Abstract
Tumor spheroids are one of the well-characterized 3D culture systems bearing close resemblance to the physiological tissue organization and complexity of avascular solid tumor stage with hypoxic core. They hold a wide-spread application in the field of pharmaceutical science and anti-cancer drug research. However, the difficulty in determining optimal technique for the generation of spheroids with uniform size and shape, evaluation of experimental outputs, or mass production often limits their usage in anti-cancer research and in high-throughput drug screening. In recent times, several studies have demonstrated various simple techniques for generating uniform-size 3D spheroids, including the hanging drop (HD), liquid overlay technique (LOT), and microfluidic approaches. Morphological alterations apart from biochemical assays, and staining techniques are suitably employed for the evaluation of experimental outcomes within 3D spheroid models. Morphological alterations in response to effective anti-cancer drug treatment in 3D tumor spheroids such as reduced spheroid size, loss of spheroid compactness and integrity or smooth surface, are highly reliable. These alterations can significantly reduce the need for biochemical assays and staining techniques, resulting in both time and cost savings. The present article specifically covers a variety of available procedures in spheroid generation. For practical applicability, we have supplemented our review study with the generation of glioblastoma U87 spheroids using HD and LOT methods. Additionally, we have also incorporated the outcome of U87 spheroid treatment with doxorubicin on spheroid morphology.
Keywords: Tumor, 3D spheroid, Reduced spheroid size, Hanging drop, Liquid overlay technique, U87
Introduction
Solid tumors accounts for over 90% of total cancer occurrences and deaths [1, 2]. Among the in vitro cancer model systems, 3D tumor spheroids represent one of the most well-characterized culture systems for cancer study [3]. 3D tumor spheroids have a close resemblance to the physiological tissue organization and complexity [4], bearing cell–cell or cell–matrix interactions, a concentric arrangement of cell populations into heterogeneous proliferating regions, or the physicochemical gradients that mimic avascular solid tumor stage with a hypoxic core [5–7]. Gene expression and signaling profile of 3D tumor spheroids bear close similarity to pathophysiological conditions [8]. 3D spheroids also exhibit drug resistance [9], with a more pronounced effect in spheroids of diameters ≥ 400 μm [10, 11]. Additional attractiveness is the inherent ability of the multicellular spheroids to generate their own extracellular matrix proteins including fibronectin, laminin, collagen, and glycosaminoglycans [12, 13]. These characteristics render them as an invaluable tool for predicting the in vivo activity of novel compounds. Several studies have demonstrated the utility of 3D tumor spheroids in a range of applications, including screening of anti-cancer drugs with anti-proliferative properties [14], assessing cytotoxicity [15], to investigate tumor invasion [16], tumor cell migration [17], tumor resistance [18], tumor penetrating ability of drugs [19], tumor angiogenesis [20], immunotherapies [21], characterizing nanoparticle penetration beyond the blood vasculature [22], and investigating the anti-cancer effect of drugs and their formulations, such as nanoparticles [23].
Despite several desirable attributes, frequency of 3D tumor spheroids’ use as a preclinical model for cancer research is relatively low. Although the concept of 3D spheroids emerged as early as the 1950s, a review of PubMed search engine using the keyword, “tumor spheroid/s,” revealed a minimal presence of about 0.5 (4901) from over 1 million articles with keyword, “tumor/s” published in the past 5 years. In a worldwide survey of in vitro pre-clinical models, 2D culture systems of cancer cell lines, accounted for over 66%, compared to 3D culture, which constituted only 10% [24]. The commonly reported limitations leading to poor utility of 3D tumor spheroids involve the challenges in generation of spheroids with uniform size and shape, variation in methods of spheroid generation, evaluation of experimental outputs, and achieving reproducible mass production [25, 26]. The techniques for generation of 3D spheroids, such as hanging drop (HD) [27], liquid overlay technique (LOT) using ultra-low attachment (ULA) microplates, or polyethylene glycol dimethacrylate hydrogel microwell arrays [28], have been exploited to create uniform size 3D multicellular tumor spheroids compatible for anti-cancer drug research and high throughput screening (HTS). This is achieved by regulating the culture media volume or cell suspension. The response of anti-cancer drug in 3D tumor spheroids can be effectively evaluated using morphological parameters, biochemical assays, and staining techniques to assess the morphology of spheroids. Morphological characterization includes determining the size, shape, volume, diameter, area, perimeter, spheroid compactness, and sphericity. These parameters can be cost-effectively evaluated using brightfield microscopy [29]. On the other hand, the biochemical assays and staining techniques used for evaluation of spheroids include Annexin V-FITC for early apoptotic cell determination [30], propidium iodide for dead cells with damaged membranes [31], Calcein AM for the quantification of live cells [32, 33], immunohistochemistry (IHC) for unique marker proteins [29], and Alamar blue for metabolically-active cells [34]. Investigators also use cell viability assays such as Trypan blue exclusion test, Perfecta 3D-cell viability assay, and the CellTiter-Glo 3D cell viability assay [3]. These techniques are either time consuming, laborious, or highly expensive.
The aim of this review is to illustrate the nature of 3D tumor spheroids, their cellular organization, and simple methods of their generation. It also highlights the different forms of cellular/morphological structuring exhibited by 3D tumor spheroids in response to effective anti-cancer drug treatment. This study directs establishment of a link between the different forms of morphological alterations exhibited by tumor spheroids and their effective drug responsiveness for future applications in the screening of anti-cancer drugs. 3D tumor spheroids can be generated with or without any scaffold or physical support. This study primarily focuses on scaffold-free spheroids or spheroids in suspension. For practical benefit, we have supplemented this review with a study on glioblastoma (GBM) U87 3D spheroid generation and their typical morphological alterations in response to the standard anti-cancer drug doxorubicin (DOX).
Cellular organization of 3D tumor spheroids
3D tumor spheroids are closely packed self-assembled cultures of tumor cells. The cellular architecture of 3D tumor spheroids, beginning around the 4th day after seeding, generally comprises of three zones: necrotic, quiescent and proliferative zone [35] (Fig. 1A). The necrotic zone forms the core, consisting of dead cells with elevated extracellular acidity, accumulation of lactate and other cellular waste, low oxygen, and poor nutrient content [35–37]. The quiescent zone represents the region between the necrotic and proliferative zone, characterized by non-proliferative cells. Oxygen and nutrient content are comparatively higher here compared to the necrotic region, gradually increasing towards the outer zone forming a gradient. The outer or the proliferative zone is composed of actively dividing cells [35, 38]. The cell concentration is densest within the core, gradually diminishing towards the periphery. The periphery or the outer proliferative zone of the spheroid simulates the actively dividing tumor cells adjoining blood vessels in in vivo tumor conditions [37]. The avascular nature of solid tumors are finely recapitulated by spheroids with diameters greater than 400 µm [39] or by 5 to 9 days old spheroids with diameters exceeding 200 µm [40].
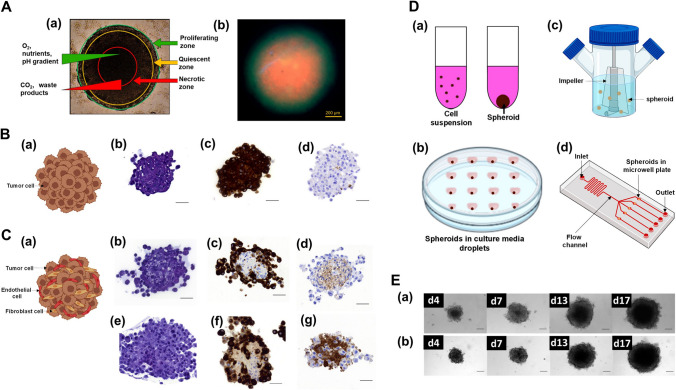
Fig. 1.
3D tumor spheroids. A Cellular organization of 3D tumor spheroids: diagrammatic representation of 3D multicellular tumor spheroid micro-zones (a); fluorescent image of live (FDA/green)/dead (PI/red) assay on breast cancer MDA-MB-231 cell spheroid (b). Adapted from [41]. B Mono-multicellular 3D tumor spheroids: schematic representation (a), histological analysis of PANC-1 3D spheroid at day 4 post seeding — hematoxylin eosin staining (b), cytokeratin AE1/AE3 (c) and fibronectin immunostaining (d), scale bars is 50 µm. Adapted from [42]. C Hetero-multicellular 3D tumor spheroids: schematic representation (a), histological analysis of PANC-1: MRC-5 3D spheroid at day 4 post seeding (b–d) — hematoxylin eosin staining (b), cytokeratin AE1/AE3 (c) and fibronectin immunostaining (d), histological analysis of PANC-1:MRC-5:HUVEC at day 4 post seeding (e–g) — hematoxylin eosin staining (e), cytokeratin AE1/AE3 (f), fibronectin immunostaining (g), scale bars is 50 µmm. Adapted from [42]. D Schematic representation of 3D spheroid generation methods: liquid-overlay technique (a); hanging-drop method (b); spinner flask (c); microfluidic device (d). E Timeline of mono-type human pancreatic cancer cell line PANC-1 spheroid from day 4 to 17 (a); timeline of hetero-type spheroid: PANC-1 co-cultured with human lung fibroblasts MRC-5 from day 4 to 17 (b), scale bar is 50 μm. Adapted from [42]. All the adapted images are licensed under the terms and conditions of Creative Commons (Creative Commons Attribution 4.0 International License). FDA, fluorescein diacetate; PI, propidium iodide
Cell type-based 3D tumor spheroids
The concept of 3D spheroids dates back to the 1950s where co-cultured chondrogenic and myogenic chick embryo cells spontaneously formed unobtrusive aggregates [43]. This concept eventually found application in cancer biomedical research and various other fields in the quest to create a more advanced in vitro model [44]. 3D spheroids can be generated using either only one type of cell (mono-multicellular) or two or more different cell types (hetero-multicellular) (Fig. 1B, C).
Mono-multicellular tumor spheroids
Mono-multicellular tumor spheroids involve culturing of target cancer cells. They serve as a valuable experimental model for avascular tumor growth stage [45]. Several studies have employed mono-multicellular spheroids to screen novel anti-cancer compounds. For example, among the five novel 2-oxo-1,2,3,4-tetrahydropyrimidines (THPMs) compound series (4c, 4d, 4f, 4 k, and 4 l), 4c and 4d exhibited the highest cytotoxic activity against cervical adenocarcinoma using 3D HeLa mono-multicellular spheroids [46]. In another study, cytotoxic activity of novel 1,2,4-triazole-3-thiol derivatives bearing a hydrazone moiety was determined to be highly effective against human melanoma using 3D mono-multicellular spheroids of human derived melanoma IGR39 cells [47].
In addition to screening of cytotoxic agents, 3D mono-multicellular tumor spheroids have demonstrated diverse application in the screening of agents that can inhibit tumor cell proliferation, metastasis, and apoptosis. The anti-proliferative activity of CUDC-907 (fimepinostat) against hepatocellular carcinoma (HCC) was showed using mono-multicellular spheroids of HCC cells, including SMMC-7721, HuH-7, SNU-449, and BEL-7402 [14]. Similarly, the anti-proliferative activity of novel synthesized THPMs, inducing cell-cycle arrest at sub G1 phase and G2/M, was determined using cervical adenocarcinoma HeLa cell mono-multicellular spheroid [46]. In the same study, the anti-invasive activity of these compounds against cervical adenocarcinoma was shown using HeLa cell spheroids. The apoptotic activity of cisplatin (CPT) against epithelial ovarian cancer was determined in A2780 cell mono-multicellular spheroids, demonstrating increased expression of caspase 3/7 positive or apoptotic cells [48].
Mono-type multicellular spheroids are the most common type of 3D tumor spheroids employed for screening of anti-cancer drugs. Their generation is cost-effective and easy to handle compared to hetero-type multicellular spheroids. However, the lack of cellular elements/components of the tumor microenvironment (TME) such as fibroblasts, endothelial cells, macrophages, immune cells [49] is the major limiting factor in the usage of these spheroids.
Hetero-multicellular spheroids
This involves the generation of 3D spheroids comprising of target tumor cells co-cultured with one or more stromal components to recapitulate TME or other type of tumor cells. The tumor cells and the surrounding stromal cells/tissues influence each other, and incorporation of TME components is critical in the screening of cancer therapeutics to increase the clinical relevance of the novel lead [50]. Fibroblast [51] and endothelial stromal cells are the most commonly co-culture cell types with target cancer cell line (75%), followed by immune cells (62.5%) and other types of cancer cells (12.5%) [24]. Human pancreatic cancer PANC-1 cells have been successfully co-cultured with human lung fibroblasts MRC-5 in ratios of 1:1; 1:2; 1:4 and 1:9 (PANC-1 cells kept constant at 500 cells/spheroid) as a model for screening of pancreatic cancer therapeutics [42]. In the same study, spheroids consisting of PANC-1 and MRC-5 cells, along with human umbilical vein endothelial cells (HUVECs), were generated in the ratio of 1:9:4 (PANC-1 cells kept constant at 500 cells/spheroid) for the same purpose. Similarly, co-cultures of MCF-7 cells with HUVECs [52], HUVECs with MDA-MB-231 [53], and human GBM cells U87 and KNS42 [54] have been established as hetero-multicellular 3D spheroid model for breast cancer studies and investigation of metastatic behavior, respectively. Hetero-multicellular spheroids are also generated from patient’s tumor biopsies [55].
Technically, the hetero-multicellular 3D spheroids have greater relevance to pathophysiological condition than mono-multicellular 3D spheroids, as they involve co-culturing with other cellular components. Additionally, few hetero-multicellular spheroids tend to exhibit resistance to anti-proliferative drugs as in pathological conditions [42]. However, hetero-multicellular spheroids are relatively expensive and challenging to constitute and difficult to analyze due to the involvement of normal cells with limited life span. Therefore, the use of mono-multicellular spheroids is highly favored [24].
Methods of 3D tumor spheroid generation
Generating 3D tumor spheroids, both of mono- and hetero-multicellular origin requires certain modifications compared to normal 2D cell culture systems. They can be generated from a single-cell proliferation or pre-aggregated cells. A crucial parameter of in vitro 3D tumor spheroids, as a model system, is ensuring the usage of spheroids with homogenous size to avoid data variability [3]. Simultaneously, determination of the optimum volume or size prior to conducting any assay is critical to the usage of 3D tumor spheroid models. Large spheroids with size exceeding 400 μm interfere in efficient drug response assessment and negate the true evaluation of drug effectiveness. For instance, 3D spheroids of GBM cell lines LN229 and U87 ≥ 400 µm did not exhibit a response to 100 µM temozolomide (TMZ), a standard chemotherapeutic drug for GBM. In contrast, spheroids within the size range of ~ 150–350 µm effectively responded to TMZ [56]. Large-sized spheroids demand sophisticated analytical tools and techniques, resulting in increased time-consumption, and hindering the penetration of fluorescence dyes to the core, thereby making the study more challenging [57]. Commonly employed scaffold-free culture methods for 3D spheroid generation, including (a) liquid-overlay technique (LOT), (b) hanging-drop (HD) method, (c) spinner bioreactors/spinner flask technique, (d) microfluidic system (as shown in Fig. 1D), along with their merits, demerits, and associated ready-to-use commercial products are described below.
Liquid-overlay technique (LOT)
This method, in principle, involves allowing cells to settle at the base of a non-adherent conical/round bottom well, facilitating their aggregation and the formation of spheroids. The most commonly used culture plate for this purpose is the 96-well ULA plate or plates fabricated with surface coatings of agar [54], agarose [58], poly-HEMA [59], and N-octanoyl glycol chitosan [60]. These non-adherent properties prevent the cells from adhering to the culture plates and promote enhanced cell–cell adhesion. Apart from 96-well plate, non-adherent 24 well [58, 61–63] and 35 mm culture plates [60] have also been employed for the generation of spheroids by LOT. This technique has been utilized to generate 3D spheroids for several tumor associated studies (Table 1).
Table 1.
