Abstract
Two strains of transgenic mice have been generated that secrete into their milk a malaria vaccine candidate, the 42-kDa C-terminal portion of Plasmodium falciparum merozoite surface protein 1 (MSP142). One strain secretes an MSP142 with an amino acid sequence homologous to that of the FVO parasite line, the other an MSP142 where two putative N-linked glycosylation sites in the FVO sequence have been removed. Both forms of MSP142 were purified from whole milk to greater than 91% homogeneity at high yields. Both proteins are recognized by a panel of monoclonal antibodies and have identical N termini, but are clearly distinguishable by some biochemical properties. These two antigens were each emulsified with Freund's adjuvant and used to vaccinate Aotus nancymai monkeys, before challenge with the homologous P. falciparum FVO parasite line. Vaccination with a positive control molecule, a glycosylated form of MSP142 produced in the baculovirus expression system, successfully protected five of six monkeys. By contrast, vaccination with the glycosylated version of milk-derived MSP142 conferred no protection compared with an adjuvant control. Vaccination with the nonglycosylated, milk-derived MSP142 successfully protected the monkeys, with 4/5 animals able to control an otherwise lethal infection with P. falciparum compared with 1/7 control animals. Analysis of the different vaccines used suggested that the differing nature of the glycosylation patterns may have played a critical role in determining efficacy. This study demonstrates the potential for producing efficacious malarial vaccines in transgenic animals.
A vaccine to combat malaria is a highly desirable public health tool to reduce morbidity and mortality in African children. It also appears technically achievable, with a number of promising candidates identified over the last 15 years eliciting effective anti-parasite responses in model systems (1, 2). Malaria vaccine development faces a major economic challenge, however. The populations that would benefit from a malaria vaccine live in the less developed countries of the world, and sub-Saharan Africa in particular. Such countries have very limited funds to expend on health care programs such as immunization; thus, the unit cost for the vaccine must be kept low whereas production methods must be capable of producing millions of doses.
Transgenic animals represent a novel technology for producing recombinant proteins for medical uses. Advantages of transgenic animal production include the ability to express complex proteins in an appropriate conformation at high yields—up to 700 liters of milk per year can be obtained from a single goat, with potential production levels of between 1 to 10 grams of protein per liter of milk (3).
To investigate whether this system could be used for the production of candidate malaria vaccine antigens, we used the 42-kDa C-terminal portion of Plasmodium falciparum merozoite surface protein 1 (MSP142; ref. 4). In P. falciparum, utilization of N- or O-linked glycans is either absent, or very limited (5, 6). Furthermore, parasite MSP1 appears to contain a glycosylphosphatidylinositol anchor moiety as the only carbohydrate modification (7, 8). Accordingly, two strains of mice were generated, one secreting transgenic (Tg) MSP142 glycosylated (G), where the amino acid sequence corresponds to the sequence of the FVO parasite line, and one secreting TgMSP142 nonglycosylated (NG), where the two putative N-linked glycosylation sites in the FVO sequence have been removed by conservative amino acid substitutions (L.-h.C., unpublished results).
Previously, transgenic animals have been used to produce recombinant proteins for therapeutic uses (3, 9), that is, for situations where the goal is to avoid an immune response against the therapeutic protein. Such recombinant antigens are of human origin, or humanized. We were interested to see whether the same system could produce high yields of a recombinant non-human vaccine candidate that would induce an efficacious anti-malarial response in a non-human primate model system.
Materials and Methods
Recombinant Protein Production.
The generation of TgMSP142-expressing transgenic mice strains is discussed elsewhere (L.-h.C., unpublished results). Mouse milk samples were obtained during lactation and frozen until sufficient quantities were obtained for purification. Whole mouse milk was diluted 2-fold in extraction buffer (2× PBS/5 mM imidazole/13 mM CHAPS, pH7.4), then centrifuged at 3000 × g for 10 min. Extraction of the pellet was repeated eight times. Histidine-tagged proteins were then purified by Ni-NTA chromatography (Qiagen, Chatsworth, CA), and desalted on a G-25 column (Amersham Pharmacia) into 10 mM sodium phosphate, 6.5 mM CHAPS (pH 6.8). This material was loaded onto a hydroxyapatite column (Bio-Rad), and MSP142 was eluted by using a salt gradient from 10 mM to 0.5 M sodium phosphate (pH 6.8). TgMSP142 was again desalted [into 10 mM sodium phosphate, 13 mM CHAPS (pH 8.0)], and loaded onto a Q Sepharose HP column (Amersham Pharmacia) running a salt gradient (0 to 1 M NaCl). Purified TgMSP142 G was dialyzed into 1× PBS (pH 7.4) and stored frozen. TgMSP142 NG was dialyzed into 1× PBS, 0.2% Tween 80 (pH 7.4) and stored frozen.
