Abstract
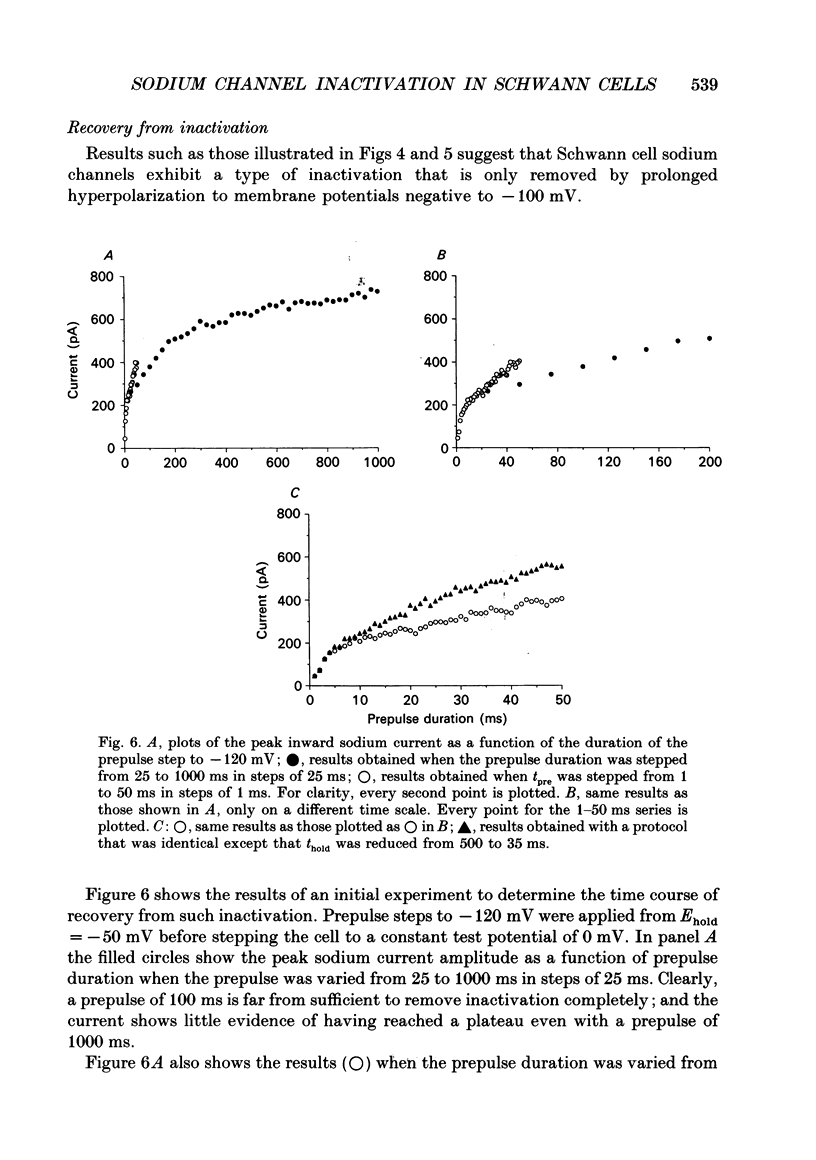
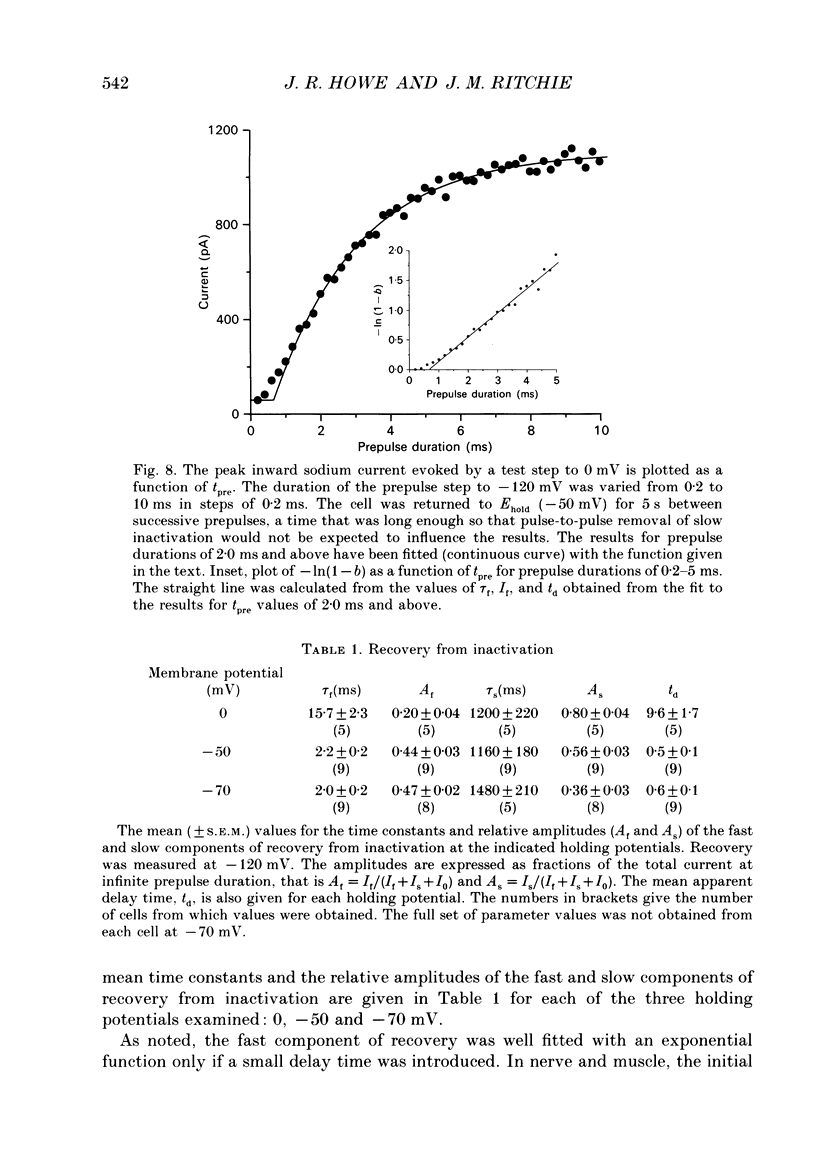
1. We have studied the kinetics of inactivation of sodium currents evoked in Schwann cells from neonatal and adult rabbits with patch-clamp recording techniques. The decay both of whole-cell currents and of ensemble currents obtained from outside-out patches was reasonably well-described by single exponential fits which gave values for the time constant, tau h, similar to those found for such currents in nerve (about 0.5 ms at 0 mV). Although inclusion of an additional exponential component usually improved the fits to the decay of these currents, the relative amplitude of the slower component (time constant 3-6 ms) was always small. 2. The time course of recovery from steady-state inactivation clearly consisted of two exponential components. At -120 mV, recovery from steady-state inactivation at -50 mV consisted of a fast component with a time constant of 2.2 +/- 0.2 ms and a much slower component with a time constant of 1.2 +/- 0.2 s (n = 9). The relative amplitude of the slow component (expressed as a fraction of the sodium current when inactivation was removed completely) was 0.56 +/- 0.03. The corresponding amplitudes of the slow component when similar experiments were done from holding potentials of 0 and -70 mV were 0.80 +/- 0.04 and 0.36 +/- 0.03, respectively (n = 5 and 8). 3. The onset of steady-state inactivation also followed a bi-exponential time course. The time constant of the slower component was similar at each potential examined (0, -50 and -70 mV), being 6-7 s. The relative amplitude of the slow component of onset depended on membrane potential, and it was similar (at each potential examined) to the corresponding amplitude of the slow component of recovery. 4. The inclusion of 40 mM-iodate ions in the pipette solution slowed the decay of whole-cell sodium currents, as did the extracellular application of venom (10 micrograms ml-1) from the scorpion Leiurus quinquestriatus. Prolonged exposure of the cells to Leiurus venom appeared to increase the steady-state amount of slow inactivation. 5. Records of single-channel sodium currents tended to cluster into records which either did, or did not, contain openings. This apparently non-random behaviour depended on membrane potential, and on the frequency at which the test steps were repeated, in the way expected if it resulted from slow inactivation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich R. W., Stevens C. F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987 Feb;7(2):418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Stanfield P. R., Stühmer W. Slow changes in currents through sodium channels in frog muscle membrane. J Physiol. 1983 Jun;339:253–271. doi: 10.1113/jphysiol.1983.sp014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989 Apr;2(4):1375–1388. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Berman M. F., Camardo J. S., Robinson R. B., Siegelbaum S. A. Single sodium channels from canine ventricular myocytes: voltage dependence and relative rates of activation and inactivation. J Physiol. 