups
The detection rates in both males and females increased in the post COVID-19 period. Similarly, the detection rates in all six age groups also increased in the post COVID-19 period (Table 3). The prevalence of pathogens in different age groups during and post the COVID-19 periods is illustrated in Fig. 5. HRV was the leading pathogen in all age groups, except in the < 1 m group, where RSV was predominant. In the ≥ 1 m, <1y and ≥ 1y, <3y groups, RSV was in the second place, followed by HPIV and HMPV, with the detection rate of RSV being significantly higher than that of HPIV and HMPV. In the ≥ 3y, <6y group, the leading four viral pathogens were HRV, HMPV, HPIV, and RSV. In ≥ 6y, < 14y, and ≥ 14y, < 18y groups, MP and influenza were the main pathogens. In the post COVID-19 period, MP increased and became a main pathogen in the ≥ 3y, <6y, ≥6y, < 14y, and ≥ 14y, < 18y groups. MP also showed an increase in the ≥ 1 m, <1y and ≥ 1y, <3y groups, becoming a main pathogen in the ≥ 1y, <3y group.
Table 3.
The detection rates by gender and different age groups during and post the COVID-19 periods
| During COVID-19(from Jan 2020 to Dec 2022) | Post COVID-19(from Jan 2023 to Jun 2024) | χ² | P | |||||
|---|---|---|---|---|---|---|---|---|
| Positive cases(n) | Overall(N) | Positive rate | Positive cases(n) | Overall(N) | Positive rate | |||
| Gender | ||||||||
| Male | 11,456 | 19,585 | 58.5% | 13,218 | 18,207 | 72.6% | 828.291 | <0.001 |
| Female | 7,394 | 13,164 | 56.2% | 9,314 | 13,076 | 71.2% | 643.352 | <0.001 |
| Age groups | ||||||||
| <1 m | 340 | 930 | 36.6% | 281 | 642 | 43.8% | 8.262 | 0.004 |
| ≥ 1 m, <1y | 5,067 | 8,692 | 58.3% | 4,689 | 6,814 | 68.8% | 181.155 | <0.001 |
| ≥ 1y, <3y | 6,206 | 9,573 | 64.8% | 5,909 | 7,496 | 78.8% | 400.033 | <0.001 |
| ≥ 3y, 6y | 5,493 | 8,862 | 62.0% | 7,007 | 8,971 | 78.1% | 552.883 | <0.001 |
| ≥ 6y, <14y | 1,688 | 4,431 | 38.1% | 6,314 | 9,251 | 68.3% | 1122.186 | <0.001 |
| ≥ 14y, <18y | 56 | 261 | 21.5% | 183 | 445 | 41.1% | 28.418 | <0.001 |
| Respiratory pathogens | ||||||||
| AdV | 1,026 | 32,749 | 3.1% | 2,663 | 31,283 | 8.5% | 852.869 | <0.001 |
| Ch | 227 | 32,749 | 0.7% | 159 | 31,283 | 0.5% | 9.128 | 0.003 |
| HBoV | 668 | 32,749 | 2.0% | 438 | 31,283 | 1.4% | 38.564 | <0.001 |
| HCoV | 690 | 32,749 | 2.1% | 572 | 31,283 | 1.8% | 6.421 | 0.011 |
| HMPV | 2,043 | 32,749 | 6.2% | 1,551 | 31,283 | 5.0% | 49.511 | <0.001 |
| HPIV | 2,432 | 32,749 | 7.4% | 2,090 | 31,283 | 6.7% | 13.539 | <0.001 |
| HRV | 7,704 | 32,749 | 23.5% | 7,160 | 31,283 | 22.9% | 3.637 | 0.057 |
| InfA(H1N1) | 41 | 32,749 | 0.1% | 1,375 | 31,283 | 4.4% | 1349.100 | <0.001 |
| InfA(H3N2) | 856 | 32,749 | 2.6% | 911 | 31,283 | 2.9% | 5.306 | 0.021 |
| InfB | 418 | 32,749 | 1.3% | 661 | 31,283 | 2.1% | 67.592 | <0.001 |
| MP | 783 | 32,749 | 2.4% | 6,226 | 31,283 | 19.9% | 5033.067 | <0.001 |
| RSV | 4,126 | 32,749 | 12.6% | 3,947 | 31,283 | 12.6% | 0.005 | 0.945 |
Fig. 5.