Subsequently, solubilization of the initial whole milk in a different buffer (1 M urea/50 mM lysine, pH 7.4) greatly simplified the first step, removing the need for repetitive extractions and the resultant large volume increases. This buffer was also more effective in dissociating the TgMSP142 from milk proteins, and consequently improved Ni-nitrilotriacetic acid (NTA) capture.
The production and purification of a recombinant form of MSP142 expressed in baculovirus bvMSP142 has been described previously (10).
Protein Characterization.
Amino acid sequencing and electron spray mass spectroscopy were performed by the Biological Resources Branch, National Institute of Allergy and Infectious Diseases. Protein concentrations were determined by BCA protein assay (Pierce, IL), and endotoxin levels by Limulus amebocyte lysate (LAL) gel clot assay (Charles River Endosafe, Charleston, SC). Glycosylation patterns were determined by using a 5-lectin DIG Glycan detection kit (Boehringer Mannheim) according to the manufacturer's instructions. For complete deglycosylation, proteins were treated with recombinant N-glycanase-PLUS (Glyko, Novato, CA) under denaturing conditions (1% wt/vol SDS) for 18 h at 37°C by using 10 mU enzyme per 100 μg antigen. For identification of glycosylation sites, proteins were treated with recombinant N-glycanase-PLUS under native conditions (1× PBS, 5 times the enzyme concentration) before HPLC purification and tryptic digestion. Tryptic digests were performed under native conditions in 1× PBS using modified trypsin (Promega) at a 1:100 wt/wt enzyme to antigen ratio for 1 h at 37°C. All HPLC purifications (post N-glycanase or trypsin treatment) were performed on a Dynamax 300 Å C8 reverse phase column (Varian) by using a 1 to 100% gradient acetonitrile into 0.1% vol/vol trifluoroacetic acid in water.
Vaccination and Challenge Infection of Malaria-Naive Aotus Monkeys.
Monkeys were housed at the Primate Research Facility, National Institutes of Health (NIH), in compliance with an NIH Animal Care and Use Committee approved protocol (LPD-8E). In the first trial, 28 monkeys were randomly assigned to groups of seven. Group assignment was masked to the primary investigators who cared for or vaccinated the animals, read films, or determined when a monkey should be drug-cured. The randomization was done to ensure that a control monkey was challenged first and last. The three vaccine groups received bvMSP142, TgMSP142 NG, and TgMSP142 G, respectively, and the fourth group placebo.
Monkeys received three vaccinations of 100 μg of the respective recombinant protein 3 wk apart, following our established protocol (10). The initial vaccinations were emulsified with complete Freund's adjuvant (Sigma), and the next two with incomplete Freund's adjuvant (Sigma). Vaccinated monkeys were challenged 15 days after the third vaccination by i.v. infusion of a freshly passaged preparation of 104 infected RBC of the highly virulent P. falciparum FVO strain. Hematocrit and Giemsa-stained thin films were made from blood collected by puncture of superficial veins in the dorsum of the calf. Parasitemia was monitored daily by Giemsa-stained thin films until treatment, and calculated based on examination of ≈2000 RBCs; if no parasites were seen, then 40 more high-power fields were examined. Monkeys were treated when parasitemia reached 5%, or their hematocrit fell below 20%. All monkeys not treated previously were treated on day 30. The treatment consisted of mefloquine administered in a single dose of 25 mg/kg of body mass by intubation.
The second Aotus challenge trial followed the protocol outlined above, with the exceptions that only two groups (TgMSP142 NG and placebo) and a larger challenge inoculum were used (1 ml of 5 × 104 pRBCs/ml).
Statistical Methods.
Aotus monkeys that control their parasitemia either self-cure or suffer anemia, requiring treatment. At this point, it is impossible to say what would have occurred to that monkey's parasite burden: it may have self-cured, or continued to control parasitemia, or lost control and suffered an acute infection. Thus, the primary endpoint includes only data up until the first monkey is treated for hematocrit rather than parasitemia. On that day, all monkeys were ranked in the following order: monkeys that were treated for parasitemia before the day of data collection ranked first, in order of first their treatment and then their cumulative parasitemia (the sum of that monkey's daily parasite burden). Then the monkey(s) that required treatment for hematocrit (so triggering the endpoint) were ranked in the same fashion. Finally, monkeys not requiring treatment up until that point were ranked in order of their cumulative parasitemias (10). A nonparametric Wilcoxon rank-sum analysis was then performed to compare test groups to the control group.
