Abstract
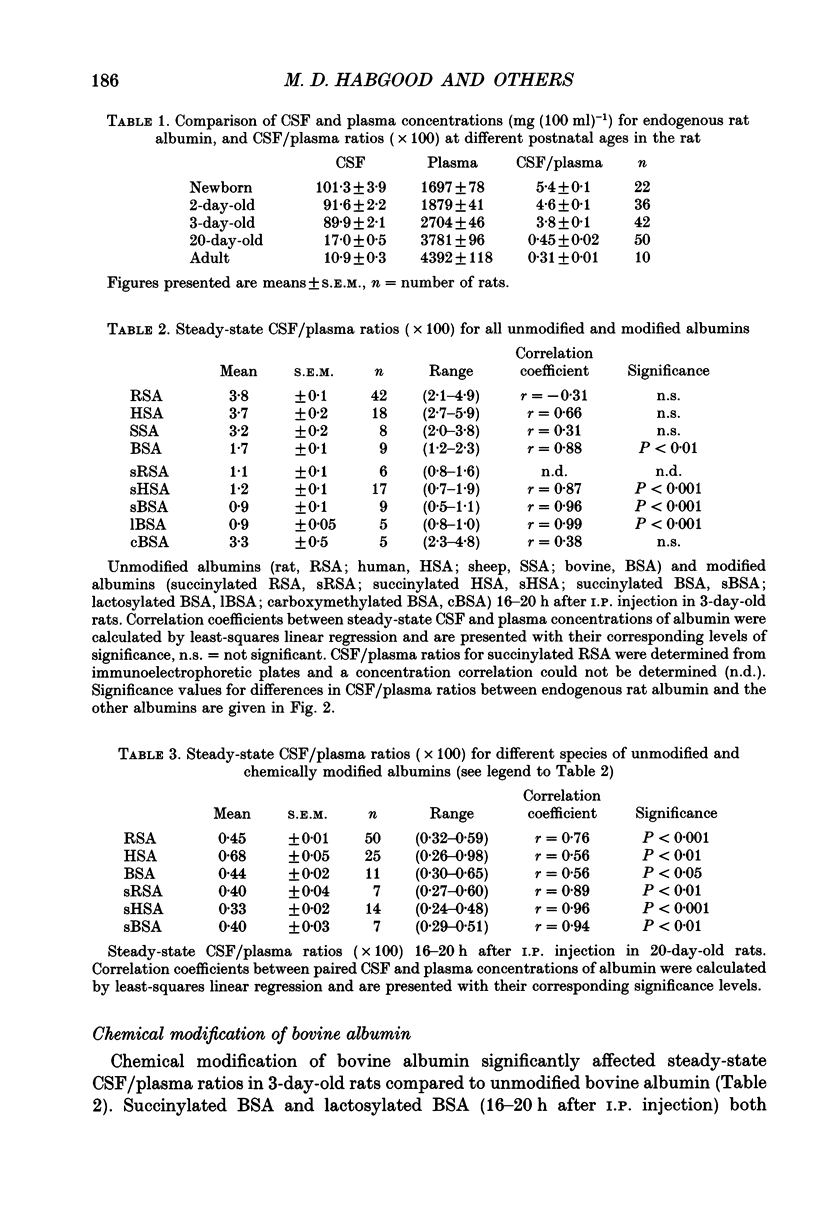
1. The transfer of albumin between the blood and the cerebrospinal fluid (CSF) has been investigated in neonatal (3 days old) and juvenile (20 days old) rats. At both stages of postnatal development, all of the albumin present in the CSF can be accounted for by transfer from the blood. Thus it is unlikely that in situ synthesis of albumin contributes to the naturally high levels of albumin in CSF in the developing brain. 2. The high concentration of albumin in CSF of the neonatal rat brain cannot be accounted for solely by diffusion from the blood. In the 3-day-old rat, only about one quarter of the albumin in CSF enters by diffusion from the blood, whilst the remainder appears to be transported into the CSF by a specific mechanism which can discriminate between different species of albumin. The specific transport component of albumin transfer between the blood and the CSF appears to be developmentally regulated and is not apparent in 20-day-old rats. 3. Chemical modification of albumin resulting in either an increase or a decrease in electrophoretic mobility (at pH 7.4), significantly reduces blood-CSF transfer of albumin in 3-day-old rats, but has little effect in the 20-day-old rat. Thus overall molecular charge does not appear to be an important feature of the species-specific blood-CSF albumin transport mechanism in neonatal rats.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Fossan G., Lorscheider F. L., Malinowska D. H., Møllgård K., Reynolds M. L., Saunders N. R., Wilkinson S. Proteins in cerebrospinal fluid and plasma of fetal sheep during development. J Physiol. 1980 Mar;300:441–455. doi: 10.1113/jphysiol.1980.sp013171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Lai P. C., Lorscheider F. L., Malinowska D. H., Møllgård K., Saunders N. R. Proteins in cerebrospinal fluid and plasma of fetal rats during development. Dev Biol. 1981 Apr 15;83(1):193–200. doi: 10.1016/s0012-1606(81)80024-3. [DOI] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds J. M., Reynolds M. L., Saunders N. R. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J Physiol. 1979 Jul;292:207–231. doi: 10.1113/jphysiol.1979.sp012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds M. L., Saunders N. R. Blood-cerebrospinal fluid transfer of plasma proteins during fetal development in the sheep. J Physiol. 1980 Mar;300:457–465. doi: 10.1113/jphysiol.1980.sp013172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Habgood M. D., Møllgård K., Stagaard M., Saunders N. R. Species-specific transfer of plasma albumin from blood into different cerebrospinal fluid compartments in the fetal sheep. J Physiol. 