Overview of studies on tumor spheroids generated by liquid-overlay technique
| Human cancer cell line type | Cell seeding density | Culture plate/matrix type | Spheroid size (diameter) at indicated day of observation | Reference |
|---|---|---|---|---|
| Breast carcinoma: MDA-MB-231 | 1 × 104 cells/well | 96-well flat bottom coated with Agarose (1.5%), GFR (10%) and Matrigel (5%) | Around 500 µm (day 6); 1 mm (day 12) | [64] |
| Breast cancer: MDA-MB-231 | 5000 cells/well | 96-well round-bottom plates coated with poly-HEMA (0.5%) | 0.42 × 0.42 mm (24 h) | [65] |
| Breast cancer: BT-474 | 3000 cells/well | 96-well round-bottom ULA plates | Approx. 250 μm (48 h) | [66] |
| Breast cancer: BT474 and EFM192A | 5 × 103 cells/well | 96-well round-bottomed plates coated with Poly-HEMA | N/A | [59] |
| Breast cancer: HCC1954 | 3 × 103 cells/well | |||
| Bladder cancer: RT4 | 1 × 103cells/well (0.5 × 104 cells/ml) | 96-well round-bottom ULA | 402.01 ± 34.01 μm (day 7) | [30] |
| 2.5 × 103 cells/well (1.25 × 104 cells/ml) | 492.14 ± 25.32 μm (day 7) | |||
| 5 × 103 cells/well (2.5104 cells/ml) | 500 μm (120 h) | |||
| 7.5 × 103 cells/well (3.75 × 104 cells/ml) | > 500 μm (7 days) | |||
| Endometrial cancer: Ishikawa, Hec-50B | 4 × 103 cells/well | 96-well ULA microplate | N/A | [67] |
| Glioblastoma: LN229 | 1 × 103 cells/well | 96-well spheroid microplates | N/A | [68] |
| Glioblastoma: U373 | 5 × 105 cells/plate | 35 mm ULA culture plates with OGC coating | 76.2 ± 20.8 μm (day 1) | [60] |
| Glioblastoma: U87 | 1000 cells/well (0.5 × 104cells/ml) | 96-well ULA plate | 300 to 500 µm (day 4) | [54] |
| 1000 cells/well (0.5 × 104cells/ml) | 96-well flat-bottom plate coated with Agar | N/A | ||
| Glioblastoma: U87 | 10–200 cells/well (2 × 103 to 4 × 104cells/ml) | 96-well low attachment plate | Diameter: 129 μm (24 h), 234 μm (48 h), 303 μm (72 h) and 357 μm (96 h) | [69] |
| Glioblastoma: U87 | 890–5 × 103 cells/well (0.89 × 104 to 5 × 104 cells/ml) | 96-well round-bottom ULA plates | Diameter: 333 ± 4 to 393 ± µm (Day 2); 818 ± 2 µm (day 12) | [70] |
| Glioblastoma: U251 | 6.0 × 105 cells/well | 24-well culture plate coated with Agarose (2.5%) | N/A | [61] |
| Glioblastoma: U251 | 2 × 103 cells/well (1 × 104cells/ml) | 96-well low attachment plate | Diameter: 135 μm (24 h), 229 μm (48 h), 323 μm (72 h) and 461 μm (96 h) | [69] |
| Glioblastoma: A172 | 2 × 103 cells/well (1 × 104cells/ml) | 96-well low attachment plate | Diameter: 71 μm (24 h), 191 μm (48 h), 240 μm (72 h) and 367 μm (96 h) | [69] |
| Glioblastoma: A172 | 890–5 × 103 cells/well (0.89 × 104 to 5 × 104 cells/ml) | 96-well round-bottom ULA plates | Diameter: 333 ± 4 to 393 ± µm (day 2); 664 ± 24 µm (day 12) | [70] |
| Hepatocellular carcinoma: Hep-G2 | 3000 cells/well | 96-well microlitre plate coated with S- layer protein | 746 ± 12 µm (day 1); 857 ± 19 µm (day 9); 810 ± 30 µm (day 12); 766 ± 51 µm (day 15); 743 ± 10 µm (day 18) | [71] |
| Neuroendocrine cell line: BON1 | 2.4 × 103 cells/well | 96-well round-bottom ULA | N/A | [62] |
| 500 cells/well | 24 well plate with cell repellent surface | |||
| Ovarian cancer: NCI-ADR-RES | 12,000 cells/well | 96-well plates coated with agar (1.5%) | 1259.0 ± 70.8 μm (day 0); 729.0 ± 46.8 μm (day 1); 523.0 ± 20.4 μm (day 3); 550.3 ± 21.0 μm (day 5); 544.3 ± 23.5 μm (day 7) | [72] |
| Ovarian carcinoma: NIH: OVCAR5, SKOV3 | 20,000 cells/well | 24-well plates coated with agarose (0.5%) | 60 to 400 μm (48 h) | [58] |
| Pharynx squamous cell carcinoma: FaDu | 5000 cells/well (5 × 104cells/ml) | 96-well plate coated with agarose (1%) | 500 µm (day 10) | [73] |
| Prostate cancer: LNCaP | 2 × 105 cells/plate | 24-well culture plate | 50–85 μm (day 7) | [63] |
| Human pancreatic cancer cells: PANC-1 co-cultured with human lung fibroblast: MRC-5 | PANC-1:MRC-5 in the ratios of 1:1, 1:2, 1:4, and 1:9 with PANC-1: 500 cells/spheroid as constant | 96-well round-bottom coated with poly-HEMA | N/A | [42] |
| Human pancreatic cancer cells: PANC-1 co-cultured with human lung fibroblast: MRC-5 & human endothelial: HUVEC | PANC-1:MRC-5:HUVEC in the ratio of 1:9:4 with PANC-1: 500 cells/spheroid as constant | 96-well round-bottom coated with poly-HEMA | N/A | [42] |
Abbreviations: GFR, growth factor reduced; Poly-HEMA, poly-2-hydroxyethyl methacrylate; N/A, data not available; OGC-N, octanoyl glycol chitosan; ULA, ultra low attachment
With LOT, spheroids of varied sizes have been generated. GBM 3D spheroids of sizes ranging from 76.2 ± 20.8 μm at day 1 for GBM U373 cell line [60] to 664 ± 24 µm for GBM A172 at day 12 [70] have been obtained. Likewise, 3D spheroids of breast cancer cell lines BT-474, EFM192A, and HCC1954, MDA-MB-231, have been generated with sizes ranging from ~ 250 μm [66] to 1 mm (1000 µm) [64] in diameter at 48 h and day 12, respectively. Conventionally, the size of the spheroids corresponds to the initial seeding density. For instance, GBM U87 cell lines seeded at a density of 10–200 cells/well resulted in spheroid size of 234 μm [69], while those seeded at a density of 890–5000 cells/well resulted in spheroids ranging in size from 333 ± 4 to 393 ± 7 µm size [70] at 48 h. Similar results have been observed in GBM A172 cell line 3D spheroids. GBM A172 cells seeded at a density of 2 × 103 cells/well yielded spheroids of 191 μm in size [69], while those seeded at 890–5000 cells/well resulted in spheroid sizes ≥ 333 ± 4 µm [70] at 48 h. So, increase in initial cell seeding number does influence the size of the spheroid formed but there is no direct correlation.
Advantages and limitation
LOT method offers a significant advantage over others in terms of ease of handling, reduced variability of the performed measurements, high reproducibility, and reduced error compared to other methods [62, 74]. Despite these tremendously appealing features for the generation of well-defined 3D tumor spheroids, there are limitations that hinder its efficient usage. One critical drawback of LOT is the generation of lesser compact spheroids compared to other techniques like HD method [75], which limit accurate evaluation of drug effectiveness. Some innovative studies are being undertaken to overcome this limitation. One approach involves incorporation of rotating mixing (nutator plates) with the conventional LOT method. Nutator plates have been observed to enhance the spheroid cellular compactness compared to conventional LOT alone, as validated in collagen staining and the exhibition of higher resistance to CPT treatment [75].
Merchandised products
There are several ready-to-use commercially-available dishes and plates designed for the generation of spheroids using LOT. These products are available under different brand names such as Corning®, Corning® Elplasia®, Corning® Matrigel®, Nunclon™ Sphera™, Akura™, and PrimeSurface® (Table 2). The dishes/plates are predispensed with low or ULA surface coatings, Matrigel matrix, Nunclon Sphera, high-efficiency ULA coating, and ultra-hydrophilic polymer preventing the cells from adhering to the well surface while imparting the consistency and stability necessary for any biological assays. Many of these plates allow spheroids to be grown and assayed directly within them. The plates have high optical clear bottom that makes them highly suitable for imaging (bright field and confocal). The black/opaque walls prevent cross-talk between wells while the white plates are well-suited for chemiluminescence assays. The availability of plates with advanced well geometry such as the 96-, 384-, and 1536-wells enables the generation of uniform single spheroids across all wells, and facilitating automated visualization and high-throughput screening [76]. In addition to the commonly used U-bottom ULA plates, V and M bottom ULA plates are also available, enabling the generation of compact spheroids [77].
Table 2.
Ready-to-use commercially available low attachment surface plates for application in LOT methods
| Type of plate | Bottom shape | Color | Volume/culture area | Surface treatment | Brand | Reference |
|---|---|---|---|---|---|---|
| 1536-well spheroid microplate | Round | Clear bottom black-walled body | 14 ml | Ultra-Low Attachment (ULA) | Corning® | [76] |
| 384-well spheroid microplate | Round | 90 μl | [78] | |||
| 96-well spheroid microplate | Round | 300 µl | [79, 80] | |||
| 24-well microcavity plate | Round | 3.47 ml | Corning® Elplasia® | [81] | ||
| 6-well microcavity plate | Round | 16.8 ml | [82] | |||
| 96-well microcavity microplate | Round | 360 µl | [83] | |||
| 12 K rectangular flask | 152 microcavities per cm2 | [84] | ||||
| 3D 384-well plate | Flat | Clear bottom black-walled body | Matrigel Matrix | Corning® Matrigel® | [85, 86] | |
| 3D 384-well Plate | Flat | Clear bottom white-walled body | [85, 87] | |||
| 3D 96-well Plate | Flat | Clear bottom black-walled body | [85, 88] | |||
| 96-Well microplate | U-shaped | Clear | 300 µl | Nunclon Sphera | Nunclon™ Sphera™ | [89–91] |
| 96-Well microplate | Flat | 400 µl | [91, 92] | |||
| 24-Well plate | Flat | 1.9 cm2 | [91, 93] | |||
| 12-Well plate | Flat | 3.8 cm2 | [91, 93] | |||
| 6-Well plate | Flat | 9.6 cm2 | [91, 92] | |||
| 90 mm dish | Flat | 56.7 cm2 | [91, 93] | |||
| 60 mm dish | Flat | 21.5 cm2 | [91, 93] | |||
| 35 mm dish | Flat | 8.8 cm2 | [91, 93] | |||
| T75 EasYFlask | 75 cm2 | [91, 94] | ||||
| T25 EasYFlask | Flat | 25 cm2 | [91, 94] | |||
| 384 spheroid microplate | Flat | Black-walled body | 40–50 µl | High-efficiency ULA coating | Akura™ | [95, 96] |
| 384 ImagePro microplate | Flat | Black-walled body | 40–50 μl | [97] | ||
| 96 spheroid microplate | Flat | Black-walled body | 70–80 µl | [95, 98] | ||
| 384-well plate | U-shaped | Clear | 106 µl | Ultra hydrophilic polymer | PrimeSurface® | [77] |
| 384-well plate | U-shaped | White | 106 µl | [77] | ||
| 96-well plate | Spindle | Clear | 200 µl | [77] | ||
| 96-well plate | U-shaped | White | 300 µl | [77] | ||
| 96-well plate | U-shaped | Clear | 300 µl | [77] | ||
| 96-well plate | V-shaped | 300 µl | [77] | |||
| 24-well plate | Flat | 1.8 cm2 | [77] | |||
| 90 mm dish | Flat | 57 cm2 | [77] | |||
| 60 mm dish | Flat | 21 cm2 | [77] | |||
| 35 mm dish | Flat | 9 cm2 | [77] |
Hanging-drop method (HD)
This method was initially developed for studying bacteria within a confined and regulated area, and later it was adapted for neuronal tissue culture by Ross Granville Harrison around the 1900s [99]. In this method, cells aggregate at the bottom of the droplets initially due to surface tension and gravitational force, eventually forming tight junctions [100, 101]. As conventionally observed in spheroid generation, several factors such as initial seeding density, duration and the type of cell lines all play an important role in determining the spheroids generated in HD method. Several studies have incorporated the HD method to generate 3D spheroids (Table 3).
Table 3.
Overview of studies of tumor spheroids generated by hanging drop technique
| Human cancer cell line type | Cell seeding density | Droplet size/volume | Spheroid size at indicated time/day of observation | Reference |
|---|---|---|---|---|
| Bladder cancer: RT4 | 5 cells/µl (0.5 × 104 cells/ml) | 40 µl | Diameter: 268.13 ± 11.80 μm (day 7) | [30] |
| 12.5 cells/µl (1.25 × 104 cells/ml) | Diameter: > 30 μm (120 h) | |||
| 25 cells/µl (2.5 × 104 cells/ml) | Diameter: 340.92 ± 16.98 to 430.57 ± 17.12 μm (day 7) | |||
| 37.5 cells/µl (3.75 × 104 cells/ml) | Diameter: 330.18 ± 14.62 to 563.97 ± 28.53 μm (day 7) | |||
| Breast cancer: MCF-7 | 40–600 cells/µl (4 × 104–6 × 105 cells/ml) | 25 µl | N/A | [102] |
| Breast cancer: MCF-7 | 16 cells/µl (1.6 × 104 cells/ml) | 30 µl | Diameter: 374.01 ± 35.07 µm (120 h) | [103] |
| Breast cancer: MCF-7 | 1 cell/µl (1000cells/ml) | 50 µl | Area: 81,968 μm2 (day 7) | [75] |
| Colon colorectal carcinoma: HCT116 | 100 cells/µl (1.0 × 105 cells/ml) | 20 µl | Volume: 0.059 ± 0.0052 mm3 (day 9) | [104] |
| Colon colorectal carcinoma: HCT116 | 0.68–45.5 cells/µl (3 × 102 to 2 × 104 cells/well of 96-well plate) | 440 µl | Diameter: 1.5 mm (30 days) | [105] |
| Glioblastoma: U251 | 250 cells/µl (2.5 × 105 cells/ml) | 20 µl | Diameter: 105 μm (24 h), 139 μm (48 h), 208 μm (72 h) and 269 μm (96 h) | [69] |
| Glioblastoma: U87 | 250 cells/µl (2.5 × 105 cells/ml) | 20 µl | Diameter: 92 μm (24 h), 143 μm (48 h), 224 μm (72 h) and 252 μm (96 h) | [69] |
| Glioblastoma: A172 | 250 cells/µl (2.5 × 105 cells/ml) | 20 µl | Diameter: 63 μm (24 h), 131 μm (48 h), 207 μm (72 h) and 265 μm (96 h) | [69] |
| Mammary adenocarcinoma: BT474 | 1000 cells/µl (1.0 × 106 cells/ml) | 20 µl | Diameter: ~ 550 µm (day 7); ≥ 750 μm (day 20) | [106] |
| Melanoma: WM1617 | 10 cells/µl (1.0 × 104 cells/ml) | 25 µl | Diameter: 420 µm (14 days) | [107] |
| Ovarian cancer: A2780 | 0.5 cells/µl (500 cells/ml) | 20 µl | Area: 12.30 ± 0.49 × 103 μm2 (day 1); 42.60 ± 1.96 × 103 μm2 (day 7) | [108] |
| Ovarian cancer: OVCAR3 | 0.5 cells/µl (500 cells/ml) | 20 µl | Area: 37.24 ± 7.61 × 103μm2 (day 7) | [108] |
| 5 cells/µl (5000 cells/ml) | Area: 281.01 ± 20.61 × 103μm2 (day 7) | |||
| Ovarian carcinoma: OVCAR8 | 1–10 cells/µl (1000 to 10000cells/ml) | 50 µl | Area: 27,595 ± 1,899 μm2 (50 cells/drop at day 7); 64,722 ± 4,186 μm2 (500 cells/drop at day 7) | [75] |
| Pancreatic: Panc-1, BxPc-3, Capab-1, MiaPaCa-2, AsPc-1 | 1000 cells/µl (1.0 × 106 cells/ml) | 20 µl | N/A | [7] |
Abbreviation: N/A, data not available
Using HD method, cell densities ranging from 10 to 20,000 cells are seeded, resulting in 3D spheroid sizes ranging from 12,300–37,240 to 302,500 μm2 in area at day 7 [106, 108], with general droplet volume of up to 50 µl. Much larger spheroid sizes of area (1.5 mm diameter) could be generated with HD methods utilizing platforms (such as 96-well plate) that can accommodate large culture media volume (about 440 µl) and sustain longer culture duration (30 days) [105]. The spheroid sizes correspond to initial cell seeding density. For example, bladder cancer RT4 cell line seeded at densities of 200, 1000, and 1500 cells, resulted in spheroid sizes of 268 ± 12 μm, 341 ± 17 to 431 ± 17 μm, and 330 ± 15 to 564 ± 29 μm, respectively at day 7 [30]. Similarly, the ovarian carcinoma OVCAR8 cell line, seeded at a density of 50 cells per droplet, generated spheroids with an area of ~ 27,595 μm2, while those seeded at 500 cells per droplet resulted in an area of ~ 64,722 m2 at day 7 [108].
Advantages and limitation
HD method is endowed with several beneficial features rendering it a highly reliable method of spheroid generation. With the HD method, uniform tightly compact spheroids are produced, characterized by a narrow coefficient of variation (10–15%), as compared to other methods (40 to 60%) [75, 109]. Spheroids generated by HD method exhibit a close physiological relevance in terms of chemoresistance to drug such as CPT treatment and robust deposition of extracellular matrix critical to better predictive outcome of in vitro drug evaluation assays [75]. Lack of requirement of specialized equipment or infrastructure makes it the most cost-effective 3D spheroid generating technique [110]. Despite these critical advantages, 3D HD method presents challenges in handling, as there is risk of displacing and disrupting the spheroids while changing the media. Additionally, inability to retain large droplet (> 50 µl) in case of the conventional HD method and lack of compatibility with most plate readers limits their usage.
Merchandised products
Interestingly, commercialized reproducible HD systems are available. Akura™ PLUS Hanging Drop System, previously known as GravityPLUS™ Hanging Drop System, is a 96-well format ready-to-use plate for spheroid generation [111]. It comprises of two plate systems, a HD plate and a spheroid plate made of polystyrene and cyclo olefin polymer. The system is designed for gravity-assisted, uniform-sized spheroids generation, with one spheroid/well. It is automation-compatible, offers secure handling with no surface attachment, and is suitable for various cell types, including those that fail to form spheroids generally or with ULA surfaces. Several studies have shown the successful generation of uniform compact spheroids using the Akura™ PLUS Hanging Drop System. For example, spheroids of breast cancer tissue from surgical resection have been successfully grown ascertained by IHC for specific markers of breast cancer such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), GATA binding protein 3 (GATA-3), and stromal vimentin and epithelial marker pan cytokeratin (panCK) [90]. Similarly, the GravityPLUS™ or Akura™ PLUS hanging drop system has been used for generation of uniform liver spheroids [112], dermis spheroids [113], and estrogen-receptor-negative (ER-) ovarian and breast cancer spheroids [114]. These models were used for drug hepatotoxicity assessment, dermatological research on the aging processes and therapy, and differentiation of ER-tumor subtypes based on Nw-hydroxy l-Arginine (NOHA) as an indicator.
Spinner bioreactors/spinner flask
This method was developed around the 1970s and involves maintaining cells in suspension by constant stirring with an impeller or magnetic stirrer bar to generate robust multicellular spheroids at a large scale [110]. A critical factor in this method is the rotation speed of the spinner (RPM-revolution per minute), as excessive slowing can lead to the settling of cell suspension, while extremely high speeds subjects the cells to pressure from the moving fluid, potentially causing cellular damage [110]. The RPM varies according to the literature, ranging from ~ 30 to 190 RPM, as shown in human hepatic adenocarcinoma SK-Hep-1 cell line [115] and mouse mammary carcinoma EMTo/Ro [116], respectively. The RPM setting for the flask also appears to differ depending on the initial seeding conditions of the cells. For instance, the RPM for mouse mammary carcinoma EMT6 cell line, when seeded as aggregates, was set at 100 [117], whereas for those seeded as single cell suspension, the RPM was set to 180 [118]. 3D spheroids as in vitro model of several cancer cell lines have been generated using spinner flask method and few representative studies are shown in Table 4.