Secondary statistical comparisons were also made. Student's t tests were used to compare antibody responses elicited to the vaccines, and nonlinear Spearman's regression analysis was performed to correlate antibody responses to protection from challenge. Nonparametric Wilcoxon rank-sum tests were also performed to compare discontinuous data between vaccine groups (e.g., days to peak parasitemia, days to treatment, peak parasitemias, and parasitemia at the time of treatment).
Measurement of Antibody Responses.
ELISAs and indirect immunofluorescent assays (IFAs) were performed as previously described (10). Serum dilutions that gave an absorbance value of 0.5 units above background were designated as the endpoint of the sera ELISA titer.
Results
Purification and Yields of TgMSP142.
Purification of recombinant proteins from the milk of transgenic animals generally commences with a centrifugation step to separate the insoluble casein phase from the recombinant protein-containing aqueous phase. In our case, TgMSP142 G fractionated about 50% into the aqueous phase and 50% into the insoluble phase, whereas TgMSP142 NG fractionated entirely with the insoluble phase (data not shown). Thus, we processed whole milk rather than the aqueous fraction. Furthermore, a stringent chromatography purification process involving four different chemistries was required, because TgMSP142 of both types formed complexes with milk proteins. We found the nonglycosylated TgMSP142 NG to be considerably more insoluble than the glycosylated TgMSP142 G, requiring the continual presence of a detergent to prevent precipitation.
Final product characteristics are shown in Table 1 and Fig. 1. The yields quoted are of final purified protein. Subsequent to the first Aotus challenge trial, the purification of TgMSP142 NG was considerably optimized and simplified. Largely, this involved an initial solubilization of the whole milk by using a lysine/urea buffer, effectively disassociating the TgMSP142 from the milk proteins, making final purification easier and yields higher (Table 1, TgMSP142 NG2).
Table 1.
Protein characteristics of purified transgenic antigens
| TgMSP142 G | TgMSP142NG | TgMSP142NG2* | |
|---|---|---|---|
| Purified protein yield per ml mouse milk | 1.6 mg/ml | 0.24 mg/ml | 0.84 mg/ml |
| Purity† | 93.3% | 91.2% | 96.7% |
| Reactivity with antibodies‡ | Strong | Strong | Strong |
| N-terminal sequence (predicted: nh2AVTPSVID) | SVIDNILSKIEN | SVIDNILSKIEN | SVIDNILSKIEN |
| Mass (gel chromatography) | 92.6 kDa | 126.7 kDa | ND |
| Endotoxin measure | <0.7 EU/mg | <2.9 EU/mg | <5 EU/mg |
The first two antigens (TgMSP142 G and TgMSP142 NG) were used in the first vaccine trial; TgMSP142 NG2 is TgMSP142 NG purified by an optimized purification method and used in the second vaccine trial (see Materials and Methods).
Amount of TgMSP142 in a single band, as a percentage of total protein. Calculated using quantitative laser scanning densitometry of Coomassie blue-stained SDS/PAGE.
A series of monoclonal antibodies recognizing conformational epitopes in MSP119 with differing specificities were used as in ref. 10: 5.2, 2D634, 13E353, 4H919, 12.1, 1E1, 12.8, 2F10.
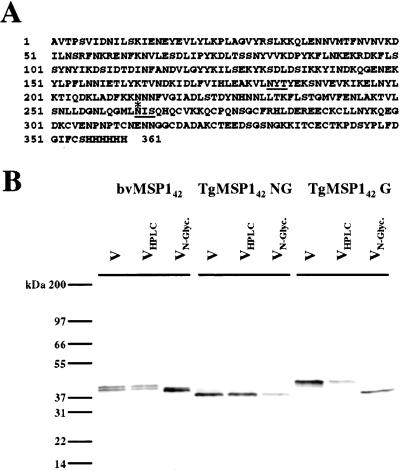
Figure 1.
Purity and glycosylation of transgenic antigens. (A) Amino acid sequence of recombinant TgMSP142 G. Underlined are the two putative N-linked glycosylation sites in the sequence. The only sequence difference between TgMSP142 G and TgMSP142 NG is the mutation of these two asparagines to glutamines. The asterisk indicates the natural parasite protease processing point for the generation of the smaller MSP119 fragment of MSP1. (B) N-linked glycosylation characteristics of the antigens. Silver-stained SDS/PAGE gel of the three vaccines given to Aotus monkeys (lanes V). Each of these vaccines was subject to reverse-phase HPLC (lanes VHPLC) before treatment with N-glycanase and repeat HPLC (lanes VN-Glyc.).
Characterization of TgMSP142.
