Abstract
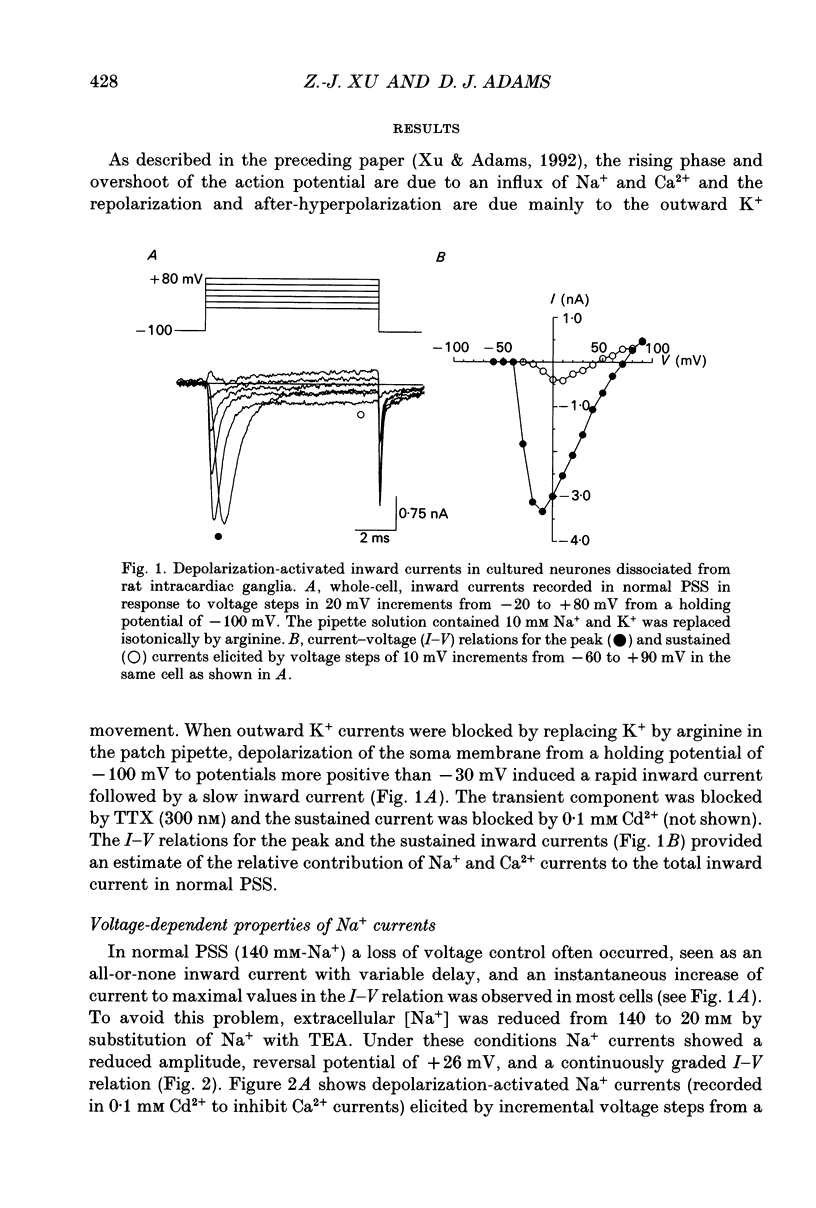
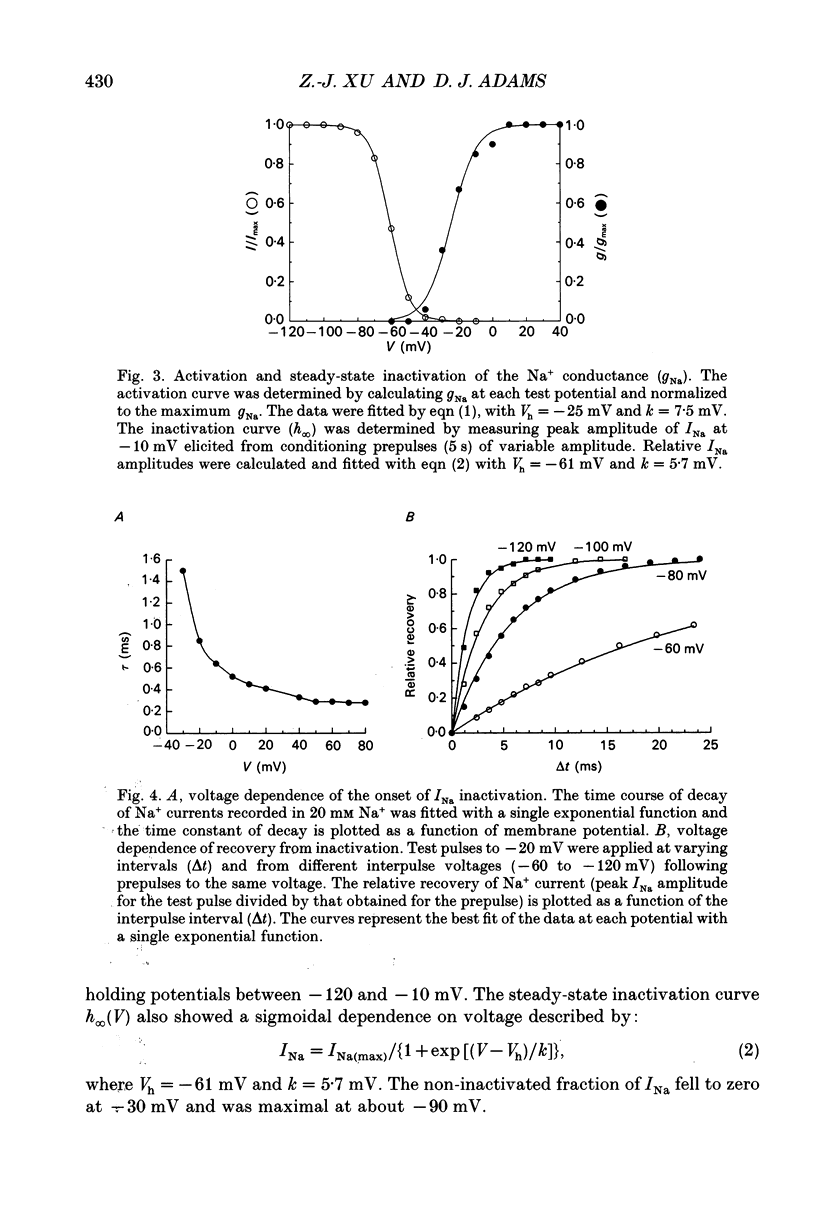
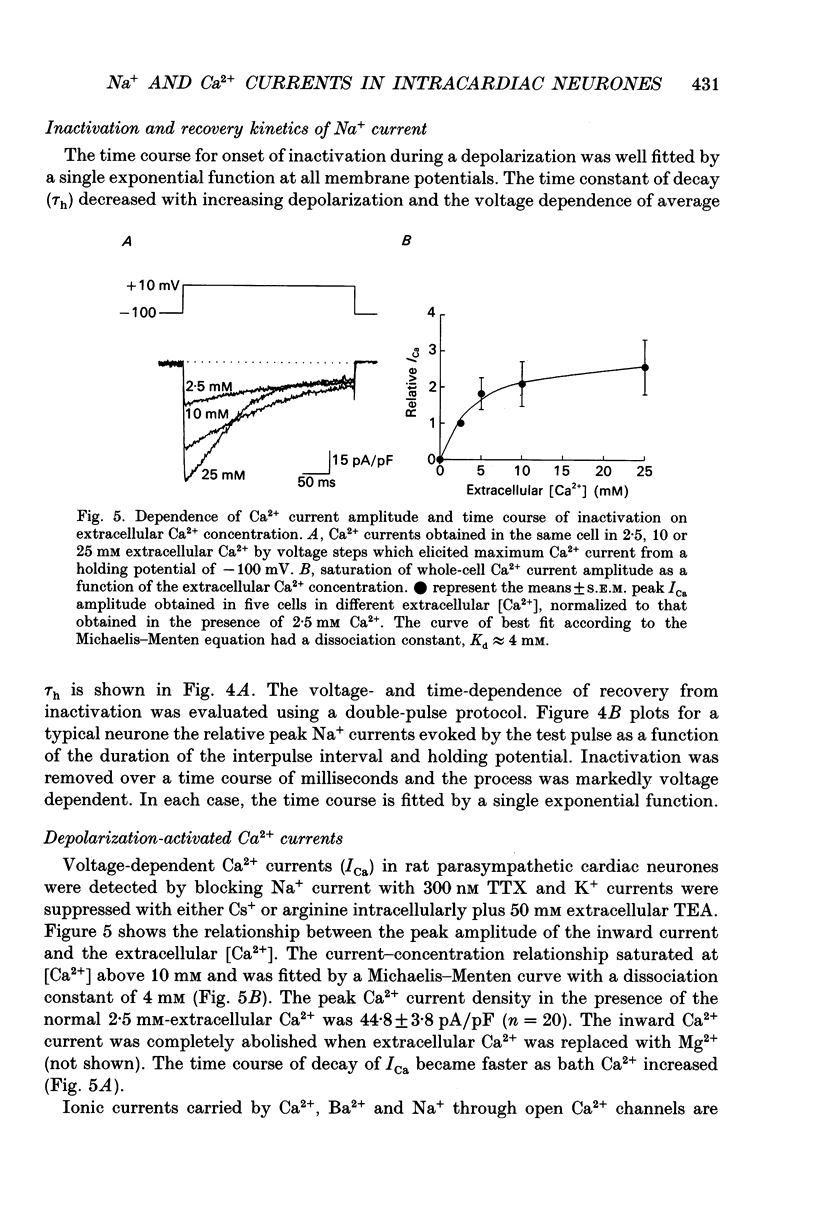
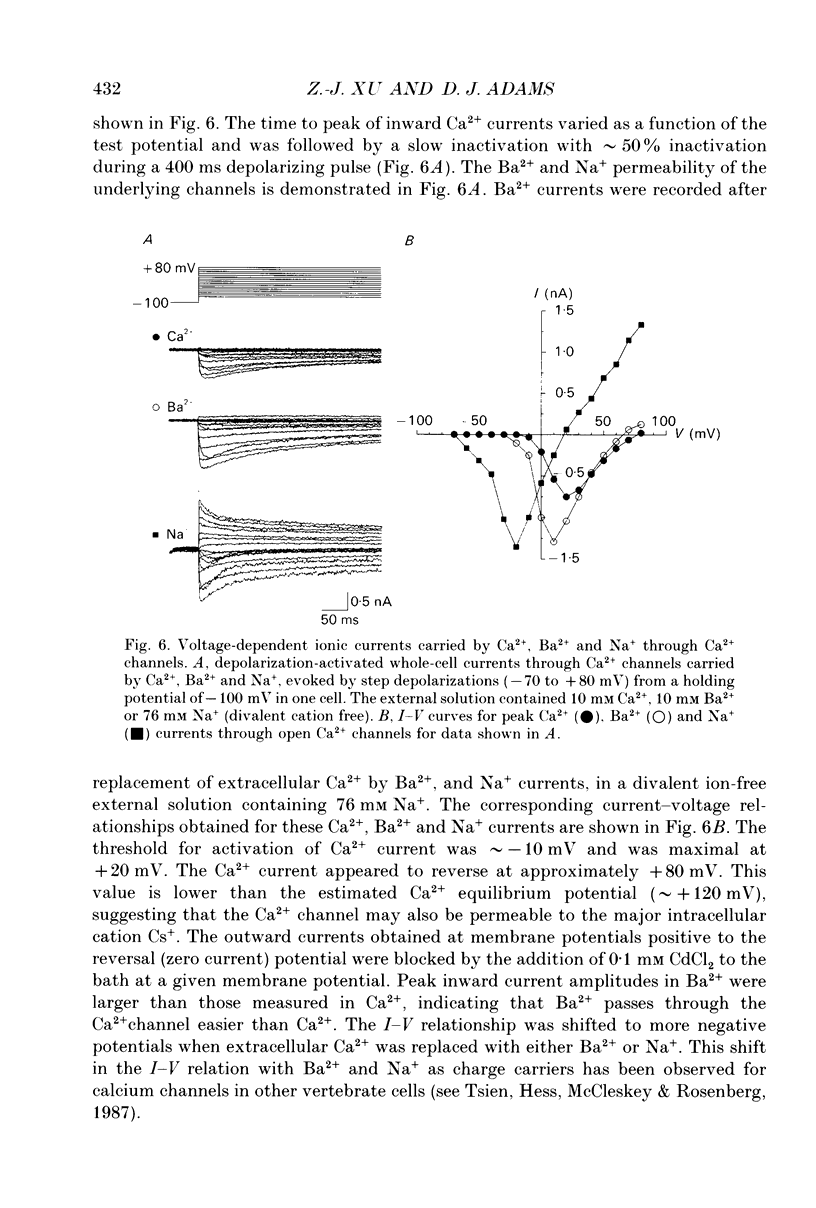
1. Depolarization-activated Na+ and Ca2+ currents underlying the rising phase of the action potential in mammalian parasympathetic ganglion cells were investigated in voltage-clamped neurones dissociated from neonatal rat intracardiac ganglia and maintained in tissue culture. 2. A current component isolated by replacing intracellular K+ with Cs+ or arginine and adding 0.1 mM Cd2+ to the external solution was dependent on extracellular [Na+] and reversibly blocked in the presence of 300 nM tetrodotoxin (TTX). Peak amplitudes