Table 4.
Overview of studies on tumor spheroids generated by Spinner flask-based technique
| Cancer cell line types | Cell seeding density | Speed | Spheroid size at indicated day of observation | Reference |
|---|---|---|---|---|
| Human breast ductal carcinoma: T-47D | 6 × 107 cells in 250 ml SF | 80 rpm | Diameter: 100 ± 50 µm (day 2), 250 ± 50 µm (day 10), 350 ± 50 µm (day 21) | [119] |
| Human bladder carcinoma: MGH-U1 | 1 to 2 spheroids/ml in 250 ml SF | 130 rpm | Diameter: 1000–1200 µm (12 days) | [120] |
| Human colorectal adenocarcinoma: HT29 | 5 × 105 cells | 150 rpm | Diameter: 30 µm increase/day | [121] |
| Human hepatic adenocarcinoma: SK-Hep-1 | 1 × 106 cells in 250 ml GM | 30 rpm | Diameter: 0.30–0.60 mm (2 weeks), 0.85–1.5 mm (3–4 weeks) | [115] |
| Rat astrocytoma/glioma:C6 | 3 × 106 cells/100 ml | 160 rpm | Diameter: 300–1000 µm (3–4 weeks) | [122] |
| Rat gliosarcoma/malignant glioma: 9L | 3 × 106 cells in 250 ml GM | 180 rpm | Diameter: 200–300 µm (week 4) | [123] |
| Mouse mammary carcinoma: EMTo/Ro | 1 × 105 cells/ml in 500 ml SF | 190 rpm | N/A | [116] |
| Mouse mammary carcinoma: EMT6 | 2000 cell aggregates were seeded in 500 ml SF | 100 rpm | Diameter: 500 µm (7 days) | [117] |
| Mouse mammary carcinoma: EMT6 | 104 cells/ml in 165 ml total volume of medium | 180 rpm | Diameter: ~ 53 µm (36–40 h) | [118] |
Abbreviation: GM, growth medium; N/A, data not available; SF, spinner flask
Spinner flask has been employed for the generation of spheroids from single cell suspension as well as from small spheroids (~ 90–100 µm) to generate larger size spheroids (≥ 1000 µm) [124]. Varied sizes of spheroids have been generated with spinner flask and the size tends to increase proportionally with duration of the generation period. Human breast ductal carcinoma T-47D cell line seeded at a density of 6 × 107 cells in 250 ml spinner flask showed a diameter of ~ 100 µm at day 2, while on days 10 and 21, spheroid sizes of ~ 250 and 350 µm were obtained, respectively [119]. Human hepatic adenocarcinoma SK-Hep-1 cell line seeded at a density of 1 × 106 cells in 250 ml growth media, generated spheroid sizes of 300–600 µm and 850–1500 µm at week 2 and 3–4 weeks, respectively [115].
Advantages and limitation
The striking features of spinner flask include the flexibility to manipulate spheroid production in larger number, with fluid movement facilitating the mass transportation of nutrients and waste in and out of the spheroids [125]. The continuous movement or mixing of the cell suspension enables the aggregation of cells to form spheroids [126], and extended culture can be achieved avoiding development of a necrotic core [127]. However, there are major limitations to this technique, including cost and variability in the size and shape of the produced spheroids. In addition, the shear forces could be harmful to low cohesive or adherent cells, constant stirring may prevent the visualization of formed spheroids [125], and the regulation of the system is difficult, apart from regulation of pH and oxygen levels [128].
Merchandised products
Different sizes/volumes of disposable spinner flasks are commercially available under the brand name Corning®, ranging from 125 ml to 3 l [129]. The body of the flask is constructed of polystyrene material, which is commensurable to the conventional glass spinner flasks used for suspension cell line culture. The flask generally comes with a paddle and integrated magnet, which helps to maintain even and smooth rotation as required. Apart from Corning®, spinner flask/bioreactors are available under the brand Eppendorf’s BioBLU® [130] and Wheaton® [131]. Literature review shows limited usage of these ready-to-use disposable spinner flasks. Disposable Corning® spinner flask of volume 125 mL and the 500 mL were used to investigate the role of shear stress levels on the growth and aggregate formation of humane telomerase reversed transcriptase (hTERT) immortalized human adipose-derived mesenchymal stem cell (hASCs) (hTERT-ASC) or the development of growth model [132]. The role of medium viscosity on the growth and aggregation of baby hamster kidney (BHK) cells in stirred tanks was analyzed using 500 cm3 Corning and 250 cm3 Wheaton spinner flasks [131]. No direct study involving the usage of spinner flasks to generate 3D tumor spheroids for novel anti-cancer drug screening or evaluation was noted in the available literature.
Microfluidics
Microfluidics is one of the most advanced innovations to overcome the shortcomings of other 3D tumor spheroid generating technologies. The fundamentals of microfluidics lie in dealing with microchannel systems to regulate small volumes of fluids as low as femtoliters [133]. The operational requirements of microfluidics system comprise a pump and a chip. The pump functions to regulate the fluid flow inside the chip with precision. The chip serves as a device or platform for processing, sensing, detecting and visualizing the fluid and its constituents. The chip is generally transparent, with microchannels connected to outside by inlet/outlet ports or holes. The chip comes in different materials and fabrications, endowed with optical clarity, high-resolution imaging, flexibility, biocompatibility, and gas permeability. These materials include acrylic, glass, acrylic glass/plexiglass (polymethyl methacrylate/PMMA), polystyrene, cyclic olefin copolymer, silicon, silicon wafer or polydimethylsiloxane (PDMS), which is a transparent silicone rubber [134].
By convention, the microfluidics system used for the generation of 3D tumor spheroids involves a chip integrated with microchannels and microwells. The microwell dimensions greatly influence the spheroid generation. The dimensions can vary from a diameter/width of about 100–800 µm to a height/depth of about 90–1000 µm. Breast adenocarcinoma MCF-7 cell line seeded at a density of 1 × 107 cells/ml in microwells of diameters 100 µm and 300 µm resulted in spheroids of diameters 100 µm and 300 µm in 48 h, respectively [135]. Microfluidic cell culture chamber size of 200 × 200 μm2 generated spheroids of human hepatocellular carcinoma cells (HepG2) with an average diameter of 130.5 μm while the larger chamber of 300 × 300 μm2 generated spheroids with average diameter of 212.7 μm [57]. Small tumor spheroids of diameter ~ 80 µm [136] could be generated with this technique [137]. It also has the leverage of generating large spheroids of diameter 530 µm upon integration with the principles of other spheroid generating techniques such as HD method [138]. Table 5 gives an overview of studies involving 3D tumor spheroids generated using microfluidic technique and their modified variants in different conditions, including GBM.
Table 5.
Overview of studies on tumor spheroid generation using microfluidic based technique
| Human cancer cell line type | Cell seeding density | Microchannel/Microwell/droplet dimensions | Spheroid size | Reference |
|---|---|---|---|---|
| Breast tumor: LCC6/Her-2 | 10 × 106 cells/ml with alginate (2%) | Closed channels of diameter 800 μm and height 90 μm | N/A | [139] |
| Breast cancer: MDA-MB-231 | 1 × 106 cells/ml | Microwell of width 100 μm and depth 100 μm | Area: 6.9 ± 0.43 × 103µm2 (overnight) | [140] |
| Breast adenocarcinoma: MCF-7 | 10 × 106 cells/ml | Circular holding sites of diameter 200 µm | N/A | [137] |
| Breast adenocarcinoma: MCF-7 | 1- 8 × 107 cells/ml | Concave microwell of diameter 800 μm and depth 150 mm | Diameter: 150 μm (3 days); spheroids remained constant on days 3–5 | [141] |
| Breast adenocarcinoma: MCF-7 | 1 × 105 cells/µl | Microwells of diameters 100 µm and 300 µm | Diameter: 100 µm and 300 µm (48 h) | [135] |
| Colon cancer: HCT116 | ~ 110 cells/microwell | Concave microwells of diameter 400 μm and depth 200 μm | Diameter: 128.1 ± 16.6 μm (day 2) | [142] |
|
Colorectal: HT-29 Normal fibroblasts: CCD-18Co |
5 × 106 cells/ml (HT-29) and 3 × 106 cells/ml (CCD-18Co) mixed with type I collagen solution | N/A | Diameter: 33% of total spheroids were > 80 μm | [136] |
| Colon carcinoma: HT-29 | 1 × 106 cells/ml | Microchambers of diameter 790 ± 60 µm | Diameter: 140 ± 35 μm (day 8); 270 ± 50 μm (day 15) | [143] |
| Colon carcinoma: HCT116 | 1 × 106 cells/ml | Microwell of diameters 500 µm and depth 200 µm | N/A | [144] |
| Glioblastoma: U87 | 1 × 105 cells/well in 250 μl MEM | An array of pillars of diameter 40 μm and height 70 μm with center-to-center distance of 140 μm | Diameter: 175 to 225 μm (3 days) | [145] |
| Hepatocellular carcinoma: HT-29, Hep-G2 | 1 × 106–5 × 106 cells/ml | Microwells of diameter 200 μm and depth 150 μm | N/A | [34] |
| Lung adenocarcinoma: A549 cells | 5 × 106 cells/ml | U shaped arrays of width 600 μm and height 1000 μm | N/A | [146] |
| Ovarian cancer: OV90 | 12,000 cells/ml | Microfluidic funnel; funnel tip diameter of 1 mm | Diameter: 230–420 μm (day 5) | [138] |
| Ovarian cancer: TOV112D | 12,000 cells/ml | Microfluidic funnel; funnel tip diameter of 1 mm | Diameter: 280–530 μm (day 5) | [138] |
| Breast cancer: MDA-MB-231 with normal mammary epithelial: MCF-10A | 2 × 106 cells/2.5 ml medium in 1:1 ratio | Microwell of diameter 200 µm and height of 220 µm | Diameter: ~ 100 µm (5–6 days) | [147] |
Abbreviations: N/A, data not available; ULA, ultra low attachment; OGC-N, octanoyl glycol chitosan; GFR, growth factor reduced
Droplet-based microfluidic is a powerful tool in recent advancements within the microfluidic domain. Tiny droplets with volumes ranging from nanoliters to femtoliters are generated and cells are entrapped within these droplets. Spheroids hence generated, are experimentally manipulated and analyzed. Usage of microfluidic-based droplet system, resulted in high-throughput generation of robust uniform-sized multicellular 3D spheroids comprising of MCF-7 and mouse fibroblast NIH-3T3 cells in various ratios for anti-cancer drug cytotoxicity/resistance evaluation [148]. Similarly, GBM U87MG cell spheroids sized ~ 99 to ~ 126 μm were generated within aqueous droplets of ~ 181 and 306 μm diameters, respectively, as model for tumor photothermal therapy [149]. The size of the spheroids was controlled by either regulating the cell seeding density, droplet size or flow rate. More than 80% of the droplets were consistent in containing one spheroid per drop. In another study, spheroids of either drug sensitive or drug resistant MCF-7 cell lines, or MCF-7 co-culture with fibroblast HS-5 cell line, were formed within alginate droplets generated in PDMS microfluidic device [137].
Other innovations in research include injection-molded plastic array 3D universal culture platform, known as U-IMPACT. U-IMPACT is a universal 3D microfluidic cell culture platform that consists of three channels with a 96-well array designed as a spheroid zone. The wells have capacity to accommodate spheroids of ~ 500 µm. The platform is suitable for setting up TME for high-throughput evaluation of vascularization, angiogenesis, and tumor cell invasion/migration [150].
Advantages and limitations
The primary advantage of microfluidics technology in 3D tumor spheroid generation is the ability to regulate fluid flows, simulating blood circulation or interstitial fluid flow (IFF) in spatial and temporal domains, mimicking in vivo like microenvironments [133, 147, 151]. In tumors, the IFF gets elevated [147] due to rise in interstitial flow pressure (IFP) that results from dysregulated angiogenesis [152–154] and accumulation of other cellular components [155]. IFF is characterized to promote cancer cell migration/metastasis, resist treatments and recurrence [156]. Considering the critical role of IFF in tumor proliferation and invasion, the microfluidics technique provides an ideal platform for precise manipulation of specific tumor environment, for flow-related studies. The fluid flow within the microfluidic device could be regulated to be directional, mixed, partition, or elaborated as desired [157]. Apart from the flexibility of regulated IFF pressure or versatility to manipulation enabling fine recapitulation of TME, the microfluidic technique is highly reliable for the generation of uniform size spheroids [158, 159]. Uniform-sized spheroid generation could be automated by controlling the flow rate. Introduction of cell suspension into the microfluidic device at a flow rate > 100 µL/min can aid in even distribution of cells across the channel [57]. This system is highly beneficial for limited samples, reagents or chemicals. Most critically, it also enables the visualization, characterization, or analyses of living cells under experimental conditions for longer periods [160]. However, recovery of the spheroids from these devices may be challenging.
Contrary to several benefits, the microfluidic technique is associated with certain limitations when used for the generation of 3D tumor spheroids. In certain setups such as non-cell adherent microfluidic culture system, the size of the spheroids is non-adjustable due to the predetermined size of the wells. While in others such as droplet-based microfluidics, the setup is quite complex. Moreover, in numerous microfluidic setups, the spheroids are initially generated in alternative culture systems before being introduced onto the microfluidic platform.
Merchandised products
Microfluidic systems and platforms are the most desirable advanced technology for application in biomedical research gaining their way into 3D cell market. The global market value of microfluidics is expected to reach USD 158.1 billion by 2031 from USD 21.7 billion in 2021 [161]. Top microfluidic companies uFluidix, Perkin Elmer, Fluidigm, Blacktrace Holdings Ltd. (Dolomite Microfluidic), Micronit, BioFluidix GmbH, Fluigent, ALine Inc., and Philips [162]. Common microfluidic device brands for 3D spheroid generation include CellASIC® ONIX2 (Merck) [163] and OMEGA 4 (eNUVIO inc.) [164]. Limited information is available regarding the generation of 3D tumor spheroids for drug screening and assessment using ready-to-use microfluidic plates. The majority of preclinical in vitro studies involve 3D tumor spheroids for drug screening and evaluation involve self-designed or fabricated microfluidic platforms.
Timeline of 3D tumor spheroid generation
The spheroidal structure depends on the type of tumor cell lines, number of cell lines, cell seeding density, and method of its generation. The cells in the initial stages of spheroid generation tend to be loosely adherent, irregularly round or oval, which eventually progress to form definite spheroid shape within a period of 24 h or longer. Breast cancer cell lines MCF7 and T-47D form compact multicellular spheroids within 24 h of incubation [65], while U87 cells spontaneously form definite spheroids within 48–96 h post incubation [165, 166]. On the other hand, HepG2 cells form definite spheroids by 6th day, post-seeding [71]. Spheroids display smooth surfaces when the cells have aggregated to form tight junctions [101]. Tightly compact 3D spheroids with round morphology are indicative of a robust cell–cell adhesion [30].
The size of the spheroid, the rate at which spheroids are formed, or morphology of the spheroids are shown to be critically dependent on cell seeding density which vary among different cell lines. Seeding density of 500 cells/well of pancreatic cancer cells (PANC-1) in 96 round-bottomed well plates showed high proliferation rate with tenfold increase in spheroid volume, i.e., from 0.03 to 0.3 mm3 (from day 4 to day 17), while seeding density of 5000 cells/well increased spheroid volume by twofold [42]. High cell seeding density of about 5000–10,000 cells tend to result in variable cell aggregation or size and shape in HepG2 cell line [71]. GBM cell lines LN229 and U87 seeded at variable density of 1000–8000 cells/well in 0.9% agarose coated round bottom 96-well plate exhibited well-defined spheroid surfaces, irrespective of the cell density seeded after 7 days post-incubation. However, greater variability was observed in their size, cell proliferation rate, and cellular organization [56]. Cells seeded in the range of 1000–2000 formed spheroids post 24 h of incubation and showed significant increase in size proportional to seeding density throughout the study period (7 days) resulting in spheroid size of ~ 400–700 µm, while cells seeded between 4000 and 8000 formed spheroids within 24 h and resulted in spheroid size of ~ 400–700 µm after 7 days with no significant increase in spheroid size throughout the study period. The study also showed increased necrotic zone in spheroids initiated from 4000 to 8000 cells.
The spheroid sizes tend to increase in a time dependent manner (Fig. 1E). Mammary adenocarcinoma BT474 cell line seeded at a density of 20,000 generated spheroids of diameter approximately 550 µm at day 7 and ≥ 750 μm at day 20 using HD method [106]. Similar pattern was observed in ovarian cancer A2780 by HD method [108] and GBM cell lines U251, U87, and A172 using ULA 96-well plates [69]. In the study involving GBM cells [69] and A2780 cells seeded at a density of 100 cells/droplet resulted in spheroids of area ~ 12 × 103 μm2 at day 1 and ~ 43 × 103 μm2 at day 7. U87 cells seeded at a density of 5000 cells/droplet resulted in spheroids of diameters 92 μm at 24 h, 143 μm at 48 h, 224 μm at 72 h and 252 μm at 96 h, respectively. U251 and A172 cells with similar experimental conditions as U87 cells resulted in spheroids of varied sizes in the following order: diameters 105 μm at 24 h > 139 μm at 48 h > 208 μm at 72 h > 269 μm at 96 h, and 63 μm (24 h) > 131 μm (48 h) > 207 μm (72 h) > 265 μm (96 h), respectively. Despite the same cell seeding density, the GBM cells resulted in varied spheroid sizes at specific culture duration time periods: U87 cells at 24 h formed spheroids of diameter 92 μm, while those of U251 and A172 showed 105 μm and 63 μm at 96 h, U87 spheroid size was 252 μm while those of U251 and A172 were 269 μm and 265 μm, respectively. Therefore, the spheroid size is dependent on inherent growth properties of the cell type, cell size, and their respective growth condition.