Both forms of TgMSP142 reacted with a panel of monoclonal antibodies (Table 1). The sequence of MSP142 from the FVO parasite has two putative N-linked glycosylation sites. In TgMSP142 NG, these two asparagines were altered to glutamines (Fig. 1A). The nature of the glycosylation for three antigens, TgMSP142 NG, TgMSP142 G, and a baculovirus-expressed form of MSP142 (bvMSP142) (10), was characterized by lectin reactivity. None of the antigens were reactive with the O-glycan-detecting peanut agglutinin. TgMSP142 NG was unreactive with all lectins tested. TgMSP142 G was reactive with the terminal sialic acid-detecting Maackia amurensis and Sambucus nigra agglutinins whereas bvMSP142 was reactive with terminal mannose detecting Galanthus nivalis agglutinin (data not shown).
To confirm that only N-linked glycosylation was occurring, all three proteins were then subjected to HPLC purification before treatment with N-glycanase (Fig. 1B). Treatment with N-glycanase caused a shift in the SDS/PAGE migration pattern for both bvMSP142 and TgMSP142 G, whereas TgMSP142 NG was unaffected.
The carboxyl terminus of MSP142 is an 11-kDa fragment made up entirely of two epidermal growth factor-like domains (EGF). This fragment migrates anomalously by SDS/PAGE at 19 kDa, and is hence known as MSP119. It has been shown previously that MSP119 is resistant to cleavage with trypsin under nonreducing conditions, with only a single cleavage occurring despite numerous potential sites (11). Tryptic cleavage of MSP142 thus releases a series of tryptic peptides from the amino region before MSP119, and MSP119 itself, which remains intact. Thus, after tryptic cleavage of MSP142, one of the two potential N-linked glycosylation sites is contained in a small tryptic peptide (it is in the region of MSP142 amino-terminal to MSP119), and the second is contained in MSP119 (Fig. 1A).
All three recombinant proteins were treated with trypsin with or without prior N-glycanase treatment. For TgMSP142 G, comparison of HPLC chromatograms of tryptic-cleaved ± N-glycanase treatment identified two peptides that shifted in elution profiles (Fig. 2). The high content of disulfide-bridged cysteines (six bridges) in MSP119 results in spectra easily distinguished from more typical protein spectra, identifying fractions 67 and 65 respectively as containing MSP119. Mass spectroscopy thus identified the glycan at this asparagine to be 2,336 Da in size. The other glycan containing peptide was identified as present in fractions 50 and 46, and to have a mass of 1,225.1 Da (Fig. 2). For bvMSP142, a similar analysis showed that insect cells also used both putative N-linked glycosylation sites during expression. Unlike TgMSP142 G, however, the glycosylation of bvMSP142 was not uniform, with peaks corresponding to one, two, up to six mannose residues detected (data not shown).
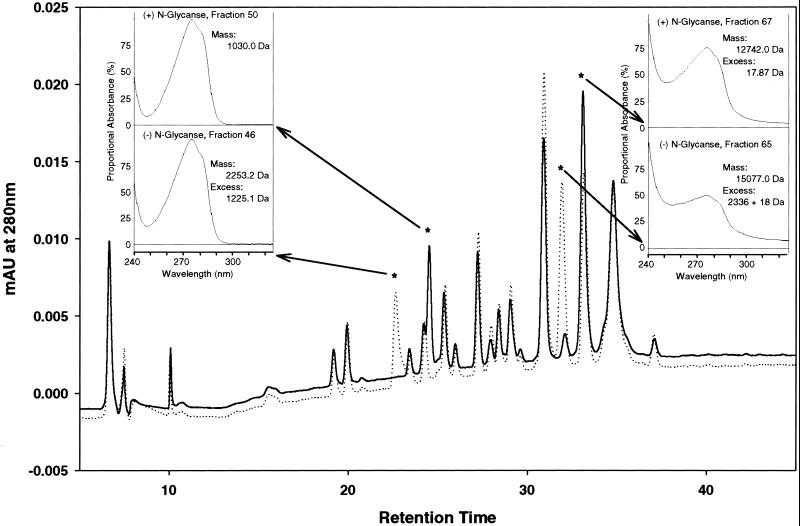
Figure 2.