The pathogens detection rates in different age groups during and post the COVID-19 periods
ICU admission rate in ARI with each pathogen during and post the COVID-19 periods
There was no significant difference in ICU admission rates during and post the COVID-19 periods, except for HBoV (Table 4). The ICU admission rate for HBoV infection was 8.7% in the post COVID-19 period, which was higher than the 4.9% during COVID-19 period. In cases of ICU admissions with Ch infection, 17 out of 23 were in the neonatal ICU.
Table 4.
ICU admission rate of each pathogen during and post the COVID-19 periods
| Respiratory pathogens | During COVID-19 | Post COVID-19 | χ² | P | ||||
|---|---|---|---|---|---|---|---|---|
| ICU admission cases | Positive_Total | ICU admission rate | ICU admission cases | Positive_Total | ICU admission rate | |||
| AdV | 12 | 1,026 | 1.2% | 53 | 2,663 | 2.0% | 2.882 | 0.09 |
| Ch | 13 | 227 | 5.7% | 10 | 159 | 6.3% | 0.053 | 0.818 |
| HBoV | 33 | 668 | 4.9% | 38 | 438 | 8.7% | 6.145 | 0.013 |
| HCoV | 20 | 690 | 2.9% | 11 | 572 | 1.9% | 1.242 | 0.265 |
| HMPV | 31 | 2,043 | 1.5% | 25 | 1,551 | 1.6% | 0.051 | 0.821 |
| HPIV | 43 | 2,432 | 1.8% | 51 | 2,090 | 2.4% | 2.494 | 0.114 |
| HRV | 203 | 7,704 | 2.6% | 187 | 7,160 | 2.6% | 0.008 | 0.929 |
| InfA(H1N1) | 1 | 41 | 2.4% | 44 | 1,375 | 3.2% | 0.075 | 0.784 |
| InfA(H3N2) | 28 | 856 | 3.3% | 35 | 911 | 3.8% | 0.418 | 0.518 |
| InfB | 6 | 418 | 1.4% | 19 | 661 | 2.9% | 2.343 | 0.126 |
| MP | 6 | 783 | 0.8% | 52 | 6,226 | 0.8% | 0.040 | 0.841 |
| RSV | 112 | 4,126 | 2.7% | 121 | 3,947 | 3.1% | 0.887 | 0.346 |
Discussion
The current study indicates that the COVID-19 pandemic and NPI measures, as well as the lifting of these restrictions, have influenced the epidemiological patterns of multiple pathogens. It corroborates and extends previous findings from our institute [10, 11], demonstrating a significant reduction in ARI admissions and prevalence of pathogens due to the enforcement of lockdown and strict NPI measures in response to the COVID-19 pandemic in early 2020. However, many pathogens, such as influenza, re-emerged irregularly relative to seasonal patterns following the gradual relaxation of these restrictions from 2021 to 2022. Consistent with the warnings proposed in previous studies [4], several months after the cessation of the “zero-COVID-19” policy and restrictions in early 2023, almost all pathogens, except Ch, re-emerged and contributed to a marked increase in ARI hospitalizations, comparable to the pre-pandemic era as documented in our previous work [11]. The marked increase in co-infection rates and the significant rise in pathogen detection rates across all age groups suggest a concurrent prevalence of multiple pathogens in the post-pandemic period. This may be associated with the reduction in pathogen exposure due to various NPI measures during the pandemic, leading to a decrease in protective antibodies and increased susceptibility upon re-exposure to pathogens [12]. The prevalence of MP and ADV remained consistent throughout the pandemic, while the seasonal epidemic peak of RSV disappeared in the last year of the pandemic; however, all of them experienced an explosive epidemic in the first year of post-pandemic. Furthermore, the epidemiological peak of MP and ADV were higher than the peaks observed during the pre-pandemic seasons [11]. The resurgence of respiratory pathogens was noted extensively in various districts of China [9, 13, 14] and globally after the lifting of restrictions [8, 15–18]. Notably, in the current study, the high levels of ARI hospitalization and pathogen detection rates continued into the second year of the post-COVID-19 period in 2024, suggesting a lasting impact on the prevalence patterns of pathogens. This underscores the importance of available vaccinations and appropriate NPI measures.
