Abstract
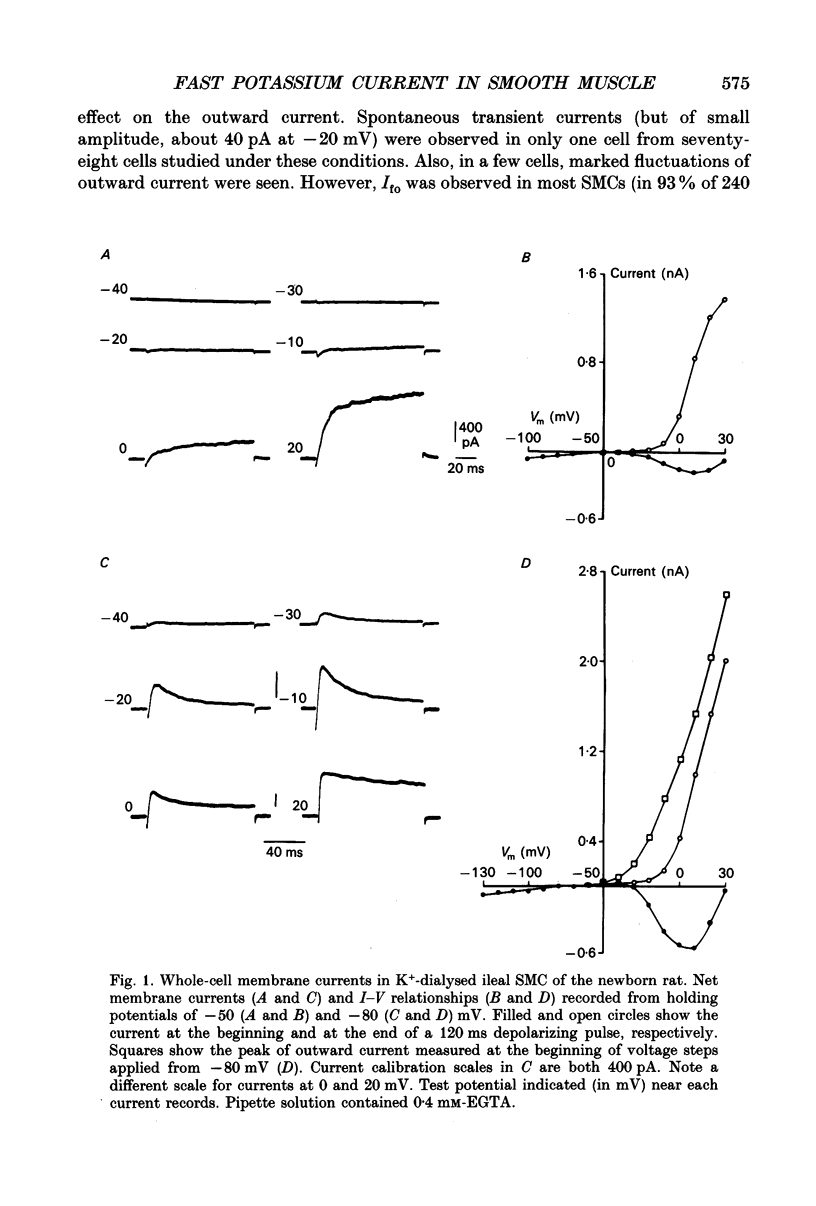
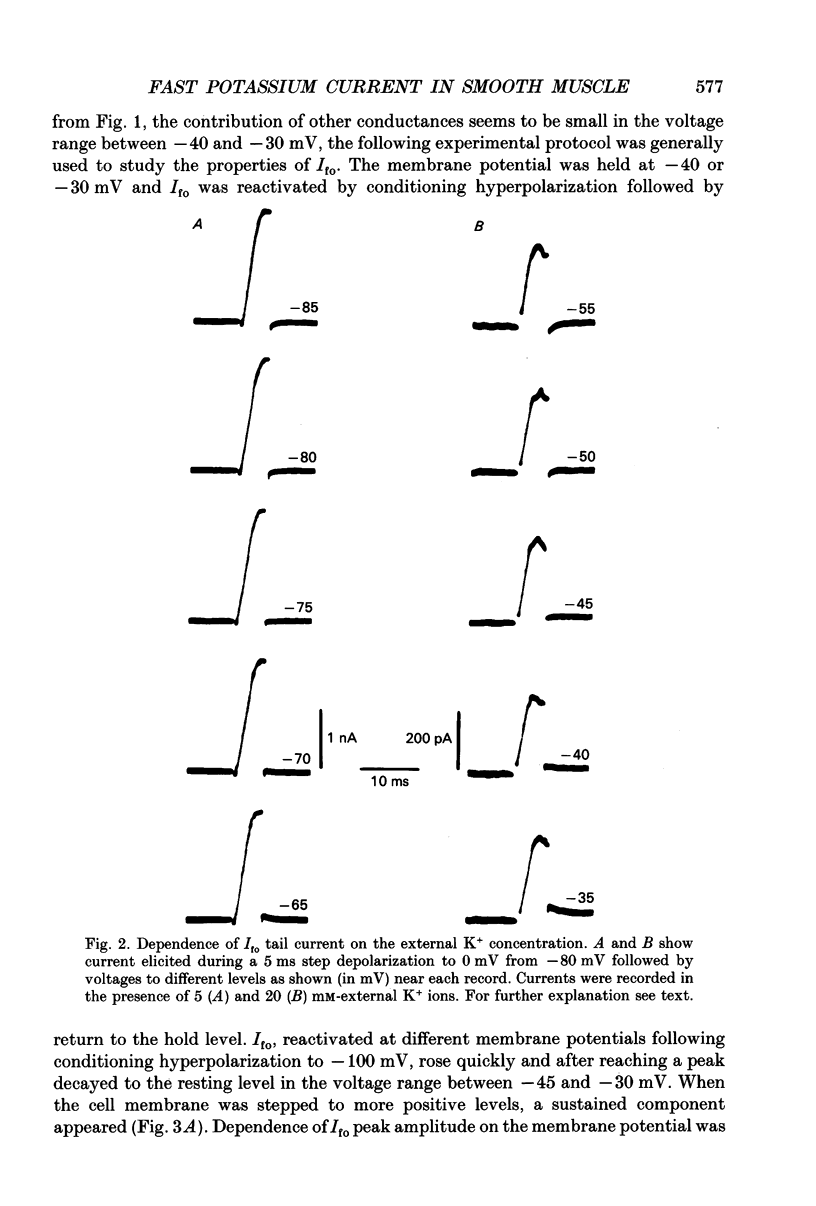
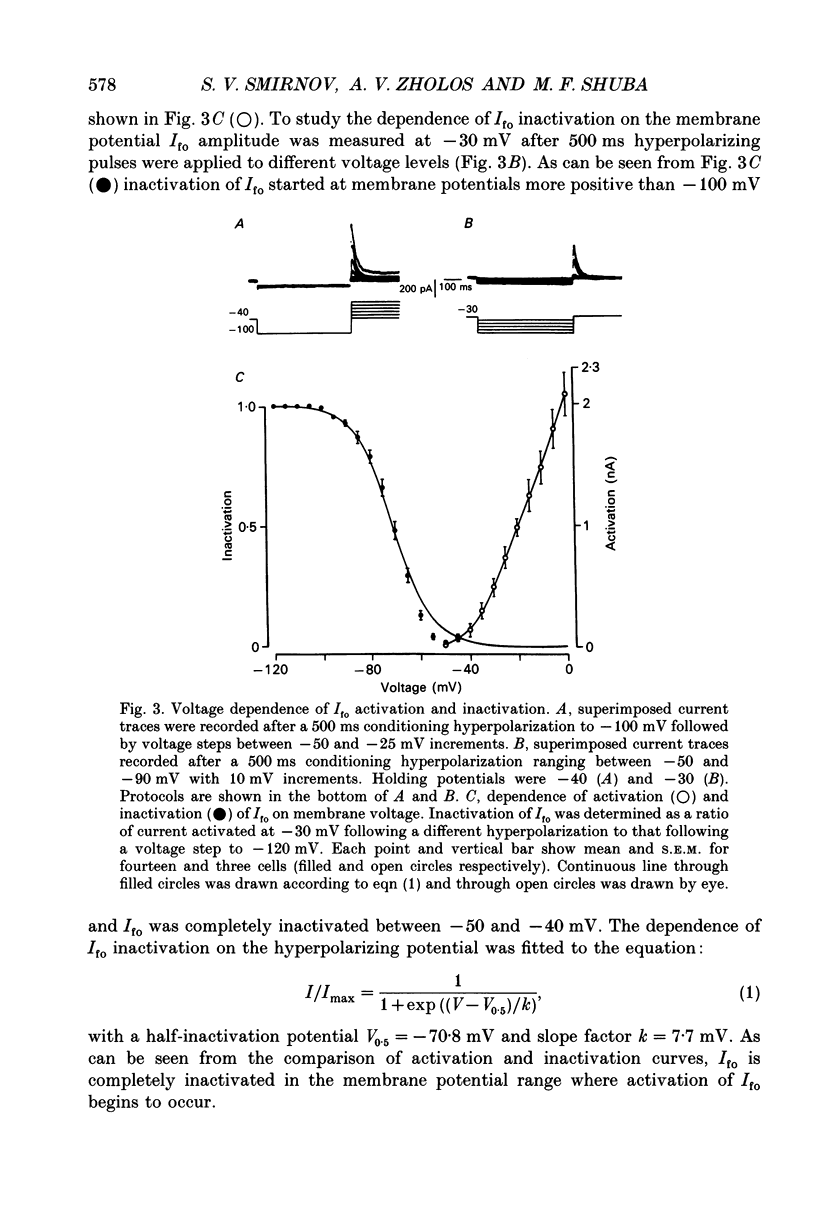
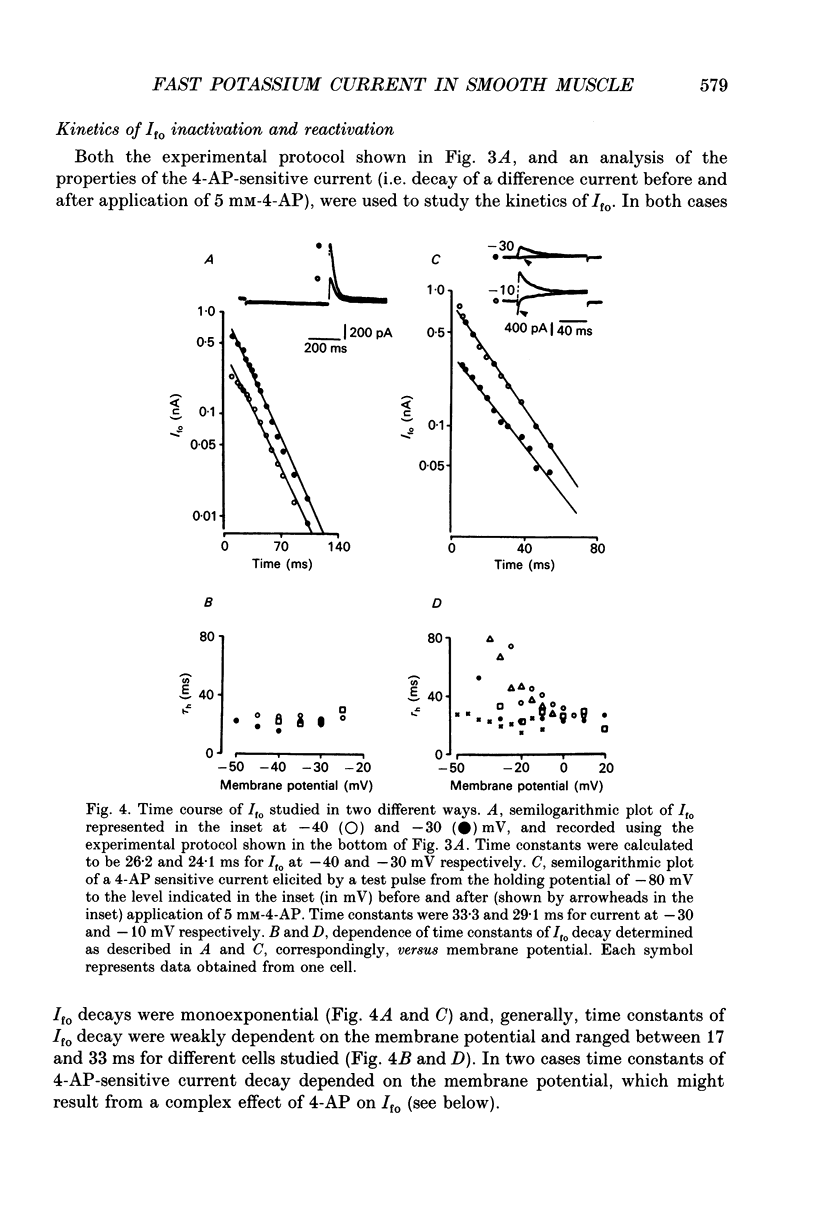
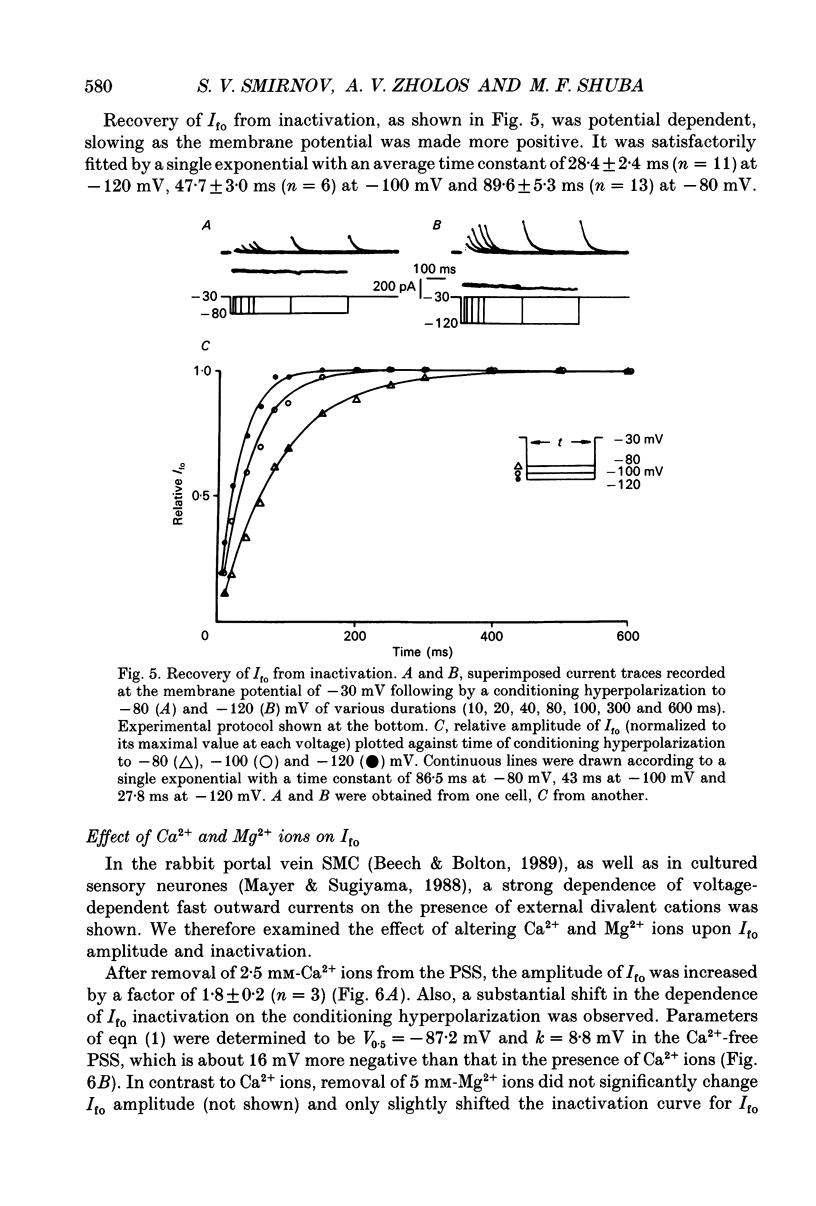
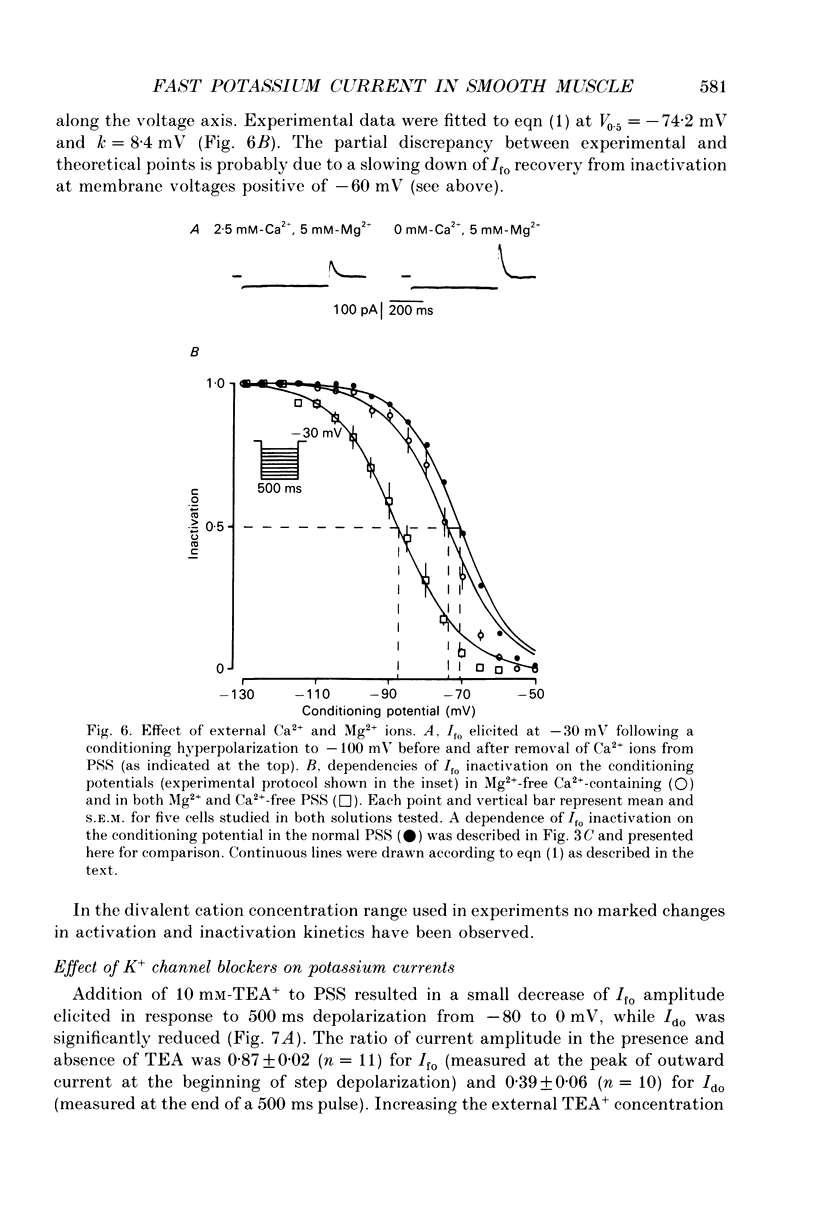
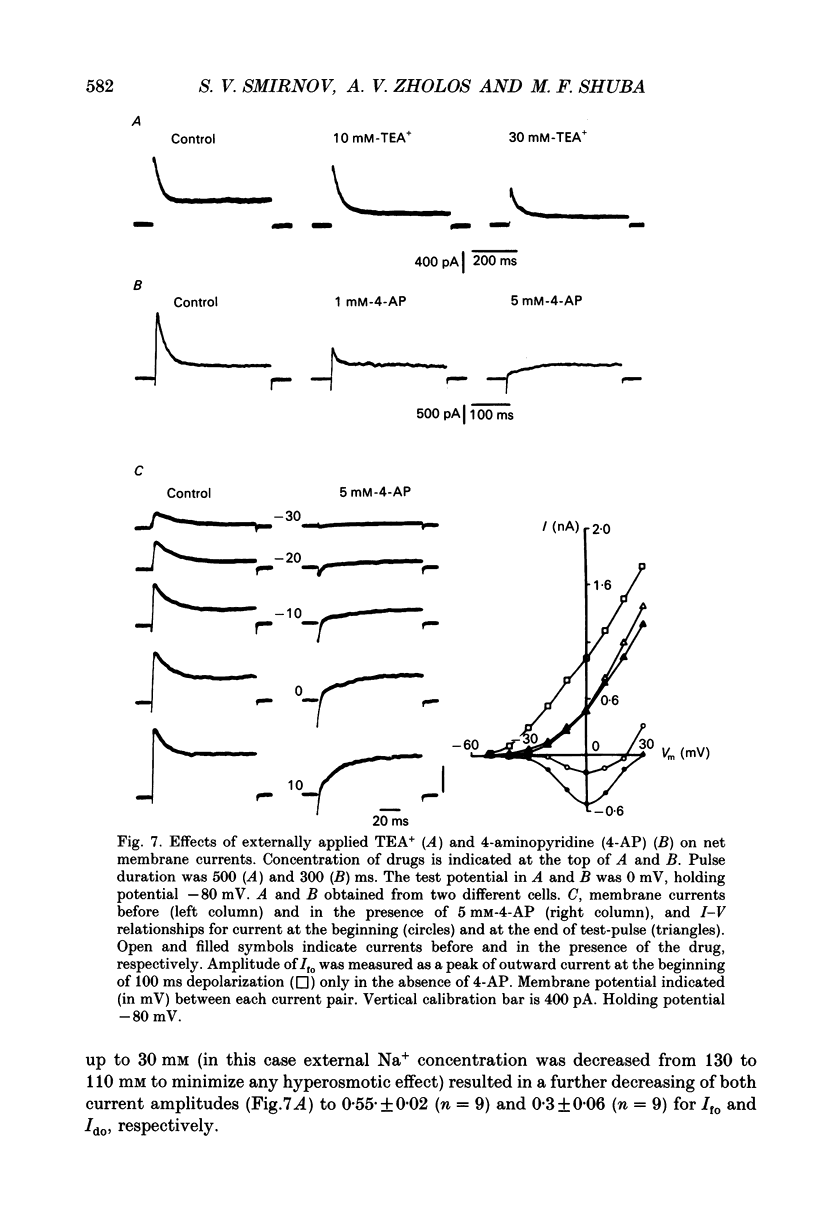
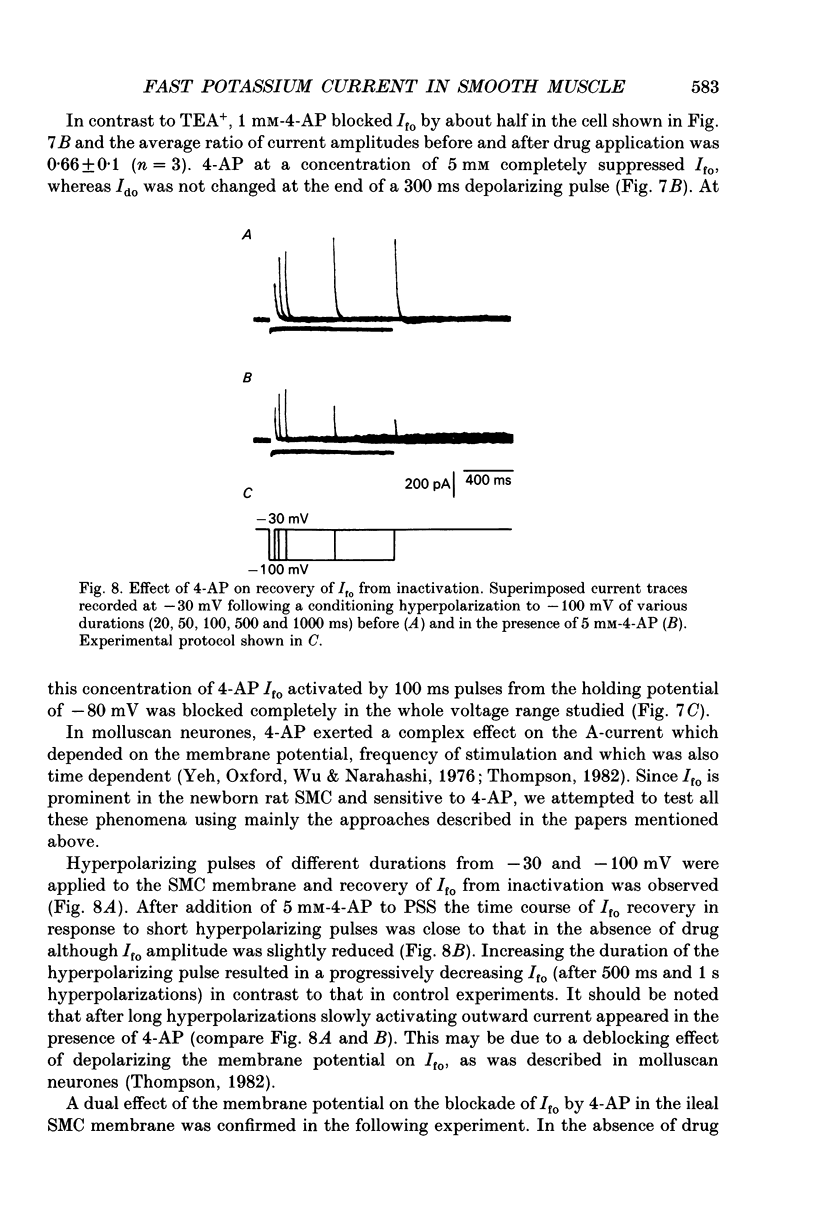
1. Whole-cell outward currents have been studied in single smooth muscle cells isolated from newborn and adult rat ileum, using fire-polished glass micropipettes. 