Characterization of N-linked glycans in TgMSP142 G. Recombinant TgMSP142 G was cleaved with trypsin and subject to reverse-phase HPLC, both before (dashed line) and after (solid line) N-glycanase treatment. (Insets) The UV/Vis-spectrum of peaks identified as shifting in retention time between the two HPLC chromatograms. Fractions 46 and 50 have UV/Vis-spectrums typical of small peptides. Fractions 65 and 67 have a very atypical spectrum, with disproportionally high absorbances at 254 nm. This result is characteristic of the presence of multiple disulfide bridges, and identified these two peptides as MSP119 (which contains six disulfide bridges in 11 kDa). The masses of the isolated peaks were determined by mass spectroscopy and are also shown in the Insets. The predicted molecular mass of the peptide containing the first N-linked glycosylation site is 1028.14 Da (1029.12 Da after N-Glycanase treatment, which converts asparagine to aspartate); the predicted mass for the MSP119 tryptic fragment is 12,723.14 Da (12,724.13 after N-Glycanase). MSP119 is known to be resistant to tryptic digest, with only one cleavage occurring (11). This cleavage increases the expected mass by 18 (for a water molecule) to 12,741.14 Da (and 12,741.13 Da). From this result, the mass of the N-linked glycans was determined as shown.
Efficacy Testing of TgMSP142.
TgMSP142 NG and G were used to each vaccinate 7 Aotus nancymai monkeys. Positive control animals received a similar amount of the known protective antigen bvMSP142 (10), and negative controls received adjuvant alone. Fifteen days after the third vaccination, all monkeys were then challenged with 104 P. falciparum parasites of the homologous FVO strain. During vaccination, three animals died (two in the TgMSP142 NG group and one in the TgMSP142 G group), unfortunately not a rare occurrence with these fragile monkeys. No animals died during the second study.
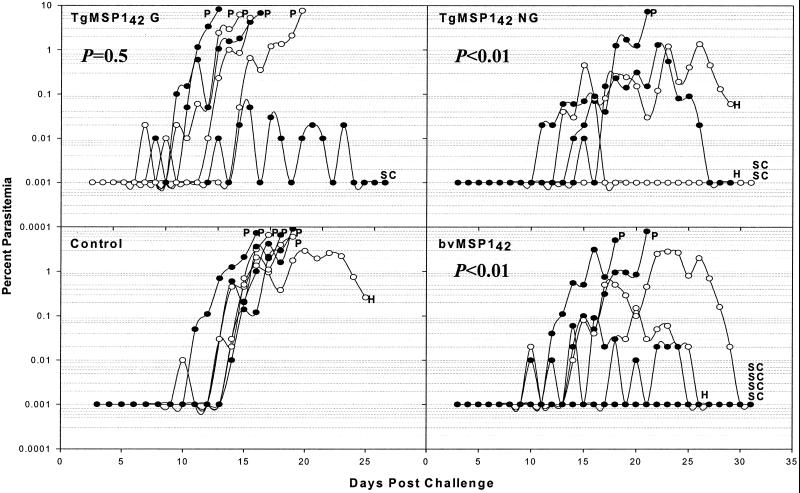
When partially protected from P. falciparum malaria, it is a characteristic of Aotus monkeys that some protected animals will suffer from anemia (10, 12). Thus, the primary measure of efficacy is to compare groups by using a Wilcoxon rank-sum test, ranking animals in order of treatment for parasitemia and cumulative parasitemia at the time the first monkey was treated for anemia (see Statistical Methods). The course of the parasitemia for each animal is shown in Fig. 3. Both bvMSP142 and TgMSP142 NG were found to be efficacious, with both groups having cumulative parasitemias significantly less then the control animals (P < 0.01 for both). TgMSP142 G was not efficacious (P = 0.5 compared with controls). At this time, 5/5 animals in the TgMSP142 NG group were untreated and 6/7 in the bvMSP142 group, compared with 2/6 for TgMSP142 G and 1/7 for controls.
Figure 3.
Course of the daily parasitemia in individual monkeys from the first Aotus vaccine trial. Monkeys were challenged on day 0 with 104 FVO P. falciparum parasitized erythrocytes, 15 days after the third vaccination. The four panels each represent one of the vaccine groups. Indicated on the graphs is the P value from a Wilcoxon rank-sum comparison of each group to the control group. Also indicated are the treatment times for uncontrolled parasitemia >5% (P), hematocrit <20% (H), or self-curing animals (SC). Alternate monkeys in a group have open and filled circles.
Other secondary markers of protection by all of the data (i.e., not just the data collected until the first animal is treated for anemia) confirmed these results. Both the TgMSP142 NG and bvMSP142 groups were significantly different from control animals when peak parasitemias, days until treatment and parasitemia at treatment were compared (for all, P < 0.05). The TgMSP142 G animals were not significantly different for any of these parameters. Overall, 6/7 control animals required treatment for parasitemia >5%, and one required treatment for a drop in hematocrit to <20%. Five of six TgMSP142 G animals required treatment for parasitemia, and one animal self-cured. Conversely, only one TgMSP142 NG animal required treatment for parasitemia, whereas two self-cured and two controlled parasitemia but required treatment for a drop in hematocrit to <20%. This result was indistinguishable from the bvMSP142 group, where two animals required treatment for parasitemia, four self-cured, and one controlled parasitemia but required treatment for a drop in hematocrit to <20%.