RSV is an important pathogen causing respiratory infections in children. In the current study, RSV was the second most common pathogen, closely following HRV, in the 1 month to 3-year-old age group. A decline of RSV during the pandemic [19], and a significant resurgence, including atypical out-of-season outbreaks, has been documented in Europe [7, 20, 21], Australia [18], Asia [22], and the United States [23, 24] following the relaxation of NPI measures. In Shenzhen, the typical season of the RSV epidemic was in the summer, as observed in this study in 2020 and 2021. However, in 2022, there was no typical seasonal RSV prevalence. An atypical seasonal epidemic occurred in March-April 2023 following the lifting of restrictions. The same pattern was documented in Zhejiang and Jiangshu province of China [12, 25], where the RSV seasonality and transmission zones were different from those in Shenzhen [26]. It is worth noting that another atypical seasonal epidemic re-emerged in February-April 2024 in the current study, indicating that the disruption of RSV prevalence patterns by the pandemic and NPI measures is not a one-time event. While the first wave of RSV epidemics following pandemic suppression exhibited unusual patterns, the second and third waves more closely resembled typical RSV patterns in many countries [27]. The onset and peak timings of future epidemics following the disruption of normal RSV dynamics need close monitoring to inform the delivery of preventive and control measures.
MP is recognized as a significant pathogen among children with CAP, particularly prevalent in those aged five years and older [1]. MP infections are known to be endemic, exhibiting periodic epidemic peaks at irregular intervals. Throughout the COVID-19 lockdown period, epidemiological surveillance consistently reported low levels of MP [11, 28]. Our findings indicate an outbreak of MP commencing in May 2023, a few months after the lifting of restrictions, continuing into 2024, not only among susceptible children over 6 years old but also with increased detection rates in children under 6 years old. A similar pattern was observed in Shanghai, Eastern China, with a peak in MP infections noted since June 2023 [29]. The extensive epidemic of MP across China has garnered significant concern [30, 31]. Nevertheless, despite the easing or discontinuation of NPI measures, a sustained decrease in MP incidence was documented across 20 countries spanning Europe, Asia, the Americas, and Oceania [32], persisting until at least September 2023 [33]. The occurrence of an MP outbreak in China, contrasted with a delayed resurgence in other countries, suggests a pathogen-specific and regionally nuanced epidemiological pattern disruption due to the pandemic.
Influenza virus prevalence was nearly eradicated during 2020 and 2021 [11], a trend consistent with findings from the United States [15] and Western Australia [34]. However, our ongoing surveillance data indicate a resurgence of InfB in the winter of 2021 and an outbreak of InfA(H3N2) from April to July 2022. A resurgence of InfA(H1N1) was observed until after the lifting of restrictions, with an outbreak occurring from February to May 2023. The epidemiological peak of InfA was higher than the peaks observed during the pre-pandemic seasons [11]. These observations suggest that the pandemic and NPI measures have disrupted the traditional seasonal circulation patterns of influenza. It is crucial to enhance epidemiological surveillance for influenza to avert potential public health crises. Notably, in the winter of 2023 and spring of 2024, InfA(H3N2), InfA(H1N1), and InfB all re-emerged.
HRV is the most common viral pathogen across all age groups except for newborns in this study, exhibiting a stable epidemiological pattern and not appearing to be affected by the pandemic and NPI measures. The detection rate of HRV remained high during the COVID-19 period, consistent with other studies [15, 35–37], which might be due to the ineffectiveness of alcohol-based hand sanitizers and the weakened inhibitory effect of face masks against rhinovirus [38, 39]. Consistent with this, Xu et al. [40] found that in 2020, the positive rates of Mp, ADV, HRV, PIV, and influenza significantly decreased compared to the pre-pandemic period. Meanwhile, HRV was the most commonly detected respiratory pathogen and the most frequent across all five age groups. Following household exposure, children were significantly less likely to become infected with SARS-CoV-2 compared to HRV (aOR 0.16), whereas this trend was opposite in adults (aOR 1.71) [41]. The epidemiological patterns of HPIV, HMPV, and HCOV were also minimally affected during the pandemic. HPIV and HMPV are common respiratory pathogens that typically follow RSV in prevalence in children aged 3 to 6 years, with the positivity rates for both being similar to those of RSV.