Key molecules in 3D spheroid formation
3D spheroid formation is dependent on cell–cell adhesion mediated via several molecules such as E-cadherin, dopamine D2 receptor (D2R), α5 integrin, α5β1-integrin, cadherin-6, and the desmosome proteins DSG2 and DSC2 (Table 6).
Table 6.
Molecules mediating 3D spheroid formation and corresponding cell types in representative studies
| Key molecules | Cell type | Reference |
|---|---|---|
| α5 integrin | Human glioblastoma cell line: U87MG | [167] |
| Human ovarian cancer cell line: OVCAR5 | [168] | |
| β1-integrin | Human ovarian cancer cell line: OVCAR5 | [168] |
| E-cadherin | Human pancreatic ductal adenocarcinoma cell lines: PANC-1 and KLM-1 | [169] |
| Mouse mammary epithelial cancer cell line (4T1); Human mammary epithelial cancer cell line (T-47D); non-tumorigenic mouse mammary epithelial cell line (HC11) | [170] | |
| Human renal carcinoma cell lines: SKRC-6, SKRC-35, SKRC-52, and SKRC-59 | [171] | |
| Human colon adenocarcinoma cell lines: HCT116 H2B–mCherry fluorescent expressing cell line | [172] | |
| Human breast cancer cell lines: MCF7, BT-474, T-47D and MDA-MB-361 | [173] | |
| Cadherin-6 | Human renal carcinoma cell lines: SKRC-6, SKRC-35, SKRC-52, and SKRC-59 | [171] |
| Dopamine D2 receptor (D2R) | Human glioblastoma cell line: U87MG | [174] |
| Desmosome proteins DSG2 and DSC2 | Human colon adenocarcinoma cell lines: HCT116 H2B–mCherry fluorescent expressing cell line | [172] |
| N-cadherin | Human breast cancer cell lines: MCF7, BT-474, T-47D and MDA-MB-361 | [173] |
Several cadherins are involved in cell–cell adhesion [175, 176]. Among them, E-cadherin plays the most significant role [177]. Inhibition of E-cadherin with DECMA-1 antibody inhibited spheroid formation in HC11, 4T1, and T-47D epithelial cell lines [170]. E-cadherin expressing renal cancer cell lines, SKRC-59 and SKRC-6, with ability to form compact type spheroids, failed to form spheroids when E-cadherin was blocked [171]. Blocking of E-cadherin binding site with anti-E-cadherin monoclonal antibody, HECD-1, reduced spheroid formation [172]. Spontaneous spheroid forming breast cancer cell lines, MCF7, BT-474, T-47D, and MDA-MB-361 showed high levels of E-cadherin expression in immunostaining and fluorescence activated cell sorting (FACS) analysis [178]. Significance of E-cadherin was further supported by increased E-cadherin mRNA level expression in pancreatic ductal adenocarcinoma (PDAC) cell lines, PANC-1 and KLM-1, that mediated spheroid formation whereas low cadherin expression in PK-45H and MIAPaCa-2 was associated with decrease in spheroid forming ability [169]. Though the role of N-cadherin in the presence of E-cadherin has been considered to be insignificant in spheroid formation [171], it mediated spheroid compactness [178]. Cadherin-6 mediated the formation of loosely packed spheroids, which were prevented in the presence of anti-cadherin-6 antibody [171].
Like E-cadherin, the role of D2R in 3D spheroid formation was established using depressed U87MG cell line spheroid formation. Spheroid forming ability of U87 cells was reduced in the presence of 100 nmol/L D2R antagonists’ haloperidol, pimozide, thioridazine, and remoxipride. On the contrary, spheroid formation was enhanced in the presence of 100 nmol/L D2R agonists PHNO, ropinirole, and sumanirole [174]. The spheroid formation is reduced in HCT116 cells in the presence of peptides corresponding to the cell adhesion recognition (CAR) sites of desmosome proteins DSG2 and DSC2 [172]. Similarly, the presence of mAb that blocks β1-integrin subunit binding, inhibited spheroid formation in ovarian carcinoma cell, NIH:OVCAR5 [168]. α5-integrin was found to mediate cell–cell cohesion during 3D spheroid formation in U87 cell line [167]. The presence of mAb against the α5-integrin subunit inhibited spheroid formation in ovarian carcinoma cells, NIH:OVCAR5 [168]. Another factor critical to spheroid formation/compaction is actin. Supplementation of the non-tumorigenic mouse mammary epithelial HC11 cell, metastatic mouse mammary epithelial cancer 4T1 cell and human mammary epithelial cancer T-47D cell cultures with cytochalasin D, known to inhibit elongation of actin filaments, inhibited spheroid formation [170, 179]. Together, these observations suggest a direct association of availability of key cell adhesion molecules like E-cadherin in spheroid formation in solid tumor cells.
Anti-cancer drug induced morphological alterations in 3D tumor spheroids
Among several techniques available, morphological parameters such as the size, shape, volume, diameter, area, perimeter, spheroid compactness, and sphericity, of the 3D tumor spheroids, emerge as one of the most reliable and cost-effective ways to determine the effectiveness of anti-cancer drugs. It is the first observable feature and require no special treatment or staining and does not affect the viability of the culture. Several studies have reported striking alterations in aforementioned morphological characters of 3D tumor spheroids in response to anti-cancer drug treatment. Types of morphological alterations to anti-cancer drugs with properties of cytotoxicity, anti-proliferation, and induction of apoptosis, anti-metastasis/anti-invasion, or anti-angiogenesis in 3D tumor spheroids are described below.
Morphological alterations in response to cytotoxic, anti-proliferative, apoptotic drugs
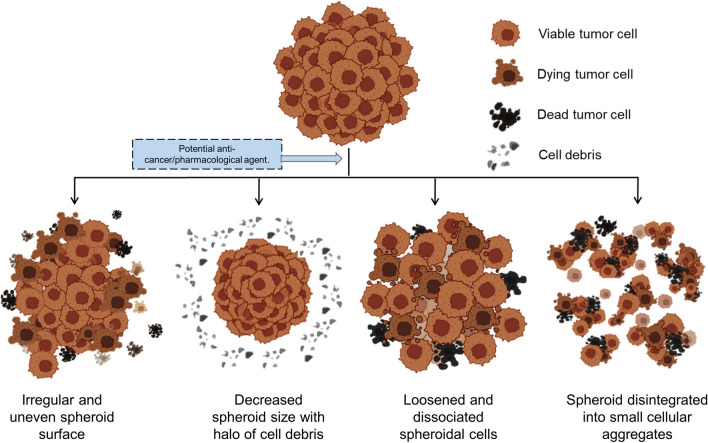
The frequently observed features with cancer targeting cytotoxic, anti-proliferative and apoptotic drugs include decreased spheroid volume/size, loosening or dissociation of cell–cell contacts, detachment of cells from surface layer or edges, frayed and uneven surface appearance, in contrast to compact smooth surface structure in the control (Fig. 2, Table 7).
Fig. 2.
Schematic diagram of morphological alterations in 3D tumor spheroids in response to drug-induced cytotoxicity. A Normal growing spheroid with viable tumor cells. B–E Variable 3D spheroid morphological alterations; irregular and uneven spheroid surface (B); decreased spheroid size with halo of cell debris (C); loosened and dissociated spheroidal cells (D); spheroid disintegrated into small cellular aggregates (E)
Table 7.
Overview of studies using 3D tumor spheroids to evaluation of anti-cancer activity of drugs and their morphological alterations in different cancer cell types
| Cell-based spheroid type (1-mono, 2-hetero) | Cancer cell origin and cell line | Anti-cancer drug/compound | Drug application timepoint | Drug-induced spheroid morphological alterations | Molecular mechanism | Reference |
|---|---|---|---|---|---|---|
| 1 | Breast adenocarcinoma: MCF7 | DOX-loaded PNIPAM-co-AA nanoparticles at | 2 days post spheroid incubation | ↓ size; detached/loosened cells; uneven surface appearance; cell debris surrounding spheroids | ND | [10] |
| 1 | Breast adenocarcinoma: MCF-7 | Tamoxifen | Day 2 post spheroid incubation | Frayed and uneven shape spheroids | ↓ cell viability/ATP | [36] |
| 1 | Cervical adenocarcinoma: HeLa | 5 novel synthesized THPMs (4c, 4d, 4f, 4 k & 4 l) | 48 h post spheroid incubation | ↓ volume; loosened cell–cell contact; detached cells from surface layer | 4d & 4c induced cell cycle arrest at subG1 phase; 4f induced arrest at G2/M | [46] |
| 1 | Colon adenocarcinoma: HT-29 | 5-fluorouracil (5-FU), oxaliplatin (OXP), folinic acid (FA) | 5th day of spheroid culture | ↓ dimensions in spheroids treated with 5-FU & OXP; altered spheroidal shape or lack of smooth contour with OXP alone and 5-FU + OXP + FA combination after 24 h | ND | [180] |
| 1 | Colorectal adenocarcinoma: HCT116 | Topotecan | 3 days post spheroid incubation | ↓ cell cluster size in a concentration-dependent manner | ND | [181] |
| 1 | Colorectal cancer: HT-29 | Trametinib, sorafinib, ponatinib & dactolisib | At 48 h of culture | ↓ size, fluffy or disintegrated spheroids | ↓ ERK1/2 phosphorylation | [182] |
| 1 | Colorectal carcinoma: HCT116 | 4 UAs: C-2028, C-2041, C-2045, C-2053 | At 72 h of culture | ↓ spheroid size compared to control; jagged or diffuse outer layer of spheroid cells | N/A | [183] |
| 1 | Colorectal cancer: HCT-116 | 5-FU, 5-FUChnps7 | After 72 h post spheroid incubation | ↑ irregularity of spherical shape in a dose-dependent manner | ND | [184] |
| 1 | Epithelial Ovarian Cancer: A2780 | CPT | Day 7 of culture | Loosened spheroidal cells |
↑ caspase 3/7 positive or apoptotic cells |
[48] |
| 1 | Glioblastoma: U87 | Temozolomide | 24 h post spheroid incubation | ↓ size; disrupted surface; disintegrated, clustered into irregular aggregates (< 50 mm) | ND | [185] |
| 1 | Glioblastoma: U87 | Temozolomide (TMZ), stiripentol (STP) | 72 h post spheroid incubation | ↓ spheroid growth/area with TMZ & STP alone or in combination (TMZ + STP) compared to control | Induced cell cycle arrest at G2M phase | [186] |
| 1 | Glioblastoma: U87 | DOX conjugated with curcumin (D-C), D-C conjugated to PEGNIO (PEGNIO/D–C), PEGNIO/D–C conjugated with t-Lyp-1 peptide (PEGNIO/D–C/t-Lyp-1) | Day 2 post spheroid incubation | ↓ spheroid diameter with D-C; loss of spheroid compactness and spheroid distortion or dissociation of cells with PEGNIO/D–C and PEGNIO/D–C/t-Lyp-1 | ND | [165] |
| 1 | Gastric cancer: HGC-27 | Family of bufadienolides | Day 4 post spheroid incubation | ↓growth of spheroids | ND | [187] |
| 1 | Head-and-neck cancer: HN3, HN4, HN9 | Sorafenib, CPT | 3 days post spheroid incubation | ↓ spheroid size compared to control | ND | [51] |
| 1 | Hepatocellular carcinoma: HepG2 | CPT, resveratrol, tirapazamine | 24 h post spheroid incubation | ↓ volume in spheroids treated 200 µm Resveratrol; loss of compactness in spheroids treated with 20 µm cisplatin and 200 µm tirapazamine | ND | [57] |
| 1 | Hepatocellular carcinoma: HepG2 | Curcumin, hydroxyurea, cisplatin | 72 h post spheroid incubation | ↓ spheroid size in a time-dependent manner for a period for 48 h duration | ND | [188] |
| 1 | Hypopharyngeal tumor: FaDu; oral squamous cell carcinoma: Cal27; Laryngeal squamous cell carcinoma: pica | CPT | Days 3 to 5 of spheroid incubation | ↓ spheroid growth and size over a period of 16 day time of all the cell lines | ND | [74] |
| 1 | Medulloblastoma: UW228-3 | Etoposide | At day 3 of culture | ↓ volume with a halo of debris and dead cells | ND | [189] |
| 1 | Malignant pleural mesothelioma: H2052/484 | CPT or CPT/pemetrexed (CPT/PMX) combination | After 3 days of seeding | ↓ size dose-dependently compared to non-treated spheroids; huge shrinkage; complete disintegration at the highest conc. (CPT = 200 µM or CPT/PMX = 200/800 µM) | ↓ intracellular ATP (ATPi) | [190] |
| 1 | Melanoma: IGR39, triple-negative breast cancer: MDA-MB-231 or pancreatic carcinoma: Panc-1 | 1,2,4 triazole-3 thiol derivatives | After 48 h of spheroid incubation | Inhibition of growth; disintegrated spheroid | ND | [47] |
| 1 | Pancreatic neuroendocrine neoplasms: BON1 | Sunitinib | 72 h post spheroid incubation | ↓ perimeter; extremely irregular and loose shape | ND | [62] |
| 1 | Prostate cancer: LNCaP | Docetaxel | 3–5 days post spheroid incubation | ↓ spheroidal size in concentration-dependent manner | ND | [55] |
| 1 | Triple negative breast cancer: MDA-MB-157 | CPT | 24 h post spheroid incubation | Disintegrated spheroid | ND | [191] |
| 2 | Head-and-neck cancer HN3, HN4, or HN9 each co-culture with fibroblast | Sorafenib, CPT | 3 days post spheroid incubation | ↓ spheroid size compared to control | ND | [51] |
Abbreviations: D, diameter; ND, not discussed/determined; PEGNIO, polyethylene glycolated niosomes; PEGNIO/D–C/t-Lyp-1, PEGNIO/D–C conjugated with t-Lyp-1 peptide; PNIPAM-co-AA, poly(N-isopropylacrylamide)-co-acrylic acid; PDMS, polydimethylsiloxane; THPMs, 2-oxo-1,2,3,4-tetrahydropyrimidines; ULA, ultra low attachment; ↓-decreased/reduced; 5-FU, 5-fluorouracil
Reduced tumor volume
Reduction in 3D tumor spheroid volume or diameter collectively represented as spheroid size is one of the most striking feature in response to effective anti-cancer drug treatment [57]. Reduced spheroid size has been observed in tumor spheroids treated with well-known anti-cancer drugs such as CPT, DOX, topotecan, 5-fluorouracil, oxaliplatin, trametinib, sorafenib, dactolisib, temozolomide, resveratrol, hydroxyurea, etoposide, pemetrexed, sunitinib, and docetaxel. Some representative studies are included here to highlight the variability of the assay outcome.
Cisplatin (CPT): CPT is an alkylating agent used in the treatment of cancers such as head and neck, lung, bladder, ovarian, cervical cancer, testicular, melanoma, and lymphomas [192]. Alkylating agents kill cancer cells by regulating the substitution of hydrogen atoms on DNA with alkyl groups resulting in cross-links of DNA chain, mismatch base pairing, breakage of DNA strand, or inhibition of DNA replication. Treatment of HepG2 spheroids with 10, 100, 500 µg/ml, and 1 mg/ml CPT for 0–48 h resulted in decreased spheroid size in a time-dependent manner [188]. CPT at 10 µg/ml, caused reduction of spheroid size from 97.3% at 0 h (initial size) to 54% at 24 h and only 38.7% at 48 h. Hypopharyngeal tumor FaDu, oral squamous cell carcinoma Cal27, and laryngeal squamous cell carcinoma PiCa spheroids treated with 5 µm CPT resulted in significant reduction of spheroid size over a period of 16 days [74]. Malignant pleural mesothelioma H2052/484 spheroids treated with 50–200 µm CPT alone or in combination with pemetrexed (PMX) as 50/200 µm, 100/400 µm, and 200/800 µm (CPT/PMX) for 17 days resulted in a dose-dependent reduction in spheroid size [190]. Treatment of head and neck cell lines (HN3, HN4, HN9 alone or each co-cultured with fibroblast) with 20 μM CPT for 3 days (72 h) resulted in a significant decrease in spheroid size compared to the control [51, 182].
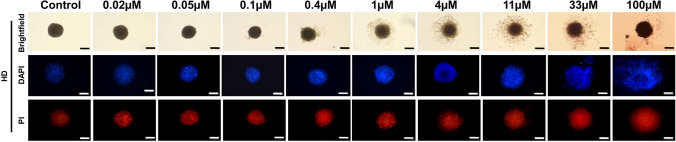
Doxorubicin (DOX): DOX is a chemotherapeutic agent having widespread clinical application [193, 194]. DOX exerts its anti-cancer activity by intercalating into DNA and inhibiting topoisomerase-II-mediated DNA repair or by release of free radicals which could damage cellular membranes, proteins, and DNA [195]. Breast adenocarcinoma MCF7 spheroids treated with DOX-loaded PNIPAM-co-AA (poly(N-isopropylacrylamide-co-acrylic acid)) nanoparticles for 96 h resulted in ~ 50% spheroid size reduction from 302 ± 11 to 153 μm [10]. Similarly, efficacy of DOX conjugates was tested on U87 GBM spheroids. The treatment resulted in reduced spheroid size by these DOX conjugates [165]. These morphometric studies revealed the potency of DOX conjugates.