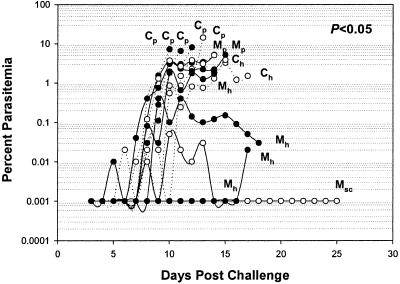
Improvements in the process development of TgMSP142 NG were made subsequent to the first Aotus challenge trial, resulting in a more robust process with higher yields (TgMSP142 NG2 in Table 1). Because 1 M of the denaturant Urea was used during this process, this material was also tested in an Aotus challenge trial, and confirmed the efficacy seen previously (Fig. 4). Again, the course of the parasitemia in the TgMSP142 NG-vaccinated monkeys was significantly different from that in the control monkeys (P < 0.05). Over the course of the infection, only 2/7 animals in the TgMSP142 NG group required treatment for parasitemia, compared with 5/7 for the controls (Fig. 4).
Figure 4.
Course of the daily parasitemia in individual monkeys from the second Aotus vaccine trial. A TgMSP142 NG group of vaccinated monkeys (M, solid lines) and a placebo group of monkeys (C, dotted lines) were challenged on day 0 with 5 × 104 FVO P. falciparum parasitized erythrocytes, 15 days after the third vaccination. Shown is the P value from a Wilcoxon rank-sum comparison of the TgMSP142 NG group to the control group. Also shown are the treatment times, for uncontrolled parasitemia >5% (Cp or Mp), hematocrit <20% (Ch or Mh), or self-curing animals (Msc). Alternate monkeys have open and filled circles.
Characterization of Immune Responses to TgMSP142.
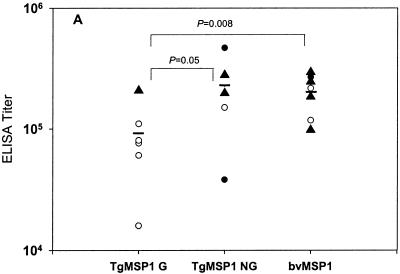
Endpoint ELISA titers against the reference antigen bvMSP142 for all animals are shown in Fig. 5. There was a significant difference in the titers to bvMSP142 between those animals vaccinated with bvMSP142 and TgMSP142 G (P = 0.008), and between those vaccinated with TgMSP142 NG and TgMSP142 G (P = 0.05). No differences in titers were observed between the bvMSP142 and TgMSP142 NG groups. No significant differences were seen in ELISA titers to other antigens (TgMSP142 NG, TgMSP142 G, or MSP119), nor were any significant differences seen in IFA titers against P. falciparum FVO parasites (data not shown). Overall, antibody titers to none of the four antigens used as ELISA capture antigens (bvMSP142, TgMSP142 NG, TgMSP142 G, or MSP119) correlated with the primary outcome of protection as defined above (cumulative parasitemia until first monkey treated for anemia). However, antibody titers to bvMSP142 did correlate with days until treatment (r2 = 0.6241, P = 0.005) and inversely with parasitemia at time of treatment (r2 = −0.4206, P = 0.05).
Figure 5.
ELISA antibody titers of individual monkeys before challenge. Shown on the x axis is the vaccine for that group of monkeys. ELISA titers are recorded as the inverse of the sera dilution corresponding to an OD405 of 0.5 against bvMSP142 as capture antigen. Animals requiring treatment for parasitemia >5% (○), hematocrit <20% (●), or self-curing (▴) are shown, as are significant differences by Student's t test between groups.
Discussion
The potential production capacity of transgenic animals is extraordinary, and each animal is in effect a product-specific production plant. Thus, transgenic animals with lactation-specific transgenes have become an attractive option for the production of high volume therapeutic products for the pharmaceutical industry in mice (13, 14), rabbits (15), pigs (16), sheep (17), goats (18), and cattle (19).
Malaria vaccines could also benefit from such low cost, high volume production methods. A single goat producing 700 liters/year of milk at the yields we obtained (0.9 g/liter of purified antigen) could supply enough antigen to vaccinate 8.4 million people annually. Because expression in the transgenic mice in this report is driven by the goat β-casein promoter, there is good reason to believe mouse production levels will hold up in goats (and perhaps improve as purification scale increases). Thus a herd of three goats could conceivably produce enough antigen to vaccinate 20 million African children per year.