The discontinuation of NPI measures increased the opportunity for pathogen infection; whether this also results in an increase in the severity remains a significant concern. In the current study, there was no significant difference in ICU admission rates for all pathogens except HBOV during and after the pandemic. The ICU admission rate of HBOV infection was 4.9% during the pandemic, close to 4.2% in our previous study with data up to January 2022 [11]. Notably, in the post-pandemic period, it increased to 8.7%. HBOV was uncommon in our study, with the highest detection rate of 4.0% in children 1–3 years old. However, studies have indicated that HBOV can cause severe ARI in children in the absence of viral and bacterial co-infections [42]. At Children’s Hospital of Soochow University in China, for lower respiratory tract infections by HBOV from January 2017 to October 2021, the rate of admission to ICU was 9.6%, which was much higher than that in the hMPV group (1%) [43]. The rate of HBOV associated admission to ICU was 7.3% [44] and 11.6% [45] in two children’s hospitals in Italy. The mechanism of severe ARI associated with HBOV and its worsening in the post-pandemic period is yet to be elucidated. This highlights the importance of persistent epidemiological surveillance of HBOV. The ICU admission rate of Ch seemed high in both the during and post-pandemic periods, though there was no difference between these two periods. It should be noted that Ch was a main pathogen in children less than one month old, and rare in other age groups, with most of the ICU admission cases being hospitalized in the neonatal ICU.
The limitations of this study include that all data were sourced from a single hospital and only included hospitalized ARI cases, which may introduce selection bias. Additionally, the study did not account for trends in bacterial pathogens. The current data indicate that the implementation of NPI measures in response to the COVID-19 pandemic has significantly reshaped the epidemiological patterns, even the clinical manifestations of some respiratory pathogens. As restrictions were lifted, continuous monitoring of these patterns is essential to understand the duration of these effects and to inform effective response strategies.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- ARI
Acute respiratory infections
- HBOV
Human Bocavirus
- MP
Mycoplasma pneumoniae
- ADV
Adenovirus
- RSV
Respiratory Syncytial Virus
- CAP
Community-acquired pneumonia
- NPI
Non-pharmaceutical interventions
- PCR
Polymerase chain reaction
- HPIV
Human parainfluenza virus
- HMPV
Human metapneumovirus
- HRV
Human rhinovirus
- HCOV
Human coronavirus
- Ch
Chlamydia
- ICU
Intensive Care Unit
Author contributions
JHC and KC conceived the study. WW, XJL and JHC analyzed the data and drafted the manuscript. ZMR, XYF, YSC, WJW, YMB and YJZ interpreted the results and revised the manuscript. All the authors have read and approved the final manuscript.
Funding
The study was supported by the Shenzhen Science and Technology Program (JCYJ20230807093820041, JCYJ20220530155811025, JCYJ20240813112420027),and Guangdong Basic and Applied Basic Research Foundation (2023A1515220156, 2022A1515220033).
Data availability
Data is provided within the manuscript or supplementary information files. The dataset generated and analyzed during the current study is not publicly available as it contains protected health information, but the de-identified dataset is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The need for informed consent was waived by the Ethics Committee of the Shenzhen Children’s Hospital because of the retrospective nature of the study.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Wang and Xiaojuan Luo contributed equally to this article and are co-first authors.
Contributor Information
Ke Cao, Email: cocoa526878@126.com.
Jiehua Chen, Email: loof11@163.com.
References
- 1.Jain S, Williams D, Arnold S, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–45. 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Respiratory M. Patterns of respiratory infections after COVID-19. Lancet Respir Med. 2024;12(1):1. 10.1016/s2213-2600(23)00472-1. [DOI] [PubMed] [Google Scholar]
- 3.Talic S, Shah s, Wild H, et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. BMJ. 2021;375:e068302. 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZJ, Yu LJ, Zhang HY, et al. Broad impacts of Coronavirus Disease 2019 (COVID-19) pandemic on Acute Respiratory infections in China: an observational study. Clin Infect Dis. 2022;75(1):e1054–62. 10.1093/cid/ciab942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023;21(3):195–210. 10.1038/s41579-022-00807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin R. From immunity debt to immunity theft-how COVID-19 might be tied to recent respiratory disease surges. JAMA. 2024;331(5):378–81. 10.1001/jama.2023.26608. [DOI] [PubMed] [Google Scholar]
- 7.Hatter L, Eathorne A, Hills T, et al. Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc Health. 2021;5(12):e44–5. 10.1016/s2352-4642(21)00333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivit C, Groen K, Jongbloed M, et al. An off-season outbreak of human metapneumovirus infections after ending of a COVID-19 lockdown. J Infect. 2022;84(5):722–46. 10.1016/j.jinf.2022.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan WS, Yau SK, To MY, et al. The seasonality of respiratory viruses in a Hong Kong Hospital, 2014–2023. Viruses. 2023;15(9). 10.3390/v15091820. [DOI] [PMC free article] [PubMed]