Others: 5-fluorouracil at 15 mM [180] and oxaliplatin at 500 µm [180] led to decrease in colon adenocarcinoma HT-29 spheroid dimensions/size in a 24 h treatment. Similar observations of reduction in tumor spheroid size for several tumor cells have been reported upon treatment with topotecan at varying concentrations (1 nm–10 µM) on treatment for 6 days in human colorectal adenocarcinoma HCT116 cell line [181] and sorafenib at 10 μM in head and neck cell lines, HN3, HN4, HN9 alone or each co-cultured with fibroblast within 3 days treatment (72 h) [51, 182]. Trametinib and dactolisib treatment alone or in combination for 6–10 days decreased the size in colorectal cancer HT-29 cells spheroids in a dose-dependent manner [182]. In GBM U87 cells, Temozolomide (TMZ) at 10–150 µM treated for 24 h [185] or in combination with stiripentol (STP) (TMZ/STP 100 µM/50 µM) in 8 days treatment [186] as well as treatment of resveratrol in HepG2 spheroids for 48 h had smaller average diameter (131.6 μm) than the corresponding controls (136.8 μm) [57]. Hydroxyurea at 10, 100, 500 µg/ml, and 1 mg/ml for 0–48 h duration in HepG2 spheroids resulted in reduction of spheroid size to 57.7% at 24 h from initial size and to 32.4% at 48 h, while, at 500 µg/ml, the spheroid size decreased to 27% (24 h) and 3.4% (48 h) from the initial size [188]. Etoposide treatment at varying concentrations ranging from 0.03 to 300 µM for 4 days in medulloblastoma UW228-3 cell spheroids exhibited an additional halo of dead cells or dead cell debris around the reduced spheroid [189]. Docetaxel at 29.4–183.7 µM in prostate cancer LNCaP cell spheroids during a period of 3 or 8 days post 12 h drug treatment [55], sunitinib at 2.5, 5, and 7 µM in pancreatic neuroendocrine neoplasms (pNEN) BON1 spheroids [62], curcumin in U87 spheroids conjugated to DOX [165], and bufadienolides at 400 nM gastric cancer HGC-27 spheroid [187] showed similar reduction in spheroid sizes. In view of the above studies, the reduction in the dimensions of the corresponding spheroids stands as a reliable factor for assessment of drug efficacy in a dose-dependent manner.
Loss of spheroid integrity/compactness
An important defining feature of viable 3D spheroids is cell–cell contact with cohesive circular or smooth surface [30]. Loosening of cell–cell contacts, compactness, or spheroid integrity is another important defining feature of cytotoxic drug effect as showed by several researchers [185].
Application of CPT alone or in combination with other drugs in several tumor spheroids has resulted in spheroid disintegration or loosening of cell–cell contacts. Treatment of triple negative breast cancer MDA-MB-157 spheroids exposed to 12.5–200 µM CPT for 4 days with a drug re-administration after 2 days resulted in spheroid disintegration attributed to the efficient penetration of CPT through the spheroids [191]. Treatment of A2780 and SK-OV-3 epithelial ovarian cancer spheroids with 50 µM CPT for 48 h resulted in loosened spheroidal cells [48]. Malignant pleural mesothelioma H2052/484 spheroids treated with 200 µM CPT alone or in combination with PMX as 200/800 µM (CPT/PMX) for 17 days resulted in complete disintegration of the spheroid [190]. HepG2 cell spheroids treated with CPT and tirapazamine (TPZ) (a member of the heterocyclic compound benzotriazines with anti-cancer activity potential by inducing oxidative DNA damage [196]) for 48 h, resulted in disruption of spheroid integrity or compactness [57].
Apart from CPT, other known anti-cancer drugs and novel compounds have shown spheroid disruption and detachment. Breast adenocarcinoma MCF7 spheroids treated with DOX-loaded PNIPAM-co-AA nanoparticles for 96 h showed characteristic spheroid disruption features [10]. GBM U87 cells treated with > 200 µM TMZ for 24 h resulted in spheroid disintegration and irregular aggregates of size < 50 µm [185]. U87 spheroids treated with improved anti-glioma therapeutic approach such as DOX-curcumin (D-C) complex encapsulated with PEGNIO (polyethylene glycolated niosomes) or PEGNIO/D–C and PEGNIO/D–C conjugated with tumor-homing peptide Lyp-1 or PEGNIO/D–C/t-Lyp-1 resulted in decreased spheroid dimension and loss of spheroid compactness, or distorted spheroids with several dissociated cells as shown in Fig. 3A [165]. Disintegration or fluffy appearance of colorectal cancer HT-29 spheroids have been observed with trametinib (< 10 nM), sorafenib (> 100 nM), ponatinib and dactolisib (> 1000 nM) alone or in combination at 4 × their IC50 [182]. Ponatinib is a benzamide, a tyrosine kinase receptor inhibitor, with antineoplastic activity [197]. Four novel asymmetrical bisacridines (C-2028, C-2041, C-2045, C-2053) shown to have cytotoxic or inhibitory effect on colorectal carcinoma HCT116 spheroids exhibited jagged or diffuse outer spheroid layer over a 14-day period compared to control [183]. Pancreatic neuroendocrine neoplasm BON1 spheroids generated in cell repellent surface 24-well plates on treatment with 5 µM sunitinib resulted in complete disintegration of spheroids after 7 days of seeding with treatment in last 72 h [62]. Novel 1,2,4 triazole-3 thiol derivatives at 10 µM reported to have cytotoxic effect against melanoma IGR39, triple-negative breast cancer MDA-MB-231 or pancreatic carcinoma Panc-1 cell lines showed disintegrated spheroid after 8 days of incubation [47]. Cytotoxic effect of 6.25–12.5 μM novel THPMs compounds in HeLa spheroid treated for 48 h resulted in loosened cell–cell contact [46]. Apart from chemotherapeutic drugs, a similar alteration in morphology was observed with other therapeutics approaches such as UV radiation that resulted in disintegration of spheroids as seen in cervicospheres as shown in Fig. 3B [198]. In conclusion, the loss of spheroid integrity emerges as another crucial defining characteristic of anti-cancer effects.
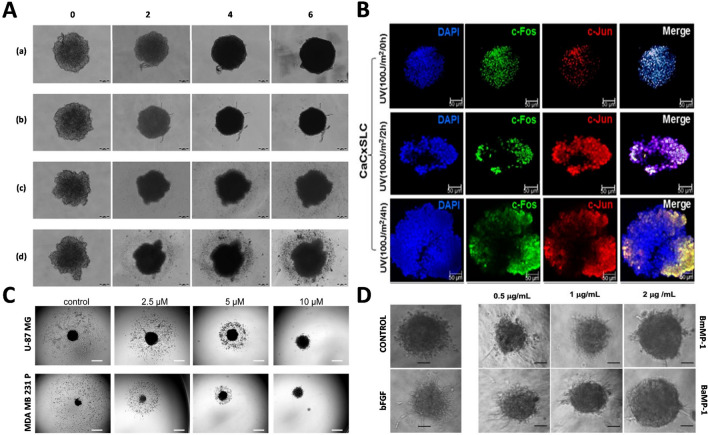
Fig. 3.
Representative images of morphological alterations in 3D tumor spheroids in response to anti-cancer agents. A U87 tumor spheroids treated with cell culture media (a), D–C (b), PEGNIO/D–C (c) and PEGNIO/D–C/t-Lyp-1 (d) resulted in decreased spheroid dimension and loss of spheroid compactness, or distorted spheroids with several dissociated cells. Adapted from [165]. B Confocal immunofluorescence image of 10 -day CaCxSLCs cultures treated with indicated exposure to UVC radiation resulted in disintegration of spheroids as seen in cervicospheres. Adapted from [198]. C Images of U-87 MG and MDA-MB-231 P spheroids showing reduced migratory behavior in response to CCT130234 treatment in a dose-dependent manner. Adapted from [54]. D Micrographs of HUVECs in 3D collagen models showing significant sprouting in the presence of bFGF, while exhibiting sprout inhibition at 2 µg/mL P-I metalloproteinases. Adapted from [211]. All the images are licensed under the terms and conditions of Creative commons (Creative Commons Attribution 4.0 International License). Abbreviations: PEGNIO, polyethylene glycolated niosomes; D-C, doxorubicin-curcumin; tLyp-1, tumor homing and penetrating peptide
Loss of even smooth spheroid surface
Few tumor spheroids tend to show frayed and uneven surface appearance without losing the core cell–cell compactness in response to toxic drug effect. Breast adenocarcinoma MCF-7 spheroid treated with 2 µM tamoxifen showed increased frayed and uneven surface along with decrease in cell viability after 7 days of treatment [36]. Treatment of MCF7 cell spheroids with DOX-loaded PNIPAM-co-AA nanoparticles for 96 h resulted in uneven spheroid surface appearance [10]. Colon adenocarcinoma HT-29 spheroids treated with 15 nM 5-FU in combination with 500 µM oxaliplatin and 2 mM folinic acid or 500 µM OXP alone, resulted in altered spheroidal shape or lack of smooth contour spheroids [180]. Colorectal cancer HCT116 spheroids treated with 4–15 μM 5-FU and 5-FU loaded chitosan nanoparticles (5-FUChnps) exhibited increased irregularity or non-spherical shape in a dose-dependent manner [184]. GBM U87 cells treated with 10–150 µM TMZ for 24 h resulted in destruction of spheroid surface [185]. Cytotoxic effect of 6.25–12.5 μM novel THPMs compounds in HeLa cell spheroid treated for 48 h resulted in detachment of cells from the spheroid surface and irregularity/unevenness in the spheroid surface [46]. Due to weaker attachment and direct exposure to drug treatments, the cells situated at the outer periphery of the spheroid experience notable impairment. This can potentially result in changes to the spheroid’s shape or the disruption of its smooth surface integrity.
Morphological alterations in response to anti-metastasis/anti-invasion drug
Metastasis or invasion are critical steps to cancer spread and serve as the key hallmark of malignant tumors. It is the major leading cause of cancer deaths (> 90%) and relapses [199]. The progression of tumor cells to metastatic form involves morphological restructuring and break down of extracellular matrix surrounding the tumor. Briefly, invasion begins as signaling pathways regulating cytoskeleton dynamics get activated in tumor cells. Once activated the cell–cell adhesion decreases, followed by cell detachment and secretion of proteolytic enzymes leading to break down of basement membrane. Through the disrupted membrane, the primary tumor cells enter into or journey through lymphatics, vascular circulation, or peritoneal space and eventually settle at a new distant site and proliferate into secondary tumor [200, 201]. Targeting of cancer cell migration could support the treatment of high-risk cancers to curable forms and prolong patient survival [202].
There are several 2D setups including Boyden chambers and scratch wound or wound healing assays to help screen or determine novel compounds with inhibitory effect on tumor cell migration. The invasive nature of the tumor cells is determined by the distance traveled. Therefore, by principle anti-invasion would involve the inhibition or reduction of the distance traveled by the cells. In addition to 2D model assays, considering the closer physiological resemblance of 3D tumor spheroids, the 3D tumor spheroids have been employed for the study/research of tumor invasion. Co-culturing of HUVECs with metastatic triple-negative breast cancer MDA-MB-231 cells in fibrin matrix preloaded with human dermal fibroblasts (HDFs) was shown to restore cellular interactions as in physiological conditions within 24 h and exhibited upstream metastatic regulators for tumor progression [53]. In a migratory study with U87 and KNS42 3D tumor spheroids, the tumor cells from the spheroids were observed to disseminate over matrigel/extracellular matrix protein coated surface in a 72 h study [54]. U87 spheroid cells exhibited rapid, single cell amoeboid type migration, while cells from KNS42 spheroids showed slow and collective radial pattern of migration. In the same study, when spheroids of U87 and MDA MB 231 P were exposed to inhibitors of phospholipase C gamma (PLCγ) and heat shock proteins 90 (HSP90) such as CCT130234 and 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), respectively, reduced migratory behavior was observed in both the tumor cell types in a dose-dependent manner, as early as 24 h as shown in Fig. 3C. Both PLCγ and HSP90 are critical to tumor cell migration. Reduction or inhibition of PLCγ and its activity resulted in the absence of cell spreading and elongation, which are pre-requisites to cancer cell migration [203, 204]. Inhibition of HSP90 led to negation of the migratory properties of colon cancer cell lines SW620, HCT116, and HT29, along with upregulation of the activating transcription factor-3 (ATF3) expression, an anti-metastatic factor [205].
Several types of 3D tumor spheroids have been used for screening of novel anti-metastatic or anti-migratory drugs for specific cancer therapeutic application. Anti-invasive effect of 6.25–12.5 μM novel THPMs compounds 4c and 4d on HeLa cell spheroids in a 48 h treatment showed significant decrease in sprout area compared to control with sprout area of approximately 277 μm2 [46]. The spheroids treated with compound 4d exhibited sprout area of about 57 μm2 at 6.25 μM and ~ 10 μm2 at 12.5 μM, and for compound 4c, the spheroids showed sprout area of ~ 15 μm2 at 6.25 μM and ~ 1 μm2 at 12.5 μM. In another study, highly invasive larynx carcinoma HLaC78 cell spheroids treated with Osmunda regalis extract exhibited reduced invaded area compared to control [206]. Similar result of reduced invasion perimeter diameter was observed in 3D spheroids of breast cancer lines MDA-MB-231 and 4T1 upon treatment with antibodies mAb-nNav1.5 and pAb-nNav1.5 in a 3D-spheroid invasion assay [207]. These studies clearly demonstrate usefulness of morphometric evaluation of changes in spheroids that could be a direct measure of drug action.
Morphological alterations in response to anti-angiogenic drug
Angiogenesis is a critical hallmark of solid tumors for growth and development. Inhibition of angiogenesis in conjuncture with cytotoxic anti-cancer drugs could greatly slow down tumor growth or progression. 3D tumor angiogenic models developed for the study of angiogenesis employ endothelial cells (ECs) as these are the main components of the blood vessel. These models are highly reliable. Co-culturing of HUVECs with MDA-MB-231 cells (9:1), in fibrin matrix preloaded with HDFs was determined to restore cellular interactions as in physiological conditions within 24 h and exhibit upstream angiogenic regulators for tumor angiogenesis [53]. In the same study, vascularised sprouts of length up to ~ 181 μm were observed to develop within 24 h. Similar morphological extensions or sprout formation was observed with HUVECs spheroids in 3D spheroid sprouting assay [208]. The significant morphological representation of angiogenesis exhibited by 3D angiogenic or tumor angiogenic models is the sprout formation.
The critical morphological alterations associated with angiogenesis inhibition using 3D tumor model include sprout length, vascularization, or EC density. HUVEC spheroids generated by mixing with 2% methylcellulose on treatment with VEGF (angiogenesis promoting factor) and breast cancer MCF-7 cell supernatant resulted in increased sprout generation around the spheroid [209]. On addition of 1 nM melatonin known to have antiangiogenic activity in breast cancer, the sprout formation was reduced. Similarly, 3D tumor spheroid model comprising of human glioma spheroids encapsulated with fibrin gel co-cultured with HUVECs showed tube-like or short cord like structures in 1–2 days. Treatment with 5–10 μM of atorvastatin significantly inhibited tube-like structure extensions with reduced number of connected and total tube length exhibited by HUVECs [210]. HUVECs in 3D collagen models showed significant sprouting in the presence of bFGF, while exhibited sprout inhibition at 2 µg/mL P-I metalloproteinases [211] as shown in Fig. 3D. In another study employing spheroid-plug approach, single spheroids of murine melanoma B16F10 and Lewis lung carcinoma (LLC) cell lines injected subcutaneously into mice when treated with axitinib (tyrosine kinase inhibitor) resulted in decreased spheroid-plug tumor size and vascularization [212]. Significant difference in development of vasculature of about 3 versus 7% at day 21 and 4 versus 9% at day 28, were observed between treated and control. Overall, the distinct morphological alterations exhibited by 3D tumor angiogenic models offer valuable insights into angiogenesis and its inhibition, highlighting the potential avenues for therapeutic interventions in cancer treatment.
Reliability of morphological alterations as interpretation to anti-cancer drug effect
Morphological changes in response to anti-cancer drug treatment is a reliable straight forward method with minimal experimental manipulation to evaluate drug responses ranging from drug effectiveness to resistance [182]. Representative studies described below support the reliability of the morphological method and warrant greater exploitation of the 3D tumor models for in vitro studies.
The quiescent/dead cells in 3D spheroids under bright field imaging is reflected as the darkest, which is generally seen at the center of the spheroid [3]. Seven days old spheroids with a dense core and brightened outer cell layer as imaged under light microscopy upon immunochemistry study with the proliferation marker Ki-67 revealed rapid proliferating tumor rim and a core with only slow proliferating cells [74]. Similar representation has been shown with cutaneous T-lymphocyte HuH-7 cell line spheroid with live/dead staining or green/red fluorescence exhibition, respectively [213]. Green fluorescence representing live cells were observed to be more concentrated on the outer cell layers, while the red fluorescence representing hypoxic and quiescent cells were confined to the center.
Several studies involving live/dead cell imaging technique using fluorescence microscope to identify live and dead cells have also shown strong correlation between the fluorescence exhibition and spheroid morphological alterations. GBM U87 cells exposed to TMZ at 100 µM or stiripentol (STP) at 50 µM alone and in combination (TMZ/STP 100 µM/50 µM) correlated with decreased cell viability using live/dead cell indicator dyes calcein AM (green) and ethidium homodimer (red), respectively [186]. Treated U87 spheroids exhibited dull green fluorescence compared to bright green fluorescence in the control and vis a vis bright red fluorescence in treated spheroids while the control exhibited dull red fluorescence. Loosened epithelial ovarian cancer A2780 and SK-OV-3 spheroid cells in response to 50 µM CPT treatment for 48 h and more significantly in A2780, corresponded with greater reduction in cell viability in A2780 compared to SK-OV-3 in a spatial evaluation of live (Calcein-AM) and dead (ethidium homodimer) cells [48] through imaging.
Spheroid size is a quantitative parameter and bears a strong correlation to molecular and biochemical assays in efficacy studies. Cell viability prediction of colorectal tumor HT-29 spheroid treated with 5 nM trametinib by biochemical PrestoBlue viability assay harmonize with HT-29 spheroid volume reduction [182]. In the same study, western blot analysis of colorectal tumor HT-29 spheroids treated with trametinib and dactolisib at 2 × IC50 concentration in combination for 24 h significantly downregulated phosphorylated ERK1/2 (~ 68%) and AKT (~ 40%), which strongly correlated with spheroid size reduction [182]. ERK1/2 and AKT are molecular factors concerned with the regulation of several stimulatory cellular processes, including cell proliferation, differentiation, survival, as well as apoptosis and stress response [214, 215]. A reduction in spheroid size in response to anti-cancer drugs 5-FU, regorafenib [216] and erlotinib [217] treatment very well correlated with decrease in metabolic activity measured by ATP level [218]. The level of ATP decreases dose-dependently with increase in drug concentration, which corresponded with disorganization of spheroid integrity and spheroid reduction in response to anti-cancer drug treatment. Cell viability measurement of triple negative breast cancer MDA-MB-157 spheroids treated with 12.5–200 µM CPT for 4 days using PrestoBlue viability assay showed strong corelation between spheroid disintegration and decreased cell viability [191]. Inhibition of malignant pleural mesothelioma H2052/484 spheroid growth or their disintegration with 200 µM CPT alone or in combination with PMX (CPT/PMX, 200/800 µM) for 17 days strongly corresponded to decrease in intracellular ATP level/cell viability, measured through an ATP-based luminescence assay [190].