Successful utilization of this potential requires that the antigens produced in the milk of transgenic animals retain biological efficacy. For vaccines as opposed to therapeutic agents, this means that they must retain appropriate immunogenicity. This report presents previously unrecorded evidence of the efficacy of a vaccine produced in transgenic animals. The nonglycosylated form of MSP142 purified from the milk of transgenic mice was equally as efficacious as the bvMSP142 used as a positive control, which itself provides the best protection we have been able to achieve in the P. falciparum Aotus challenge model (10). Hence, in a biological model of efficacy, a vaccine antigen produced in transgenic mice, as opposed to the more usual therapeutic proteins, was capable of inducing a protective immune response.
The failure of the glycosylated form of the antigen, TgMSP142 G, to also confer protection was surprising. bvMSP142 is glycosylated yet protective, TgMSP142 G was more easily purified than TgMSP142 NG, and the reactivity by confirmation-specific monoclonal antibodies did not appear to differ significantly among the three proteins. We thus expected TgMSP142 G to be the preferred candidate. Vaccination with TgMSP142 G did elicit antibody titers to three forms of MSP1 (MSP119, TgMSP142 G, and TgMSP142 NG by ELISA) and to parasites (by IFA) that were not significantly lower than vaccination with either bvMSP142 or TgMSP142 NG. However, antibody titers as measured by ELISA to bvMSP142 were significantly lower, and vaccination with TgMSP142 G gave no protection.
The lack of a clear correlation between reduced antibody titers to TgMSP142 G and reduced protection suggests that it was not simply a lack of immunogenicity that resulted in reduced protection. And although containing a somewhat lower endotoxin content than TgMSP142 NG (0.7 EU/mg compared with 2.9 EU/mg), the TgMSP142 did not contain less endotoxin than the bvMSP142 (0.8 EU/mg; ref. 10).
This result tends to suggest that the large mammalian glycans on TgMSP142 G may have been playing a complex role in effecting efficacy. The glycans on TgMSP142 G are complex N-linked sugars with terminal sialic acids. The mass of the sugar at each of the asparagines is distinct. This result is in marked contrast to the bvMSP142, where the sugars are of the high mannose type and heterogeneous in length. It is possible the large sugars on TgMSP142 G block the presentation of protective epitopes, where antibodies known to inhibit the parasite's essential proteolytic processing of MSP1 bind (20), especially because the second asparagine in MSP142 is the naturally occurring first amino acid of MSP119. The processing of MSP142 to MSP119 by P. falciparum is known to be a requirement for successful red cell invasion by the parasite (21). Why the glycosylation of this (or the first) asparagine in bvMSP142 would not cause a similar phenomenon is unclear; the heterogeneity of the bvMSP142 glycosylation, however, does leave a proportion of molecules unglycosylated. Alternately, terminal sialic sugars on mammalian carbohydrates may have altered the conformation or binding of antibodies to TgMSP142 G, especially because P. falciparum, while possibly performing very limited N- and O-linked glycosylation (5, 6), lacks sialic acid (22).
Regardless of the mechanism, it is apparent that both TgMSP142 NG and TgMSP142 G appeared to be equally strong candidates before the Aotus challenge. This result reemphasizes the central importance such challenge studies have in malaria vaccine research (23). Furthermore, the design of successful DNA vaccines for the treatment of malaria may also need to take the glycosylation results into account.
This study successfully demonstrates that recombinant proteins obtained from the milk of transgenic animals in high yield can elicit protective immunity in a simian model of malaria immunity. Thus, transgenic animal production may have significant utility for the manufacturing of vaccines against malaria.
Acknowledgments
We would like to gratefully acknowledge our debt to the following people for their assistance: members of the Malaria Vaccine Development Unit animal care unit (Joshua Reece, Brian Keegan, and Laura Corvette) for their exemplary care of the animals used in this study; Maria-Elena Fabucci for PCR analysis of monkey blood; Olga Muratova for IFA studies; and Aaron Miles, Michael Whitmore, Jeff Lyons, and Anthony Holder for testing of the proteins with monoclonal antibodies. We also would like to acknowledge the large debt the success of this project owes to Dr. B. Fenton Hall, Project Officer with the Parasitology and International Programs Branch, National Institute of Allergy and Infectious Diseases.