1989 Aug;415:503–531. doi: 10.1113/jphysiol.1989.sp017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Slow mechanism for sodium permeability inactivation in myelinated nerve fibre of Xenopus laevis. J Physiol. 1977 Sep;270(2):283–297. doi: 10.1113/jphysiol.1977.sp011952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975 Jan;244(2):511–534. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Ritchie J. M., Rogart R. B., Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979 Jul;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y., Schrager P., Ritchie J. M. Neuronal-type Na+ and K+ channels in rabbit cultured Schwann cells. Nature. 1984 Sep 13;311(5982):156–157. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Sodium currents in axon-associated Schwann cells from adult rabbits. J Physiol. 1987 May;386:181–203. doi: 10.1113/jphysiol.1987.sp016529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. A., Rojas E., Suarez-Isla B. A. Activation and inactivation characteristics of the sodium permeability in muscle fibres from Rana temporaria. J Physiol. 1982 Mar;324:297–318. doi: 10.1113/jphysiol.1982.sp014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Conductance of the sodium channel in myelinated nerve fibres with modified sodium inactivation. J Physiol. 1976 Nov;262(3):729–742. doi: 10.1113/jphysiol.1976.sp011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. M. Ultra-slow inactivation of the ionic currents through the membrane of myelinated nerve. Biochim Biophys Acta. 1976 Mar 5;426(2):232–244. doi: 10.1016/0005-2736(76)90334-5. [DOI] [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. On the active form of 4-aminopyridine: block of K+ currents in rabbit Schwann cells. J Physiol. 1991 Feb;433:183–205. doi: 10.1113/jphysiol.1991.sp018421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Sodium currents in Schwann cells from myelinated and non-myelinated nerves of neonatal and adult rabbits. J Physiol. 1990 Jun;425:169–210. doi: 10.1113/jphysiol.1990.sp018098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Two types of potassium current in rabbit cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):19–27. doi: 10.1098/rspb.1988.0061. [DOI] [PubMed] [Google Scholar]
- Kiss T., Nagy K. Interaction between sodium channels in mouse neuroblastoma cells. Eur Biophys J. 1985;12(1):13–18. doi: 10.1007/BF00254090. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Die Wirkung von Skorpiongift auf die Ionenströme des Ranvierschen Schnürrings. II. Unvollständiage Natrium-Inaktivierung. Pflugers Arch. 1968;303(2):150–161. doi: 10.1007/BF00592632. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K., Kiss T., Hof D. Single Na channels in mouse neuroblastoma cell membrane. Indications for two open states. Pflugers Arch. 1983 Dec;399(4):302–308. doi: 10.1007/BF00652757. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz J. R., Stämpfli R. A comparison of sodium currents in rat and frog myelinated nerve: normal and modified sodium inactivation. J Physiol. 