Reduced disseminated area of tumor cells around the 3D tumor models in anti-metastatic research studies strongly corresponded to reduced or decreased metastasis supporting molecules. Decreased MMP2 and MMP9 gene level expression was recorded with anti-invasive behavior exhibited by HeLa cell spheroids in response to novel THPMs compounds [46]. In another study, reduced invaded area exhibited by highly invasive larynx carcinoma HLaC78 cell spheroids on treatment with Osmunda regalis extract compared to control corresponded with the downregulation of c-Met signaling [206]. c-Met signaling plays a significant role in the activation of pathways involved in invasion [219]. Similarly, decrease in tube like structure formation (angiogenic behavior) by HUVECs in 3D tumor spheroid in response to atorvastatin treatment corresponded with reduced levels of angiogenic markers such as VEGF, CD31, and Bcl-2 as well as induced apoptotic regulator caspase-3 expression [210]. These observations collectively indicate functional relevance of cell migration out of the spheroid as direct measure of metastatic/ anti-metastatic effects.
The morphological alterations seen in tumor spheroids in response to effective anti-cancer drug might differ from cell to cell. In several studies, loss of spheroid integrity or cell–cell contact often leads to increase in spheroid size/area [57]. Treatment of A2780 and SK-OV-3 epithelial ovarian cancer spheroids with 50 µM CPT for 48 h resulted in loosened spheroidal cells leading to increased spheroidal mean diameter from 2194 ± 130 µm and 1305 ± 203 µm to 2477 ± 168 µm and 2093 ± 37 µm respectively [48]. HepG2 cell spheroids treated with CPT and TPZ alone for 48 h resulted in loss of spheroid compactness resulting in increased spheroid area compared to the corresponding control [57]. Therefore, thorough knowledge about the nature/behavior of the specific tumor cell as 3D model under study is warranted before making a logical conclusion about drug action. This study also emphasizes the need to take into consideration multi-morphological alterations in a study as discussed. Therefore, cumulative analysis of all the altered morphological features encompassing the spheroid integrity along with, the compactness, expression of cell adhesion molecules will prove valuable in evaluation of drug efficiency.
Practical demonstration: generation of U87 spheroid and their treatment with DOX
U87 (Uppsala 87) is an epithelial-like GBM cell line originally established in the year 1968 at Uppsala University, Sweden, from a 44-year-old astrocytoma-GBM female patient belonging to the Caucasian race [220]. It is one of the most commonly used cell lines in preclinical research as experimental models of GBM. GBM is one of the most therapeutically challenging and incurable forms of cancer. It generally arises in the anterior subcortical region of the brain [221], with idiosyncrasies of diffuse invasion and aggressive migration to neighboring areas of the brain, making it impossible for complete resection of the neoplastic cells resulting in 100% relapse [222]. Making it worse is the fact that there are no preventive steps to stop its development or improve its outcome. Treatment to GBM is faced with several challenges unlike other tumors and the lack of cost-effective techniques/models closely recapitulating native pathophysiological conditions, further widens and deepens the need of study/research in understanding GBM at the preclinical stage. This also stands as one of the limiting factors to discovery of effective and efficient GBM therapeutics. 3D tumor spheroids having a close resemblance to the native cellular organization of GBM and other solid tumors, can effectively aid in filling this gap in a cost-effective manner.
U87 3D spheroids were successfully generated under standardized conditions using the gravity-enforced HD and LOT based on a non-adherent surface that prevents cellular adhesion. With the help of live microscopic observation and micrographic images, it was observed that U87 cells easily aggregated within 10 h of incubation and formed definite spheroids by day 5 (96 h post incubation). In the HD method, the spheroids showed gradual increase in spheroid volume of 2881–3038 mm3 at day 2 to about 4522 mm3 at day 12 and gradual decline thereafter. In LOT, the spheroids ranged in volume from 8038–8651 mm3 at day 2 to 12,721 ± 294 mm3 at day 11 (Fig. 4A–C). The spheroids were incubated by HD method using 100 × 20 mm cell culture plate (Fig. 4D) and LOT using U-bottomed ULA plate (Fig. 4E). From days 13 in HD and 11 in LOT, reduction in spheroid volume was observed. Larger spheroid volumes were obtained in LOT compare to HD technique.
Fig. 4.
Timeline of U87 spheroids. A U87 spheroids generated using HD method. B U87 spheroids generated using LOT. C Graphical representation of the spheroid volume within 14-day period. D Hanging drop method using petri-plate. E LOT using ULA 96-well plate. The graph was plotted using Microsoft 10 Excel and the data expressed as mean ± SD
With the help of live microscopic observation and micrographic images, the results of U87 spheroids treated with different concentrations of DOX (0.02, 0.05, 0.1, 0.4, 1, 4, 11, 33, and 100 µM) for 72 h showed reduced spheroid diameter at 0.05 and 0.1 µm treatments compared to control (treated with only culture media). In spheroids treated with higher DOX concentration, loss of compactness, cellular dissociation, and cell debris around the core tumor spheroid could be observed. Additionally, bright fluorescence with propidium iodide (PI) as red and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) as blue in a dose-dependent manner was observed (Fig. 5).
Fig. 5.
Images of U87 spheroids treated with different concentrations of DOX using HD method. Brightfield images (upper row) along with DAPI (middle row) and PI (lower row) images of corresponding treatment concentrations
Methodology of 3D U87 spheroid generation and their application in the evaluation of drug cytotoxicity
U87 cell culture
Human GBM U87(MG) cell line was purchased from National Centre for Cell Science (NCCS), Pune, India, and cultured in MEM(E)/DMEM supplemented with 1 mM Sodium Pyruvate, 1% Non-Essential Amino Acid, 2% Pen strep Glutamine, 10% (v/v) fetal bovine serum (FBS), inside humidified CO2 incubator (Galaxy 170S) at 37 °C and 5% CO2. Established cells were harvested and checked for viability with the help of Trypan blue. Manual cell counting was performed for seeding cells to develop spheroids.
3D spheroid generation
For the generation of U87 3D spheroids, trypsinized single cells were suspended in the media at a density of 250 cells/µl. For HD, 20 µl of the above cell suspension containing 5 × 103 U87 cells each were placed sufficiently apart onto the lid of the culture vessel. The lid with drops of cells was inverted over the bottom chamber of the dish, containing 1 × PBS (to keep the cells moisten). For LOT, ULA plates were used. Into the well, 20 µl of cell suspension containing 5 × 103 U87 cells was placed in each well in triplicates. The spheroids were maintained at standard conditions of 5% CO2 and 37 °C. The culture was replenished with 10 µl fresh media on alternate days. The culture was observed for 14 days (the first day of incubation was considered as day 0). On day 15, the spheroids were imaged using inverted microscope (Nikon Eclipse Ts2R). The spheroid volumes were calculated using the formula: spheroid volume, V = 0.5 * Length * Width2 [223].
Drug treatment
The treatment regimen involved a single DOX treatment at varying concentrations (0.02 to 100 µM) for 72 h. On day 5 of spheroid generation (4 days old spheroids), the culture media were completely removed and replaced with DOX at different concentrations which was placed in each well in triplicates. Spheroids with no treatments were considered control. After 72 h, the spheroids were imaged using inverted microscope (Nikon Eclipse Ts2R) and 3D image analyzed for morphometric alterations (size/volume, loss of spheroid integrity, degradation of spheroids, etc.).
Validation of 3D tumor spheroid viability
The morphometric alterations in response to drug treatment or cell death was further validated by dead cell stains, such as PI and DAPI, as they are more permeable to dead cell membrane [224, 225]. PI intercalates between double stranded DNA base pairs, excited between 400 and 600 nm and emits light between 600 and 700 nm wavelengths [226]. DAPI on entering the cell membrane binds with DNA and forms a DAPI-DNA complex exhibiting blue fluorescence (wavelength of excitation and emission of the complex = 360 nm and 460 nm respectively) [227, 228]. For both PI (working conc. 10 mg/ml PBS) and DAPI (working conc. 5 µg/ml PBS) staining, 5 µl was added to each of the spheroid droplets of the hanging drop or ULA plate wells. The mixture was incubated for about 5–10 min in the dark at room temperature, observed under the microscope and images were taken.
Conclusion and future direction
The screening of anti-cancer drugs is the core principle in the treatment of cancer. 3D tumor spheroids exhibit a strong analogy to in vivo pathophysiological conditions and demonstrate morphological alterations such as reduced spheroid size, loss of spheroid compactness and integrity in response to drug effectiveness. These alterations serve as reliable parameters, and the incorporation of morphological features to analyze drug effectiveness for 3D tumor spheroids would greatly complement the use of biochemical assays. Additionally, the application of spheroid staining techniques would lead to improved visualization. These morphological parameters hold significant potential in determining the anti-cancer drugs, serving as initial indicators of drug response and facilitating the assessment of drug efficacy in a cost-effective and physiologically more relevant manner. Furthermore, the utilization of 3D tumor spheroids will aid in overcoming the limitations of 2D cultures, reduce the efforts and cost of in vivo drug screening, expedite the process of drug pre-screening, and enhance the efficiency of drug discovery by effectively predicting therapeutic efficacy in vivo.
Acknowledgements
We are thankful to Prof. Savita Roy, Principal, Daulat Ram College, University of Delhi, for providing constant support and lab facility throughout the investigation and Late Dr Amar Jyoti for the initial efforts to conceptualize.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- 5-FU
5-Fluorouracil
- 5-FUChnps
5-FU loaded chitosan nanoparticles
- CAR
Cell adhesion recognition
- CPT
Cisplatin
- D
Diameter
- D2R
Dopamine D2 receptor
- DAPI
4′,6-Diamidino-2-phenylindole dihydrochloride
- DOX
µ
- FBS
Fetal bovine serum
- GBM
Glioblastoma multiforme
- GFR
Growth factor reduced
- H
Hour
- HD
Hanging drop
- IFF
Interstitial fluid flow
- LOT
Liquid overlay technique
- NA
Not applicable
- N/A
Data not available
- NCCS
National Centre for Cell Science
- OGC
N-octanoyl glycol chitosan
- PDAC
Pancreatic ductal adenocarcinoma
- PDMS
Polydimethylsiloxane
- PEGNIO
Polyethylene glycolated niosomes
- PMX
Pemetrexed
- PEGNIO
Polyethylene glycolated niosomes
- PEGNIO/D–C/t-Lyp-1
PEGNIO/D–C conjugated with t-Lyp-1 peptide
- PNIPAM-co-AA
Poly(N-isopropylacrylamide-co-acrylic acid)
- PI
Propidium iodide
- RPM
Revolution per minute
- STP
Stiripentol
- THPMs
2-Oxo-1,2,3,4-tetrahydropyrimidines
- TMZ
Temozolomide
- TPZ
Tirapazamine
- TME
Tumor microenvironment
- ULA
Ultra low attachment
- ↓
Decreased/reduced
Author contribution
Conceptualization: ACB and AS. Data curation: AS and ACB. Formal analysis: AS, SL, JK, NG, DJ, TT, NA, AC, JY, and AC. Funding acquisition: ACB. Investigation: AS, SL, JK, NG, DJ, and ACB. Methodology: AS, SL, JK, NG, DJ, TT, NA, AC, JY, and AC. Project administration: ACB. Resources: ACB and AS. Supervision: ACB. Validation: ACB. Visualization: AS, DJ, TT, NA, and ACB. Writing original draft: AS and ACB. Review and editing: ACB.
Funding
The study was supported by research grants from Indian Council of Medical Research (ICMR-ICRC) to ACB (No.5/13/4/ACB/ICRC/2020/NCD-III), grant from Institution of Eminence University of Delhi (Ref. No./IoE/2021/12/FRP) to ACB, grant from CSIR-UGC to A. Chhokar [573(CSIR-UGC NET JUNE 2017)]; grant from CSIR to NA (09/045(1622)/2019-EMR-I); grant from CSIR to JY (09/045(1629)/2019-EMR-I); grant from CSIR-UGC to TT (764/(CSIR-UGC NET JUNE 2019); grant from CSIR to DJ (09/0045/(11635)/2021-EMR-1) and grant from CSIR to A. Chaudhary (09/0045(12901)/2022-EMR-1). This study was partly supported by the UGC-FRPS grant [Ref. F.30–400/2017(BSR F.D.Dy.No.5326] & SERB-ECR grant (Ref.ECR/2016/000897) to late Dr Amar Jyoti, Neuropharmacology and Drug Delivery Laboratory, Daulat Ram College, University of Delhi, Delhi.
Data availability
The data sets used and or/analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
NA
Consent for publication
All authors have read the final manuscript and agreed to the publication.
Competing interests
The authors declare no competing interests.
References
- 1.Cooper GM, Hausman RE. The development and causes of cancer. The cell: A molecular approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Available from https://www.ncbi.nlm.nih.gov/books/NBK9963/.
- 2.Fontebasso Y, Dubinett SM. Drug development for metastasis prevention. Crit Rev Oncog. 2015;20(5–6):449–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanoni M, et al. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep. 2016;6:19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cwikiel W, et al. Malignant esophageal strictures: treatment with a self-expanding nitinol stent. Radiology. 1993;187(3):661–5. [DOI] [PubMed] [Google Scholar]
- 5.Jacobi N, et al. Organotypic three-dimensional cancer cell cultures mirror drug responses in vivo: lessons learned from the inhibition of EGFR signaling. Oncotarget. 2017;8(64):107423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellor HR, Ferguson DJ, Callaghan R. A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer. 2005;93(3):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware MJ, et al. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng Part C Methods. 2016;22(4):312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma HL, et al. Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Mol Imaging. 2012;11(6):487–98. [PubMed] [Google Scholar]
- 9.Karlsson H, et al. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp Cell Res. 2012;318(13):1577–85. [DOI] [PubMed] [Google Scholar]
- 10.Kang A, et al. Concave microwell array-mediated three-dimensional tumor model for screening anticancer drug-loaded nanoparticles. Nanomedicine. 2015;11(5):1153–61. [DOI] [PubMed] [Google Scholar]
- 11.Filipiak-Duliban A, Brodaczewska K, Kajdasz A, Kieda C. Spheroid culture differentially affects cancer cell sensitivity to drugs in melanoma and rcc models. Int J Mol Sci. 2022;23(3):1166. [DOI] [PMC free article] [PubMed]
- 12.Nederman T, et al. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984;44(7):3090–7. [PubMed] [Google Scholar]
- 13.Miyazaki T, et al. Formation of proteoglycan and collagen-rich scaffold-free stiff cartilaginous tissue using two-step culture methods with combinations of growth factors. Tissue Eng Part A. 2010;16(5):1575–84. [DOI] [PubMed] [Google Scholar]
- 14.Liao W, et al. Therapeutic potential of CUDC-907 (Fimepinostat) for hepatocarcinoma treatment revealed by tumor spheroids-based drug screening. Front Pharmacol. 2021;12:658197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanigasekara J, et al. Plasma induced reactive oxygen species-dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Processes Polym. 2022;19(4):2100157. [Google Scholar]
- 16.Zraikat M, Alshelleh T. Comparison between different 3D spheroid tumor invasion models. Assay Drug Dev Technol. 2020;18(5):239–42. [DOI] [PubMed] [Google Scholar]
- 17.Vinci M, et al. Tumor spheroid-based migration assays for evaluation of therapeutic agents. Methods Mol Biol. 2013;986:253–66. [DOI] [PubMed] [Google Scholar]
- 18.Nunes AS, et al. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019;116(1):206–26. [DOI] [PubMed] [Google Scholar]
- 19.Baranyai Z, et al. Cellular internalization and inhibition capacity of new anti-glioma peptide conjugates: physicochemical characterization and evaluation on various monolayer- and 3D-spheroid-based in vitro platforms. J Med Chem. 2021;64(6):2982–3005. [DOI] [PubMed] [Google Scholar]
- 20.Chaddad H, et al. Combining 2D angiogenesis and 3D osteosarcoma microtissues to improve vascularization. Exp Cell Res. 2017;360(2):138–45. [DOI] [PubMed] [Google Scholar]
- 21.Boucherit N, Gorvel L, Olive D. 3D tumor models and their use for the testing of immunotherapies. Front Immunol. 2020;11:603640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchoryk A, et al. Penetration and uptake of nanoparticles in 3D tumor spheroids. Bioconjug Chem. 2019;30(5):1371–84. [DOI] [PubMed] [Google Scholar]
- 23.Singh MS, et al. An ovarian spheroid based tumor model that represents vascularized tumors and enables the investigation of nanomedicine therapeutics. Nanoscale. 2020;12(3):1894–903. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Pacheco S, O'Driscoll L. Pre-clinical in vitro models used in cancer research: results of a worldwide survey. Cancers (Basel). 2021;13(23):6033. [DOI] [PMC free article] [PubMed]
- 25.Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan AQ. Animal Models in Cancer Drug Discovery || Role of 3D tissue engineering models for human cancer and drug development. Animal Models in Cancer Drug Discovery, pp 309–322.