Abbreviations
- MSP1
merozoite surface protein 1
- Tg
transgenic
- NG
nonglycosylated
- G
glycosylated
- bv
baculovirus
- IFA
immunofluorescent assay
References
- 1.Miller L H, Hoffman S L. Nat Med. 1998;4:520–524. doi: 10.1038/nm0598supp-520. [DOI] [PubMed] [Google Scholar]
- 2.Good M F, Kaslow D C, Miller L H. Annu Rev Immunol. 1998;16:57–87. doi: 10.1146/annurev.immunol.16.1.57. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph N S. Trends Biotechnol. 1999;17:367–374. doi: 10.1016/s0167-7799(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 4.Holder A A, Blackman M J, Burghaus P A, Chappel J A, Ling I T, McCallum-Deighton N, Shai S. Mem Inst Oswaldo Cruz. 1992;87:37–42. doi: 10.1590/s0074-02761992000700004. [DOI] [PubMed] [Google Scholar]
- 5.Gowda D C, Gupta P, Davidson E A. J Biol Chem. 1997;272:6428–6439. doi: 10.1074/jbc.272.10.6428. [DOI] [PubMed] [Google Scholar]
- 6.Gowda D C, Davidson E A. Parasitol Today. 1999;15:147–152. doi: 10.1016/s0169-4758(99)01412-x. [DOI] [PubMed] [Google Scholar]
- 7.Gerold P, Schofield L, Blackman M J, Holder A A, Schwarz R T. Mol Biochem Parasitol. 1996;75:131–143. doi: 10.1016/0166-6851(95)02518-9. [DOI] [PubMed] [Google Scholar]
- 8.Schofield L, Hackett F. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollock D P, Kutzko J P, Birck-Wilson E, Williams J L, Echelard Y, Meade H M. J Immunol Methods. 1999;231:147–157. doi: 10.1016/S0022-1759(99)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowers A W, Cioce V, Shimp R L, Lawson M, Hui G, Muratova O, Kaslow D C, Robinson R, Long C A, Miller L H. Infect Immun. 2001;69:1536–1546. doi: 10.1128/IAI.69.3.1536-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stowers A W, Zhang Y, Shimp R L, Kaslow D C. Yeast. 2001;18:137–150. doi: 10.1002/1097-0061(20010130)18:2<137::AID-YEA657>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Egan A F, Blackman M J, Kaslow D C. Infect Immun. 2000;68:1418–1427. doi: 10.1128/iai.68.3.1418-1427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon K, Lee E, Vitale J A, Smith A E, Westphal H, Hennighausen L. Biotechnology. 1987;24:425–428. [PubMed] [Google Scholar]
- 14.Kerr D E, Plaut K, Bramley A J, Williamson C M, Lax A J, Moore K, Wells K D, Wall R J. Nat Biotechnol. 2001;19:66–70. doi: 10.1038/83540. [DOI] [PubMed] [Google Scholar]
- 15.Zinovieva N, Lassnig C, Schams D, Besenfelder U, Wolf E, Muller S, Frenyo L, Seregi J, Muller M, Brem G. Transgenic Res. 1998;7:437–447. doi: 10.1023/a:1008831028620. [DOI] [PubMed] [Google Scholar]
- 16.Wall R J, Pursel V G, Shamay A, McKnight R A, Pittius C W, Hennighausen L. Proc Natl Acad Sci USA. 1991;88:1696–1700. doi: 10.1073/pnas.88.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright G, Carver A, Cottom D, Reeves D, Scott A, Simons P, Wilmut I, Garner I, Colman A. Biotechnology (NY) 1991;9:830–834. doi: 10.1038/nbt0991-830. [DOI] [PubMed] [Google Scholar]
- 18.Ebert K M, Selgrath J P, DiTullio P, Denman J, Smith T E, Memon M A, Schindler J E, Monastersky G M, Vitale J A, Gordon K. Biotechnology (NY) 1991;9:835–838. doi: 10.1038/nbt0991-835. [DOI] [PubMed] [Google Scholar]
- 19.Krimpenfort P, Rademakers A, Eyestone W, van der Schans A, van den Broek S, Kooiman P, Kootwijk E, Platenburg G, Pieper F, Strijker R, et al. Biotechnology (NY) 1991;9:844–847. doi: 10.1038/nbt0991-844. [DOI] [PubMed] [Google Scholar]
- 20.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackman M J, Whittle H, Holder A A. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 22.Schauer R, Wember M, Howard R J. Hoppe Seyler's Z Physiol Chem. 1984;365:185–194. doi: 10.1515/bchm2.1984.365.1.185. [DOI] [PubMed] [Google Scholar]
- 23.Stowers A W, Miller L H. Trends Parasitol. 2001;17:415–419. doi: 10.1016/s1471-4922(01)02011-6. [DOI] [PubMed] [Google Scholar]