2. Two major outward currents, delayed (I(do)) and fast inactivating potential-dependent (I(fo)), have been observed in the newborn rat ileal cells. I(fo) is activated between -50 and -40 mV from the holding potential of -80 mV, whereas I(do) usually starts to activate at membrane potentials positive to -20 mV. Activation of I(do) was fast, its time-to-peak decreased from 10.8 +/- 0.9 ms (n = 5) at -30 mV to 4.5 +/- 0.7 ms (n = 4) at 20 mV. 3. I(fo) decay was monoexponential and its time constant did not depend on the membrane potential. Dependence of I(fo) inactivation on membrane voltage in normal physiological salt solutions (PSS) could be described by the Boltzmann equation with the following parameters: a half-inactivation potential, V0.5 = -70.8 mV and slope factor, k = 7.7 mV. 4. Recovery of I(fo) from inactivation was fitted by a single exponential and was potential dependent. The average time constant was 28.4 +/- 2.4 ms (n = 11) at -120 mV, 47.7 +/- 3.0 ms (n = 6) at -100 mV and 89.6 +/- 5.3 ms (n = 13) at -80 mV. 5. Removal of Ca2+ ions from the PSS (in the presence of 5 mM-Mg2+) increased I(fo) amplitude by about two times, and shifted its voltage dependence of inactivation towards negative membrane potentials by about 16 mV (V0.5 = -87.2 mV). Removal of Mg2+ from the PSS (in the presence of 2.5 mM-Ca2+) exerted no effects upon either inactivation dependence (V0.5 = -74.2 mV) or I(fo) amplitude. 6. I(do) and I(fo) had different sensitivities to K+ channel blockers. With 10 mM-external TEA+ I(do), was preferentially suppressed, while 5 mM-4-aminopyridine (4-AP) completely blocked I(fo). I(fo) was also partially blocked by a higher TEA+ concentration (30 mM), which suppressed I(fo) to 0.55 +/- 0.02 (n = 9). The blocking effect of 4-AP on I(fo) was potential, use and time dependent. 7. Ileal cells isolated from the adult rat demonstrated the presence of two populations of smooth muscle cells. One has an outward current which seems to be similar to that described in the newborn rat. However, in other cells spontaneous transient outward currents, well described in other single smooth muscle cells, but not found in newborn rat ileal cells, have been observed.