- 10.Li L, Wang H, Liu A, et al. Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in Shenzhen, China. Virol J. 2021;18(1):202. 10.1186/s12985-021-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zheng Y, De Jonge MI, et al. Lockdown measures during the COVID-19 pandemic strongly impacted the circulation of respiratory pathogens in Southern China. Sci Rep. 2022;12(1):16926. 10.1038/s41598-022-21430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang W, Xu L, Wang Y, et al. Exploring immunity debt: dynamic alterations in RSV antibody levels in children under 5 years during the COVID-19 pandemic. J Infect. 2024;88(1):53–6. 10.1016/j.jinf.2023.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Yu J, Wang Y, et al. Cocirculation and coinfection of multiple respiratory viruses during autumn and winter seasons of 2023 in Beijing, China: a retrospective study. J Med Virol. 2024;96(4):e29602. 10.1002/jmv.29602. [DOI] [PubMed] [Google Scholar]
- 14.Wei M, Li S, Lu X, et al. Changing respiratory pathogens infection patterns after COVID-19 pandemic in Shanghai, China. J Med Virol. 2024;96(4):e29616. 10.1002/jmv.29616. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Fries AC, Demarcus LS, et al. Circulating trends of Influenza and other Seasonal Respiratory viruses among the US Department of Defense Personnel in the United States: impact of the COVID-19 pandemic. Int J Environ Res Public Health. 2022;19(10). 10.3390/ijerph19105942. [DOI] [PMC free article] [PubMed]
- 16.Kuitunen I, Artama M, Haapanen M, et al. Respiratory virus circulation in children after relaxation of COVID-19 restrictions in fall 2021-A nationwide register study in Finland. J Med Virol. 2022;94(9):4528–32. 10.1002/jmv.27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumley SF, Richens N, Lees E, et al. Changes in paediatric respiratory infections at a UK teaching hospital 2016–2021; impact of the SARS-CoV-2 pandemic. J Infect. 2022;84(1):40–7. 10.1016/j.jinf.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eden JS, Sikazwe C, Xie R, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13(1):2884. 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong B, Koç U, Bandeira T, et al. Changes in the global hospitalisation burden of respiratory syncytial virus in young children during the COVID-19 pandemic: a systematic analysis. Lancet Infect Dis. 2023. 10.1016/s1473-3099(23)00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández-Rivas L, Pedraz T, Calvo C, et al. Respiratory syncytial virus outbreak during the COVID-19 pandemic. How has it changed?. Enferm Infecc Microbiol Clin. 2023;41(6):352–5. 10.1016/j.eimc.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nygaard U, Hartling UB, Nielsen J, et al. Hospital admissions and need for mechanical ventilation in children with respiratory syncytial virus before and during the COVID-19 pandemic: a Danish nationwide cohort study. Lancet Child Adolesc Health. 2023;7(3):171–9. 10.1016/s2352-4642(22)00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CM, Wahab AA, Ali A. Assessing the impact of COVID-19 on epidemiological changes of severe pediatric respiratory syncytial virus infections in Malaysia. Front Public Health. 2024;12:1246921. 10.3389/fpubh.2024.1246921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burks A, King W, Orr M. The changing virology and trends in resource utilization for bronchiolitis since COVID-19. Pediatr Pulmonol. 2023;58(11):3171–8. 10.1002/ppul.26640. [DOI] [PubMed] [Google Scholar]
- 24.Mcmorrow ML, Moline HL, Toepfer AP, et al. Respiratory Syncytial Virus-Associated hospitalizations in Children < 5 years: 2016–2022. Pediatrics. 2024. 10.1542/peds.2023-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Ru X, Chen S, et al. Analysis of the prevalence and clinical features of respiratory syncytial virus infection in a pediatric hospital in Zhejiang Province from 2019 to 2023. J Med Virol. 2024;96(6):e29758. 10.1002/jmv.29758. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, Deng S, Sun S, et al. Respiratory syncytial virus seasonality, transmission zones, and implications for seasonal prevention strategy in China: a systematic analysis. Lancet Glob Health. 2024;12(6):e1005–16. 10.1016/s2214-109x(24)00090-1. [DOI] [PubMed] [Google Scholar]
- 27.Thindwa D, Li K, Cooper-Wootton D, et al. Global patterns of rebound to normal RSV dynamics following COVID-19 suppression. BMC Infect Dis. 2024;24(1):635. 10.1186/s12879-024-09509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrick MMS, Michael LB, Søren AU, et al. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: results of a global survey, 2017 to 2021. Euro Surveill. 2022;27. 10.2807/1560-7917.Es.2022.27.19.2100746. [DOI] [PMC free article] [PubMed]
- 29.Xiao-Bo Z, Wen H, Yong-Hao G, et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: unusual pneumonia caused by usual pathogen. World J Pediatr. 2024;20. 10.1007/s12519-023-00793-9. [DOI] [PubMed]
- 30.Gemma C. What’s behind China’s mysterious wave of childhood pneumonia?. Nature. 2023. 10.1038/d41586-023-03732-w. [DOI] [PubMed] [Google Scholar]
- 31.Team WM. WHO statement on reported clusters of respiratory illness in children in northern China [Internet]. 2023 [cited 2023 Dec 6]. Available from: https://www.who.int/news/item/22-11-2023-who-statement-on-reported-clusters-of-respiratory-illness-in-children-in-northern-china.