- 27.Bartosh TJ, Ylostalo JH. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr Protoc Stem Cell Biol. 2014;28:2B 6 1-2B 6 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sant S, Johnston PA. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol. 2017;23:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebhard C, Gabriel C, Walter I. Morphological and immunohistochemical characterization of canine osteosarcoma spheroid cell cultures. Anat Histol Embryol. 2016;45(3):219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaral RLF, et al. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol. 2017;8:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruningk SC, et al. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci Rep. 2020;10(1):1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirenko O, et al. High-content assays for characterizing the viability and morphology of 3D cancer spheroid cultures. Assay Drug Dev Technol. 2015;13(7):402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessel S, et al. High-throughput 3D tumor spheroid screening method for cancer drug discovery using celigo image cytometry. SLAS Technol. 2017;22(4):454–65. [DOI] [PubMed] [Google Scholar]
- 34.Kwapiszewska K, et al. A microfluidic-based platform for tumour spheroid culture, monitoring and drug screening. Lab Chip. 2014;14(12):2096–104. [DOI] [PubMed] [Google Scholar]
- 35.Nath S, Devi GR. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther. 2016;163:94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolver MG, Elingaard-Larsen LO, Pedersen SF. Assessing cell viability and death in 3D spheroid cultures of cancer cells. J Vis Exp. 2019;(148):e59714. [DOI] [PubMed]
- 37.Hirschhaeuser F, et al. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3–15. [DOI] [PubMed] [Google Scholar]
- 38.Van Zundert I, Fortuni B, Rocha S. From 2D to 3D cancer cell models-the enigmas of drug delivery research. nanomaterials (Basel). 2020;10(11):2236. [DOI] [PMC free article] [PubMed]
- 39.Riffle S, Hegde RS. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res. 2017;36(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland RM, et al. A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(5):491–5. [DOI] [PubMed] [Google Scholar]
- 41.Abolhassani H, et al. Rapid generation of homogenous tumor spheroid microtissues in a scaffold-free platform for high-throughput screening of a novel combination nanomedicine. PLoS ONE. 2023;18(2):e0282064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazzari G, et al. Multicellular spheroid based on a triple co-culture: a novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018;78:296–307. [DOI] [PubMed] [Google Scholar]
- 43.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86(3):287–301. [PMC free article] [PubMed] [Google Scholar]
- 44.Xu S, Gao J. Invasiveness and metastasis of tumor spheroid aggregates of human giant cell carcinoma (lung clone strain PLA801-95D) in vitro and in vivo. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1991;13(5):353–8. [PubMed] [Google Scholar]
- 45.Browning AP, Sharp JA, Murphy RJ, Gunasingh G, Lawson B, Burrage K, et al. Quantitative analysis of tumour spheroid structure. Elife. 2021;10:10:e73020. [DOI] [PMC free article] [PubMed]
- 46.Crnogorac MD, et al. 3D HeLa spheroids as a model for investigating the anticancer activity of Biginelli-hybrids. Chem Biol Interact. 2021;345:109565. [DOI] [PubMed] [Google Scholar]
- 47.Sermuksnyte A, Kantminiene K, Jonuskiene I, Tumosiene I, Petrikaite V. The effect of 1,2,4-Triazole-3-thiol derivatives bearing hydrazone moiety on cancer cell migration and growth of melanoma, breast, and pancreatic cancer spheroids. Pharmaceuticals (Basel). 2022;15(8):1026. [DOI] [PMC free article] [PubMed]
- 48.Gupta P, Miller A, Olayanju A, Madhuri TK, Velliou E. A systematic comparative assessment of the response of ovarian cancer cells to the chemotherapeutic cisplatin in 3D Models of various structural and biochemical configurations-does one model type fit all? Cancers (Basel). 2022;14(5):1274. [DOI] [PMC free article] [PubMed]
- 49.Varesano S, Zocchi MR, Poggi A. Zoledronate triggers Vdelta2 T cells to destroy and kill spheroids of colon carcinoma: quantitative image analysis of three-dimensional cultures. Front Immunol. 2018;9:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J, Shin D, Roh JL. Development of an in vitro cell-sheet cancer model for chemotherapeutic screening. Theranostics. 2018;8(14):3964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzarito G et al. Mammary epithelial and endothelial cell spheroids as a potential functional in vitro model for breast cancer research. J Vis Exp. 2021;(173). [DOI] [PubMed]
- 53.Dey M, et al. Studying tumor angiogenesis and cancer invasion in a three-dimensional vascularized breast cancer micro-environment. Adv Biol (Weinh). 2021;5(7):e2100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinci M, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulholland T, et al. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci Rep. 2018;8(1):14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh SK, et al. Critical role of three-dimensional tumorsphere size on experimental outcome. Biotechniques. 2020;69(5):333–8. [DOI] [PubMed] [Google Scholar]
- 57.Patra B, et al. Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device. Sci Rep. 2016;6:21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey RC, et al. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159(6):2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breslin S, O’Driscoll L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016;7(29):45745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae Y, et al. Preparation and characterization of 3D human glioblastoma spheroids using an N-octanoyl glycol chitosan hydrogel. Int J Biol Macromol. 2021;185:87–97. [DOI] [PubMed] [Google Scholar]
- 61.Mirab F, Kang YJ, Majd S. Preparation and characterization of size-controlled glioma spheroids using agarose hydrogel microwells. PLoS ONE. 2019;14(1):e0211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bresciani G, et al. Evaluation of spheroid 3D culture methods to study a pancreatic neuroendocrine neoplasm cell line. Front Endocrinol (Lausanne). 2019;10:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X, et al. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33(35):9049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, et al. 3D models of epithelial-mesenchymal transition in breast cancer metastasis: high-throughput screening assay development, validation, and pilot screen. J Biomol Screen. 2011;16(2):141–54. [DOI] [PubMed] [Google Scholar]
- 65.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–32. [DOI] [PubMed] [Google Scholar]
- 66.Howes AL, et al. 3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PLoS ONE. 2014;9(9):e108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edwards K, Yao S, Pisano S, Feltracco V, Brusehafer K, Samanta S, et al. Hyaluronic acid-functionalized nanomicelles enhance SAHA efficacy in 3D endometrial cancer models. Cancers (Basel). 2021;13(16):4032. [DOI] [PMC free article] [PubMed]
- 68.Sherman H, Rossi AE. A novel three-dimensional glioma blood-brain barrier model for high-throughput testing of tumoricidal capability. Front Oncol. 2019;9:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wanigasekara J, et al. Three-dimensional (3D) in vitro cell culture protocols to enhance glioblastoma research. PLoS ONE. 2023;18(2):e0276248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alves SR, et al. Characterization of glioblastoma spheroid models for drug screening and phototherapy assays. OpenNano. 2023;9:100116. [Google Scholar]
- 71.Eilenberger C, et al. Effect of spheroidal age on sorafenib diffusivity and toxicity in a 3D HepG2 spheroid model. Sci Rep. 2019;9(1):4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarisozen C, Abouzeid AH, Torchilin VP. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm. 2014;88(2):539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yakavets I, Jenard S, Francois A, Maklygina Y, Loschenov V, Lassalle HP, et al. Stroma-rich co-culture multicellular tumor spheroids as a tool for photoactive drugs screening. J Clin Med. 2019;8(10):1686. [DOI] [PMC free article] [PubMed]
- 74.Hagemann J, et al. Spheroid-based 3D cell cultures enable personalized therapy testing and drug discovery in head and neck cancer. Anticancer Res. 2017;37(5):2201–10. [DOI] [PubMed] [Google Scholar]
- 75.Raghavan S, et al. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7(13):16948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corning®1536-well. Corning® 1536-well black/clear round bottom ultra-low attachment spheroid microplate, with lid, sterile. [cited 2023 23rd July]; Available from: 4527 | Corning® 1536-well Black/Clear Round Bottom Ultra-low Attachment Spheroid Microplate, with Lid, Sterile | Corning.
- 77.PrimeSurface®3D-Culture-Spheroid-plates. PrimeSurface® 3D culture spheroid plates: ultra-low attachment (ULA) plates. [cited 2023 8th July]; Available from: 356257 | Corning® Matrigel® Matrix - 3D Plate, 384-well, White/Clear Square Bottom, Phenol Red-Free, Individually Wrapped, with Lid, 5/Cs | Corning.
- 78.Corning®384-well. Corning® 384-well Spheroid Microplates. [cited 2023 22nd July]; Available from: Corning® 384-well Spheroid Microplates | Corning.
- 79.Sun Y, Hu J, Zhu M. in Emerging molecular mechanisms of cell cycle regulation in cancer: functions and potential applications. 2022, Frontiers in Oncology and Frontiers in Cell and Developmental Biology, p 168.
- 80.Corning®96-well. Corning® 96-well black/clear round bottom ultra-low attachment spheroid microplate, with lid, sterile. 2023 [cited 2023 22nd July]; Available from: 4515 | Corning® 96-well Black/Clear Round Bottom Ultra-Low Attachment Spheroid Microplate, with Lid, Sterile | Corning.
- 81.Corning®Elplasia®24-well. Corning® Elplasia® 24-well black/clear round bottom ultra-low attachment, microcavity plate, with lid. [cited 2023 23rd July]; Available from: 4441 | Corning® Elplasia® 24-well Black/Clear Round Bottom Ultra-Low Attachment, Microcavity Plate, with Lid | Corning.
- 82.Corning®Elplasia®6-well. Corning® Elplasia® 6-well black/clear round bottom ultra-low attachment, microcavity plate, with lid. [cited 2023 24th July]; Available from: 4440 | Corning® Elplasia® 6-well Black/Clear Round Bottom Ultra-Low Attachment, Microcavity Plate, with Lid | Corning.
- 83.Corning®Elplasia®96-well. Corning® Elplasia® 96-well black/clear round bottom ultra-low attachment, microcavity microplate, with lid. [cited 2023 19th July]; Available from: 4442 | Corning® Elplasia® 96-well Black/Clear Round Bottom Ultra-Low Attachment, Microcavity Microplate, with Lid | Corning.
- 84.Corning®Elplasia®12KFlask. Corning® Elplasia® 12K flask. Available from: Corning® Elplasia® 12K Flask | Corning.
- 85.Sherman H, Shyu J, Kennebunk M. Corning® Matrigel® Matrix-3D Plates for High Throughput 3D Assays. Available from: https://www.corning.com/catalog/cls/documents/application-notes/CLS-AN-572-A4.pdf
- 86.Corning®Matrigel®Matrix-3D-Plate-384-well. Corning® Matrigel® Matrix - 3D plate, 384-well, black/clear square bottom, phenol red-free, individually wrapped, with lid, 5/Cs. [cited 2023 21st July]; Available from: 356256 | Corning® Matrigel® Matrix - 3D Plate, 384-well, Black/Clear Square Bottom, Phenol Red-Free, Individually Wrapped, with Lid, 5/Cs | Corning.
- 87.Plate-384-well, C.M.M.-D. Corning® Matrigel® Matrix - 3D Plate, 384-well, White/Clear Square Bottom, Phenol Red-Free, Individually Wrapped, with Lid, 5/Cs. [cited 2023 8th July]; Available from: 356257 | Corning® Matrigel® Matrix - 3D Plate, 384-well, White/Clear Square Bottom, Phenol Red-Free, Individually Wrapped, with Lid, 5/Cs | Corning.
- 88.Corning®Matrigel®Matrix-3D-Plate-96-well. Corning® Matrigel® Matrix - 3D plate, 96-well, phenol red-free, black/clear, individually wrapped, with lid, 1/Cs. [cited 2023 20th July]; Available from: 356259 | Corning® Matrigel® Matrix - 3D Plate, 96-well, Phenol Red-Free, Black/Clear, Individually Wrapped, with Lid, 1/Cs | Corning.
- 89.Nunclon™Sphera™96-Well. Nunclon™ Sphera™ 96-well, nunclon sphera-treated, U-shaped-bottom microplate. [cited 2023 19th July]; Available from: Nunclon™ Sphera™ 96-Well, Nunclon Sphera-Treated, U-Shaped-Bottom Microplate (thermofisher.com).
- 90.Hofmann S, et al. Patient-derived tumor spheroid cultures as a promising tool to assist personalized therapeutic decisions in breast cancer. Transl Cancer Res. 2022;11(1):134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaarn L, Marwood T, Scott R, Carter S, Granchelli J, Neeley C. The Nunclon Sphera surface supports formation of three dimensional cancer spheroids in suspension. Available from: https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/technical-documents/application-notes/thermo-scientific-sphera-cancer-application-note.pdf
- 92.ThermoScientific™96-well. 96 well plate, sphera low-attachment surface, pack of 1. [cited 2023 19th July]; Available from: 96 Well Plate, Sphera Low-Attachment Surface, Pack of 1 (thermofisher.com)
- 93.Nunclon™Sphera™Dishes. Nunclon™ Sphera™ Dishes. [cited 2023 19th July]; Available from: https://www.thermofisher.com/order/catalog/product/174943.
- 94.Nunclon™Sphera™Flasks. Nunclon™ Sphera™ Flasks. [cited 2023 19th July]; Available from: Nunclon™ Sphera™ Flasks (thermofisher.com).
- 95.Wardwell-Swanson J, et al. A framework for optimizing high-content imaging of 3D models for drug discovery. SLAS Discov. 2020;25(7):709–22. [DOI] [PubMed] [Google Scholar]
- 96.Akura™384-Spheroid-Microplate. Akura™ 384 spheroid microplate [cited 2023 8th July]; Available from: https://shop.insphero.com/products/akura-384-spheroid-microplate-10-pack?pr_prod_strat=copurchase_transfer_learning&pr_rec_id=847ff397b&pr_rec_pid=7645112139937&pr_ref_pid=6917606047905&pr_seq=uniform.
- 97.Akura™384-ImagePro. Akura™ 384 ImagePro. [cited 2023 8th July]; Available from: Akura™ 384 ImagePro (10/pack) – InSphero.
- 98.Akura™96-Spheroid-Microplate. Akura™ 96 spheroid microplate. [cited 2023 10th July]; Available from: Akura™ 96 Spheroid Microplate (20/pack) – InSphero.
- 99.Navis, A.R., Hanging drop tissue culture. Embryo Project Encyclopedia, 2012.
- 100.Gutierrez L, et al. A hanging drop culture method to study terminal erythroid differentiation. Exp Hematol. 2005;33(10):1083–91. [DOI] [PubMed] [Google Scholar]
- 101.Liu X et al. A novel SimpleDrop chip for 3D spheroid formation and anti-cancer drug assay. Micromachines (Basel). 2021;12(6). [DOI] [PMC free article] [PubMed]
- 102.Zhang W, et al. Optimization of the formation of embedded multicellular spheroids of MCF-7 cells: how to reliably produce a biomimetic 3D model. Anal Biochem. 2016;515:47–54. [DOI] [PubMed] [Google Scholar]
- 103.Shujaa Edin HY, et al. Recombinant human erythropoietin enhanced the cytotoxic effects of tamoxifen toward the spheroid MCF-7 breast cancer cells. Saudi J Biol Sci. 2021;28(9):5214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Low LTHTLE et al. A reliable and affordable 3D tumor spheroid model for natural product drug discovery: a case study of curcumin Prog Drug Discov Biomed Sci. 2019;1(1).
- 105.Jeong Y, Tin A, Irudayaraj J. Flipped well-plate hanging-drop technique for growing three-dimensional tumors. Front Bioeng Biotechnol. 2022;10:898699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriguez CE, et al. Autophagy protects from trastuzumab-induced cytotoxicity in HER2 overexpressing breast tumor spheroids. PLoS ONE. 2015;10(9):e0137920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monico DA, et al. Melanoma spheroid-containing artificial dermis as an alternative approach to in vivo models. Exp Cell Res. 2022;417(1):113207. [DOI] [PubMed] [Google Scholar]
- 108.Raghavan S, et al. Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol Oncol. 2015;138(1):181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelm JM, et al. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83(2):173–80. [DOI] [PubMed] [Google Scholar]
- 110.Li L, LaBarbera D. 3D high-content screening of organoids for drug discovery. Comprehensive Med Chem. 2017;III:2. [Google Scholar]
- 111.Akura™PLUS-Hanging-Drop-System. [cited 2023 25th July ]; Available from: https://shop.insphero.com/products/akura-plus-spheroid-hanging-drop-system.
- 112.Lee G, et al. Generation of uniform liver spheroids from human pluripotent stem cells for imaging-based drug toxicity analysis. Biomaterials. 2021;269:120529. [DOI] [PubMed] [Google Scholar]
- 113.Rescigno F, Ceriotti L, Meloni M. Extra cellular matrix deposition and assembly in dermis spheroids. Clin Cosmet Investig Dermatol. 2021;14:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohan S, et al. Assessing the predictive response of a simple and sensitive blood-based biomarker between estrogen-negative solid tumors. Adv Med Sci. 2020;65(2):424–8. [DOI] [PubMed] [Google Scholar]
- 115.Pawlik TM, et al. Amino acid uptake and regulation in multicellular hepatoma spheroids. J Surg Res. 2000;91(1):15–25. [DOI] [PubMed] [Google Scholar]
- 116.Casciari JJ, Sotirchos SV, Sutherland RM. Glucose diffusivity in multicellular tumor spheroids. Cancer Res. 1988;48(14):3905–9. [PubMed] [Google Scholar]
- 117.Foster TH, et al. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993;53(6):1249–54. [PubMed] [Google Scholar]
- 118.Wigle JC, Sutherland RM. Increased thermoresistance developed during growth of small multicellular spheroids. J Cell Physiol. 1985;122(2):281–9. [DOI] [PubMed] [Google Scholar]
- 119.Ronen S, Degani H. Studies of the metabolism of human breast cancer spheroids by NMR. Magn Reson Med. 1989;12(2):274–81. [DOI] [PubMed] [Google Scholar]
- 120.Erlichman C, Tannock IF. Growth and characterization of multicellular tumor spheroids of human bladder carcinoma origin. In Vitro Cell Dev Biol. 1986;22(8):449–56. [DOI] [PubMed] [Google Scholar]
- 121.Mueller-Klieser RMSBSJBHGBBW. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46(10):5320–9. [PubMed] [Google Scholar]
- 122.Bauman GS, et al. Effects of radiation on a three-dimensional model of malignant glioma invasion. Int J Dev Neurosci. 1999;17(5–6):643–51. [DOI] [PubMed] [Google Scholar]
- 123.Deen DF, et al. Development of a 9L rat brain tumor cell multicellular spheroid system and its response to 1,3-bis(2-chloroethyl)-1-nitrosourea and radiation. J Natl Cancer Inst. 1980;64(6):1373–82. [DOI] [PubMed] [Google Scholar]
- 124.Rofstad EK, Sutherland RM. Growth and radiation sensitivity of the MLS human ovarian carcinoma cell line grown as multicellular spheroids and xenografted tumours. Br J Cancer. 1989;59(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12(10):1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakthivel K, Hoorfar M, Kim K. High-throughput three-dimensional cellular platforms for screening biophysical microenvironmental signals. In: Micro and Nano Systems for Biophysical Studies of Cells and Small Organisms. Elsevier; 2021. p. 125–52. [Google Scholar]
- 127.Santos JM, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pinto DS, da Silva CL, Cabral JM. Scalable expansion of mesenchymal stem/stromal cells in bioreactors. A Focus on Hydrodynamic Characterization. 2019:537.