1987 Jan;382:175–191. doi: 10.1113/jphysiol.1987.sp016362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Modification of sodium inactivation in myelinated nerve by Anemonia toxin II and iodate. Analysis of current fluctuations and current relaxations. Biochim Biophys Acta. 1980 Aug 4;600(2):456–466. doi: 10.1016/0005-2736(80)90448-4. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Sodium currents and sodium-current fluctuations in rat myelinated nerve fibres. J Physiol. 1982 Aug;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B. Modal gating behavior of cardiac sodium channels in cell-free membrane patches. Biophys J. 1988 Jun;53(6):857–862. doi: 10.1016/S0006-3495(88)83166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peganov E. M., Khodorov B. I., Shishkova L. D. Medlennaia natrievaia inaktivatsiia v membrane perekhvata Ranve. Rol' naruzhnogo kaliia. Biull Eksp Biol Med. 1973 Sep;76(9):15–19. [PubMed] [Google Scholar]
- Quandt F. N. Burst kinetics of sodium channels which lack fast inactivation in mouse neuroblastoma cells. J Physiol. 1987 Nov;392:563–585. doi: 10.1113/jphysiol.1987.sp016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Slow inactivation of currents in cardiac Purkinje fibres. J Physiol. 1968 Jul;197(1):233–253. doi: 10.1113/jphysiol.1968.sp008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Inactivation in Myxicola giant axons responsible for slow and accumulative adaptation phenomena. J Physiol. 1981 Mar;312:531–549. doi: 10.1113/jphysiol.1981.sp013642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L., Simoncini L., Stühmer W. Comparison between slow sodium channel inactivation in rat slow- and fast-twitch muscle. J Physiol. 1987 Feb;383:339–348. doi: 10.1113/jphysiol.1987.sp016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Slow sodium inactivation in Myxicola axons. Evidence for a second inactive state. Biophys J. 1976 Jul;16(7):771–778. doi: 10.1016/S0006-3495(76)85727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayer J. Behaviour of chemically modified sodium channels in frog nerve supports a three-state model of inactivation. Pflugers Arch. 1985 May;404(1):21–28. doi: 10.1007/BF00581486. [DOI] [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Covariance of nonstationary sodium current fluctuations at the node of Ranvier. Biophys J. 1981 Apr;34(1):111–133. doi: 10.1016/S0006-3495(81)84840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini L., Stühmer W. Slow sodium channel inactivation in rat fast-twitch muscle. J Physiol. 1987 Feb;383:327–337. doi: 10.1113/jphysiol.1987.sp016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stämpfli R. Intraaxonal iodate inhibits sodium inactivation. Experientia. 1974 May 15;30(5):505–508. doi: 10.1007/BF01926319. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The inactivation of sodium channels in the node of Ranvier and its chemical modification. Ion Channels. 1990;2:123–168. doi: 10.1007/978-1-4615-7305-0_4. [DOI] [PubMed] [Google Scholar]
- Wang G. K., Strichartz G. R. Purification and physiological characterization of neurotoxins from venoms of the scorpions centruroides sculpturatus and leiurus quinquestriatus. Mol Pharmacol. 1983 Mar;23(2):519–533. [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]