- 32.Meyer Sauteur PM, Chalker VJ, Berger C, et al. Mycoplasma pneumoniae beyond the COVID-19 pandemic: where is it?. Lancet Microbe. 2022;3(12):e897. 10.1016/s2666-5247(22)00190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer Sauteur P, Beeton M. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. 2024;5(2):e100–1. 10.1016/s2666-5247(23)00344-0. [DOI] [PubMed] [Google Scholar]
- 34.Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of Coronavirus Disease 2019 Public Health Measures on detections of Influenza and Respiratory Syncytial Virus in Children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199–202. 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nenna R, Matera L, Pierangeli A, et al. First COVID-19 lockdown resulted in most respiratory viruses disappearing among hospitalised children, with the exception of rhinoviruses. Acta Paediatr. 2022;111(7):1399–403. 10.1111/apa.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao R, Du Y, Tong J, et al. Influence of COVID-19 pandemic on the virus spectrum in children with respiratory infection in Xuzhou, China: a long-term active surveillance study from 2015 to 2021. BMC Infect Dis. 2023;23(1):467. 10.1186/s12879-023-08247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi HJ, Kim NY, Eom SA, et al. J Korean Med Sci. 2022;37(21):e172. 10.3346/jkms.2022.37.e172. Effects of Non-Pharmacological Interventions on Respiratory Viruses Other Than SARS-CoV-2: Analysis of Laboratory Surveillance and Literature Review From 2018 to 2021. [DOI] [PMC free article] [PubMed]
- 38.Jia R, Lu L, Li S, et al. Human rhinoviruses prevailed among children in the setting of wearing face masks in Shanghai, 2020. BMC Infect Dis. 2022;22(1):253. 10.1186/s12879-022-07225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–80. 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M, Liu P, Su L, et al. Comparison of respiratory pathogens in Children with Lower Respiratory Tract infections before and during the COVID-19 pandemic in Shanghai, China. Front Pediatr. 2022;10:881224. 10.3389/fped.2022.881224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boom TT, De Hoog MLA, Westerhof I, et al. Age-specific SARS-CoV-2 transmission differed from human rhinovirus in households during the early COVID-19 pandemic. J Infect. 2024;89(2):106218. 10.1016/j.jinf.2024.106218. [DOI] [PubMed] [Google Scholar]
- 42.Moesker FM, Van Kampen JJ, Van der Eijk AA, et al. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect. 2015;21(10):e964961–968. 10.1016/j.cmi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X, Dai G, Wang T, et al. Comparison of the clinical features of human bocavirus and metapneumovirus lower respiratory tract infections in hospitalized children in Suzhou, China. Front Pediatr. 2022;10:1074484. 10.3389/fped.2022.1074484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporizzi A, Ravidà F, Barneschi S, et al. Analysis of a cohort of 165 Pediatric patients with human Bocavirus infection and comparison between mono-infection and respiratory co-infections: a retrospective study. Pathogens. 2024;13(1). 10.3390/pathogens13010055. [DOI] [PMC free article] [PubMed]
- 45.Petrarca L, Nenna R, Frassanito A, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World J Pediatr. 2020;16(3):293–8. 10.1007/s12519-019-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Shi HJ, Kim NY, Eom SA, et al. J Korean Med Sci. 2022;37(21):e172. 10.3346/jkms.2022.37.e172. Effects of Non-Pharmacological Interventions on Respiratory Viruses Other Than SARS-CoV-2: Analysis of Laboratory Surveillance and Literature Review From 2018 to 2021. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data is provided within the manuscript or supplementary information files. The dataset generated and analyzed during the current study is not publicly available as it contains protected health information, but the de-identified dataset is available from the corresponding author on reasonable request.