- 129.Corning®Disposable-Spinner-Flasks. Corning® disposable spinner flasks. [cited 2023 1st August 2023]; Available from: https://ecatalog.corning.com/life-sciences/b2c/US/en/Bioprocess-and-Scale-up/Disposable-Spinner-Flasks/Corning%C2%AE-Disposable-Spinner-Flasks/p/corningDisposableSpinnerFlasks.
- 130.Teale M, et al. Chemically defined, xeno-free expansion of human mesenchymal stem cells (hMSCs) on benchtop-scale using a stirred single-use bioreactor. Methods Mol Biol. 2022;2436:83–111. [DOI] [PubMed] [Google Scholar]
- 131.Moreira JL, et al. Effect of viscosity upon hydrodynamically controlled natural aggregates of animal cells grown in stirred vessels. Biotechnol Prog. 1995;11(5):575–83. [DOI] [PubMed] [Google Scholar]
- 132.Jossen V, Eibl R, Kraume M, Eibl D. Growth behavior of human adipose tissue-derived stromal/stem cells at small scale: numerical and experimental investigations. Bioengineering (Basel). 2018;5(4):106. [DOI] [PMC free article] [PubMed]
- 133.Chiesa E, Dorati R, Pisani S, Conti B, Bergamini G, Modena T, et al. The microfluidic technique and the manufacturing of polysaccharide nanoparticles. Pharmaceutics. 2018;10(4):267. [DOI] [PMC free article] [PubMed]
- 134.Nielsen JB, et al. Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem. 2020;92(1):150–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prince E, et al. Microfluidic arrays of breast tumor spheroids for drug screening and personalized cancer therapies. Adv Healthc Mater. 2022;11(1):e2101085. [DOI] [PubMed] [Google Scholar]
- 136.Takeuchi T. Medical checking of the aged. 13. Orthopedic diseases (2). Hokenfu Zasshi. 1989;45(2):128–9. [PubMed] [Google Scholar]
- 137.Sabhachandani P, et al. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip. 2016;16(3):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marimuthu M, et al. Multi-size spheroid formation using microfluidic funnels. Lab Chip. 2018;18(2):304–14. [DOI] [PubMed] [Google Scholar]
- 139.Chen MC, Gupta M, Cheung KC. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed Microdevices. 2010;12(4):647–54. [DOI] [PubMed] [Google Scholar]
- 140.Au Ieong K et al. Investigation of drug cocktail effects on cancer cell-spheroids using a microfluidic drug-screening assay. Micromachines. 2017;8(6).
- 141.Sun D, et al. A novel three-dimensional microfluidic platform for on chip multicellular tumor spheroid formation and culture. Microfluid Nanofluid. 2014;17(5):831–42. [Google Scholar]
- 142.Lim W, Park S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules. 2018;23(12). [DOI] [PMC free article] [PubMed]
- 143.Ziółkowska K, et al. Development of a three-dimensional microfluidic system for long-term tumor spheroid culture. Sens Actuators, B Chem. 2012;173:908–13. [Google Scholar]
- 144.Chen Y, et al. Drug cytotoxicity and signaling pathway analysis with three-dimensional tumor spheroids in a microwell-based microfluidic chip for drug screening. Anal Chim Acta. 2015;898:85–92. [DOI] [PubMed] [Google Scholar]
- 145.Lim W, et al. Formation of size-controllable tumour spheroids using a microfluidic pillar array (muFPA) device. Analyst. 2018;143(23):5841–8. [DOI] [PubMed] [Google Scholar]
- 146.Barisam M, Niavol FR, Kinj MA, Saidi MS, Ghanbarian H, Kashaninejad N. Enrichment of cancer stem-like cells by controlling oxygen, glucose and fluid shear stress in a microfluidic spheroid culture device. J Sci: Adv Mater Device. 2022;7(2):100439.
- 147.Huang YL, et al. Tumor spheroids under perfusion within a 3D microfluidic platform reveal critical roles of cell-cell adhesion in tumor invasion. Sci Rep. 2020;10(1):9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee SI, Choi YY, Kang SG, Kim TH, Choi JW, Kim YJ, et al. 3D Multicellular tumor spheroids in a microfluidic droplet system for investigation of drug resistance. Polymers (Basel). 2022;14(18):3752. [DOI] [PMC free article] [PubMed]
- 149.Lee JM, et al. Generation of tumor spheroids using a droplet-based microfluidic device for photothermal therapy. Microsyst Nanoeng. 2020;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee SR, et al. U-IMPACT: a universal 3D microfluidic cell culture platform. Microsyst Nanoeng. 2022;8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee SW, et al. In vitro lung cancer multicellular tumor spheroid formation using a microfluidic device. Biotechnol Bioeng. 2019;116(11):3041–52. [DOI] [PubMed] [Google Scholar]
- 152.Lunt SJ, et al. Interstitial fluid pressure in tumors: therapeutic barrier and biomarker of angiogenesis. Future Oncol. 2008;4(6):793–802. [DOI] [PubMed] [Google Scholar]
- 153.Milosevic M, Fyles A, Hill R. Interstitial fluid pressure in cervical cancer: guide to targeted therapy. Am J Clin Oncol. 2001;24(5):516–21. [DOI] [PubMed] [Google Scholar]
- 154.Yeo SG, et al. Interstitial fluid pressure as a prognostic factor in cervical cancer following radiation therapy. Clin Cancer Res. 2009;15(19):6201–7. [DOI] [PubMed] [Google Scholar]
- 155.Boucher Y, et al. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer. 1997;75(6):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Munson JM, Shieh AC. Interstitial fluid flow in cancer: implications for disease progression and treatment. Cancer Manag Res. 2014;6:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aryasomayajula A, Bayat P, Rezai P, Selvaganapathy PR. Microfluidic devices and their applications. In: Bhushan B, edetor. Springer Handbook of Nanotechnology. Springer Handbooks. Berlin, Heidelberg: Springer; 2017. p.487–536.
- 158.Sun Q, et al. Microfluidic formation of coculture tumor spheroids with stromal cells as a novel 3D tumor model for drug testing. ACS Biomater Sci Eng. 2018;4(12):4425–33. [DOI] [PubMed] [Google Scholar]
- 159.Tsai HF, Trubelja A, Shen AQ, Bao G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14(131):20170137. [DOI] [PMC free article] [PubMed]
- 160.Uhl CG, Liu Y. Microfluidic device for expedited tumor growth towards drug evaluation. Lab Chip. 2019;19(8):1458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alliedmarketresearch. Microfluidic devices market research, 2031. [cited 2023 20230812]; Available from: https://www.alliedmarketresearch.com/microfluidic-devices-market-A17085.
- 162.Fortunebusinessinsights. Medical device / microfluidic devices market. [cited 2023 20230812]; Available from: https://www.fortunebusinessinsights.com/industry-reports/microfluidic-devices-market-101098.
- 163.MERCK-MO4. CellASIC ONIX switching plate mammalian cells (4 chamber). [cited 2023 12th August]; Available from: https://www.sigmaaldrich.com/IN/en/product/mm/m04s035pk.
- 164.eNUVIO. microfluidics. [cited 2023 12th August]; Available from: https://enuvio.com/product-tag/microfluidics/.
- 165.Seleci DA, et al. Tumor homing and penetrating peptide-conjugated niosomes as multi-drug carriers for tumor-targeted drug delivery. RSC Adv. 2017;7(53):33378–84. [Google Scholar]
- 166.Sivakumar H, et al. Multi-cell type glioblastoma tumor spheroids for evaluating sub-population-specific drug response. Front Bioeng Biotechnol. 2020;8:538663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blandin AF, et al. Glioma cell dispersion is driven by alpha5 integrin-mediated cell-matrix and cell-cell interactions. Cancer Lett. 2016;376(2):328–38. [DOI] [PubMed] [Google Scholar]
- 168.Casey RC, et al. β1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159(6):2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matsuda Y, et al. Morphological and cytoskeletal changes of pancreatic cancer cells in three-dimensional spheroidal culture. Med Mol Morphol. 2010;43(4):211–7. [DOI] [PubMed] [Google Scholar]
- 170.Smyrek I et al. E-cadherin, actin, microtubules and FAK dominate different spheroid formation phases and important elements of tissue integrity. Biol Open. 2019;8(1). [DOI] [PMC free article] [PubMed]
- 171.Shimazui T, et al. Role of complex cadherins in cell-cell adhesion evaluated by.pdf. Oncol Rep. 2004;11:357–60. [PubMed] [Google Scholar]
- 172.Saias L, et al. Cell-cell adhesion and cytoskeleton tension oppose each other in regulating tumor cell aggregation. Cancer Res. 2015;75(12):2426–33. [DOI] [PubMed] [Google Scholar]
- 173.Ivascu A, Kubbies M. Diversity of cell-mediated adhesions in breast cancer spheroids.pdf. Int J Oncol. 2007;31:1403–13. [PubMed] [Google Scholar]
- 174.Weissenrieder JS, et al. The dopamine D2 receptor contributes to the spheroid formation behavior of U87 glioblastoma cells. Pharmacology. 2020;105(1–2):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marie PJ, et al. Cadherin-mediated cell-cell adhesion and signaling in the skeleton. Calcif Tissue Int. 2014;94(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maitre JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23(14):R626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–57. [DOI] [PubMed] [Google Scholar]
- 178.Ivascu A, Kubbies M. Diversity of cell-mediated adhesions in breast cancer spheroids. Int J Oncol. 2007;31(6):1403–13. [PubMed] [Google Scholar]
- 179.Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982;92(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Galateanu B, et al. Impact of multicellular tumor spheroids as an in vivolike tumor model on anticancer drug response. Int J Oncol. 2016;48(6):2295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gomes A, et al. Evaluation by quantitative image analysis of anticancer drug activity on multicellular spheroids grown in 3D matrices. Oncol Lett. 2016;12(6):4371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thakuri PS, et al. Quantitative size-based analysis of tumor spheroids and responses to therapeutics. Assay Drug Dev Technol. 2019;17(3):140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kulesza J, Pawłowska M, Augustin E. The influence of antitumor unsymmetrical bisacridines on 3D cancer spheroids growth and viability. Molecules. 2021;26(20):6262. [DOI] [PMC free article] [PubMed]
- 184.Smith T, Affram K, Bulumko E, Agyare E. Evaluation of in-vitro cytotoxic effect of 5-FU loaded-chitosan nanoparticles against spheroid models. J Nat Sci. 2018;4(10):e535. [PMC free article] [PubMed]
- 185.Akasov R, et al. Formation of multicellular tumor spheroids induced by cyclic RGD-peptides and use for anticancer drug testing in vitro. Int J Pharm. 2016;506(1–2):148–57. [DOI] [PubMed] [Google Scholar]
- 186.Yadav A, et al. Repurposing an antiepileptic drug for the treatment of glioblastoma. Pharm Res. 2022;39(11):2871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang J, et al. Anti-gastric cancer activity in three-dimensional tumor spheroids of bufadienolides. Sci Rep. 2016;6:24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim CH, et al. Vertically coated graphene oxide micro-well arrays for highly efficient cancer spheroid formation and drug screening. Adv Healthc Mater. 2020;9(7):e1901751. [DOI] [PubMed] [Google Scholar]
- 189.Ivanov DP, et al. Multiplexing spheroid volume, resazurin and acid phosphatase viability assays for high-throughput screening of tumour spheroids and stem cell neurospheres. PLoS ONE. 2014;9(8):e103817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gendre DAJ, et al. Optimization of tumor spheroid model in mesothelioma and lung cancers and anti-cancer drug testing in H2052/484 spheroids. Oncotarget. 2021;12(24):2375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lemmo S, et al. Optimization of aqueous biphasic tumor spheroid microtechnology for anti-cancer drug testing in 3D culture. Cell Mol Bioeng. 2014;7(3):344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925. [DOI] [PubMed] [Google Scholar]
- 193.Johnson-Arbor K, Dubey R. Doxorubicin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459232/.
- 194.Carvalho C, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267–85. [DOI] [PubMed] [Google Scholar]
- 195.Thorn CF, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Moriwaki T, et al. Cytotoxicity of tirapazamine (3-amino-1,2,4-benzotriazine-1,4-dioxide)-induced DNA damage in chicken DT40 cells. Chem Res Toxicol. 2017;30(2):699–704. [DOI] [PubMed] [Google Scholar]
- 197.Massaro F, Molica M, Breccia M. Ponatinib: a review of efficacy and safety. Curr Cancer Drug Targets. 2018;18(9):847–56. [DOI] [PubMed] [Google Scholar]
- 198.Tyagi A, et al. Cervical cancer stem cells manifest radioresistance: association with upregulated AP-1 activity. Sci Rep. 2017;7(1):4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ganesh K, Massague J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: molecular and cellular perspective. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK164700/.
- 201.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10 Suppl 1(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stoletov K, Beatty PH, Lewis JD. Novel therapeutic targets for cancer metastasis. Expert Rev Anticancer Ther. 2020;20(2):97–109. [DOI] [PubMed] [Google Scholar]
- 203.Jones NP, et al. PLCgamma1 is essential for early events in integrin signalling required for cell motility. J Cell Sci. 2005;118(Pt 12):2695–706. [DOI] [PubMed] [Google Scholar]
- 204.Tomas NM, et al. Akt and phospholipase Cgamma are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res Notes. 2012;5:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moser C, et al. Blocking heat shock protein-90 inhibits the invasive properties and hepatic growth of human colon cancer cells and improves the efficacy of oxaliplatin in p53-deficient colon cancer tumors in vivo. Mol Cancer Ther. 2007;6(11):2868–78. [DOI] [PubMed] [Google Scholar]
- 206.Schmidt M, et al. The influence of Osmunda regalis root extract on head and neck cancer cell proliferation, invasion and gene expression. BMC Complement Altern Med. 2017;17(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sharudin NA, et al. Invasion and metastasis suppression by anti-neonatal Nav1.5 antibodies in breast cancer. Asian Pac J Cancer Prev. 2022;23(9):2953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu X, et al. ITGA5 promotes tumor angiogenesis in cervical cancer. Cancer Med. 2023;12(10):11983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laborda-Illanes A, et al. Development of in vitro and in vivo tools to evaluate the antiangiogenic potential of melatonin to neutralize the angiogenic effects of VEGF and breast cancer cells: CAM assay and 3D endothelial cell spheroids. Biomed Pharmacother. 2023;157:114041. [DOI] [PubMed] [Google Scholar]
- 210.Bayat N, et al. The anti-angiogenic effect of atorvastatin in glioblastoma spheroids tumor cultured in fibrin gel: in 3D in vitro model. Asian Pac J Cancer Prev. 2018;19(9):2553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bhat SK, et al. P-I metalloproteinases and L-amino acid oxidases from Bothrops species inhibit angiogenesis. J Venom Anim Toxins Incl Trop Dis. 2021;27: e20200180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Szade K, et al. Spheroid-plug model as a tool to study tumor development, angiogenesis, and heterogeneity in vivo. Tumour Biol. 2016;37(2):2481–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Han M, et al. Enhanced percolation and gene expression in tumor hypoxia by PEGylated polyplex micelles. Mol Ther. 2009;17(8):1404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wortzel I, Seger R. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer. 2011;2(3):195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nitulescu GM, et al. The Akt pathway in oncology therapy and beyond (Review). Int J Oncol. 2018;53(6):2319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res. 2018;211:45–56. [DOI] [PubMed] [Google Scholar]
- 217.Abdelgalil AA, Al-Kahtani HM, Al-Jenoobi FI. Erlotinib. Profiles Drug Subst Excip Relat Methodol. 2020;45:93–117. [DOI] [PubMed] [Google Scholar]
- 218.Zoetemelk M, et al. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep. 2019;9(1):7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Expasy. Cellosaurus U-87MG Uppsala (CVCL_GP63). 12-Jan-2021 [cited 2022 22nd November]; Available from: https://www.cellosaurus.org/CVCL_GP63.
- 221.Larjavaara S, et al. Incidence of gliomas by anatomic location. Neuro Oncol. 2007;9(3):319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Belousov A, et al. The extracellular matrix and biocompatible materials in glioblastoma treatment. Front Bioeng Biotechnol. 2019;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen W, Wong C, Vosburgh E, Levine AJ, Foran DJ, Xu EY. High-throughput image analysis of tumor spheroids: a user-friendly software application to measure the size of spheroids automatically and accurately. J Vis Exp. 2014;(89):51639. [DOI] [PMC free article] [PubMed]
- 224.Wallberg F, Tenev T, Meier P. Analysis of apoptosis and necroptosis by fluorescence-activated cell sorting. Cold Spring Harb Protoc. 2016;2016(4):pdb prot087387. [DOI] [PubMed] [Google Scholar]
- 225.Crowley LC, Marfell BJ, Waterhouse NJ. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc. 2016;2016(9). 10.1101/pdb.prot087205. [DOI] [PubMed]
- 226.Crowley LC et al. Measuring cell death by propidium iodide uptake and flow cytometry. Cold Spring Harb Protoc. 2016;2016(7). 10.1101/pdb.prot087163. [DOI] [PubMed]
- 227.Chen H, et al. Clinical significance of ALDH1 combined with DAPI expression in patients with esophageal carcinoma. Oncol Lett. 2017;14(4):4878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Otto FJ. High-resolution analysis of nuclear DNA employing the fluorochrome DAPI. Methods Cell Biol. 1994;41:211–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and or/analyzed during the current study are available from the corresponding author upon reasonable request.