1991 Aug;439:215–237. doi: 10.1113/jphysiol.1991.sp018664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Saunders N. R., Schejter E. J., Zakut H., Zevin-Sonkin D., Zisling R., Soreq H. Synthesis of plasma proteins in fetal, adult, and neoplastic human brain tissue. Dev Biol. 1986 May;115(1):93–104. doi: 10.1016/0012-1606(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr. 1974 Dec 15;52(24):1158–1164. doi: 10.1007/BF01466734. [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Rubin J. B., Handrahan J. V., Connor J. R., Fine R. E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res. 1987;18(2):299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Giffels J. Study of protein characteristics that influence entry into the cerebrospinal fluid of normal mice and mice with encephalitis. J Clin Invest. 1982 Aug;70(2):289–295. doi: 10.1172/JCI110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Michel C. C. The Malpighi lecture. Vascular permeability--the consequence of Malpighi's hypothesis. Int J Microcirc Clin Exp. 1985;4(3):265–284. [PubMed] [Google Scholar]
- Mooradian A. D. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988 Jan-Feb;9(1):31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- Reynolds M. L., Evans C. A., Reynolds E. O., Saunders N. R., Durbin G. M., Wigglesworth J. S. Intracranial haemorrhage in the preterm sheep fetus. Early Hum Dev. 1979 Jul;3(2):163–186. doi: 10.1016/0378-3782(79)90005-7. [DOI] [PubMed] [Google Scholar]
- Shimon-Hophy M., Wadhwani K. C., Chandrasekaran K., Larson D., Smith Q. R., Rapoport S. I. Regional blood-brain barrier transport of cationized bovine serum albumin in awake rats. Am J Physiol. 1991 Aug;261(2 Pt 2):R478–R483. doi: 10.1152/ajpregu.1991.261.2.R478. [DOI] [PubMed] [Google Scholar]
- Triguero D., Buciak J. B., Yang J., Pardridge W. M. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4761–4765. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorbrodt A. W., Lossinsky A. S., Dobrogowska D. H., Wisniewski H. M. Sequential appearance of anionic domains in the developing blood-brain barrier. Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):31–37. doi: 10.1016/0165-3806(90)90219-o. [DOI] [PubMed] [Google Scholar]
- Webber S. E., Widdicombe J. G. The transport of albumin across the ferret in vitro whole trachea. J Physiol. 1989 Jan;408:457–472. doi: 10.1113/jphysiol.1989.sp017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]