Full text
PDF


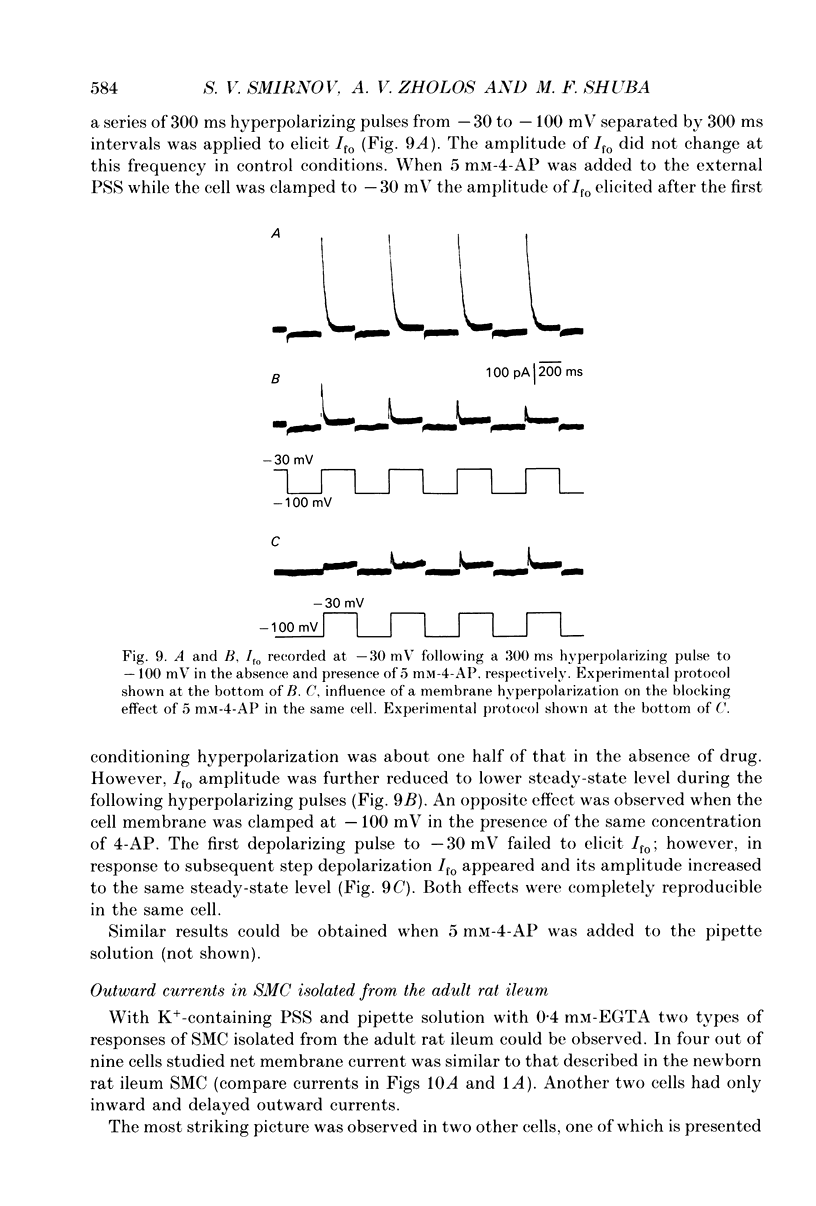
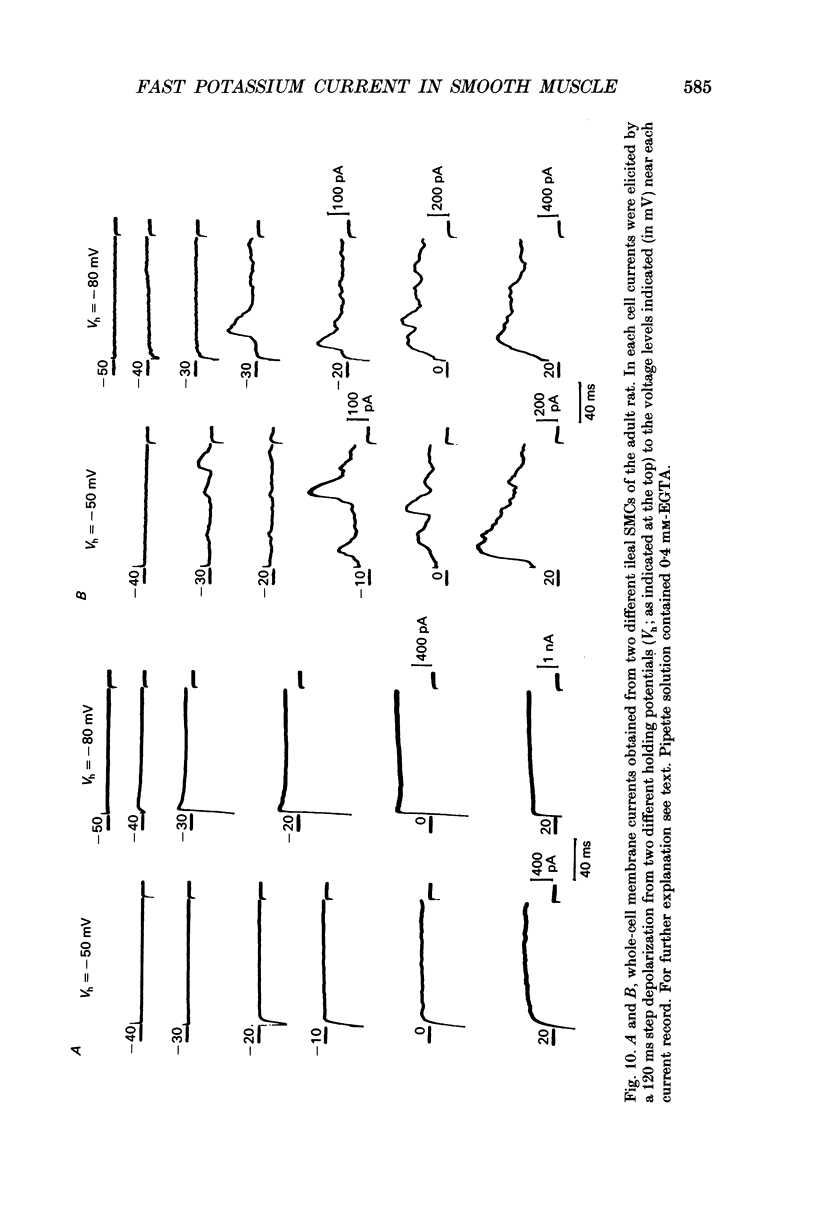




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld D. R., Hume J. R., Krier J. Action potentials and membrane currents of isolated single smooth muscle cells of cat and rabbit colon. Pflugers Arch. 1990 Mar;415(6):678–687. doi: 10.1007/BF02584005. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T., Kurachi Y., Sugimoto T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflugers Arch. 1990 Apr;416(1-2):151–161. doi: 10.1007/BF00370237. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1990 Aug;427:301–324. doi: 10.1113/jphysiol.1990.sp018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kitamura K., Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987 Aug;409(6):561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Shuba M. F. Tetrodotoksinchuvstvitel'nye natrievye kanaly v membrane gladkomyshechnoi kletki kishechnika krysy. Dokl Akad Nauk SSSR. 1989;308(6):1485–1489. [PubMed] [Google Scholar]
- Smirnov S. V., Zholos A. V., Shuba M. F. Potential-dependent inward currents in single isolated smooth muscle cells of the rat ileum. J Physiol. 1992 Aug;454:549–571. doi: 10.1113/jphysiol.1992.sp019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. Aminopyridine block of transient potassium current. J Gen Physiol. 1982 Jul;80(1):1–18. doi: 10.1085/jgp.80.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Hu S. L., Kao C. Y. Outward current in single smooth muscle cells of the guinea pig taenia coli. J Gen Physiol. 1989 Mar;93(3):551–564. doi: 10.1085/jgp.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