of Na+ currents elicited by step depolarization from a holding potential of -100 mV were 351 +/- 18 pA/pF (140 mM extracellular Na+). 3. The sodium current-voltage (I-V) curve exhibited a threshold for activation at -40 mV and reached a maximum at -10 mV. The Na+ conductance increased sigmoidally with increasing depolarization reaching half-maximal activation at -25 mV, with a maximum slope corresponding to 7.5 mV per e-fold change in conductance. 4. During a maintained depolarization, Na+ currents turned on and then decayed (inactivated) with an exponential time course. The time constant of inactivation was voltage dependent decreasing from 0.85 ms at -20 mV to 0.3 ms at +60 mV (23 degrees C). The steady-state inactivation of the Na+ conductance was voltage-dependent with half-inactivation occurring at -61 mV and near-complete inactivation at -20 mV. Recovery from inactivation also followed an exponential time course with a time constant that increased at depolarized membrane potentials. 5. A voltage- and Ca(2+)-dependent current was isolated by replacement of intracellular K+ with either Cs+ or arginine and of extracellular Na+ with tetraethylammonium and the addition of TTX. Extracellular Ba2+ or Na+ (in the absence of external divalent cation) could substitute for Ca2+. Peak Ca2+ current increased with increasing extracellular [Ca2+] and above 10 mM (Kd approximately 4 mM) approached saturation. The peak Ca2+ current density was 45 +/- 4 pA/pF (2.5 mM-extracellular Ca2+). 6. The Ca2+ I-V relation exhibited a high threshold for activation (-20 mV) and reached a maximum at +20 mV. Changing the holding potential from -100 to -40 mV did not alter the I-V relationship. Peak Ca2+ conductance increased sigmoidally with increasing depolarization reaching half-maximal activation at -4 mV, with a maximal slope of 4 mV per e-fold change in Ca2+ conductance. 7. The kinetics of activation and inactivation of the Ca2+ current were voltage dependent and the time course of inactivation was fitted by the sum of two exponentials.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
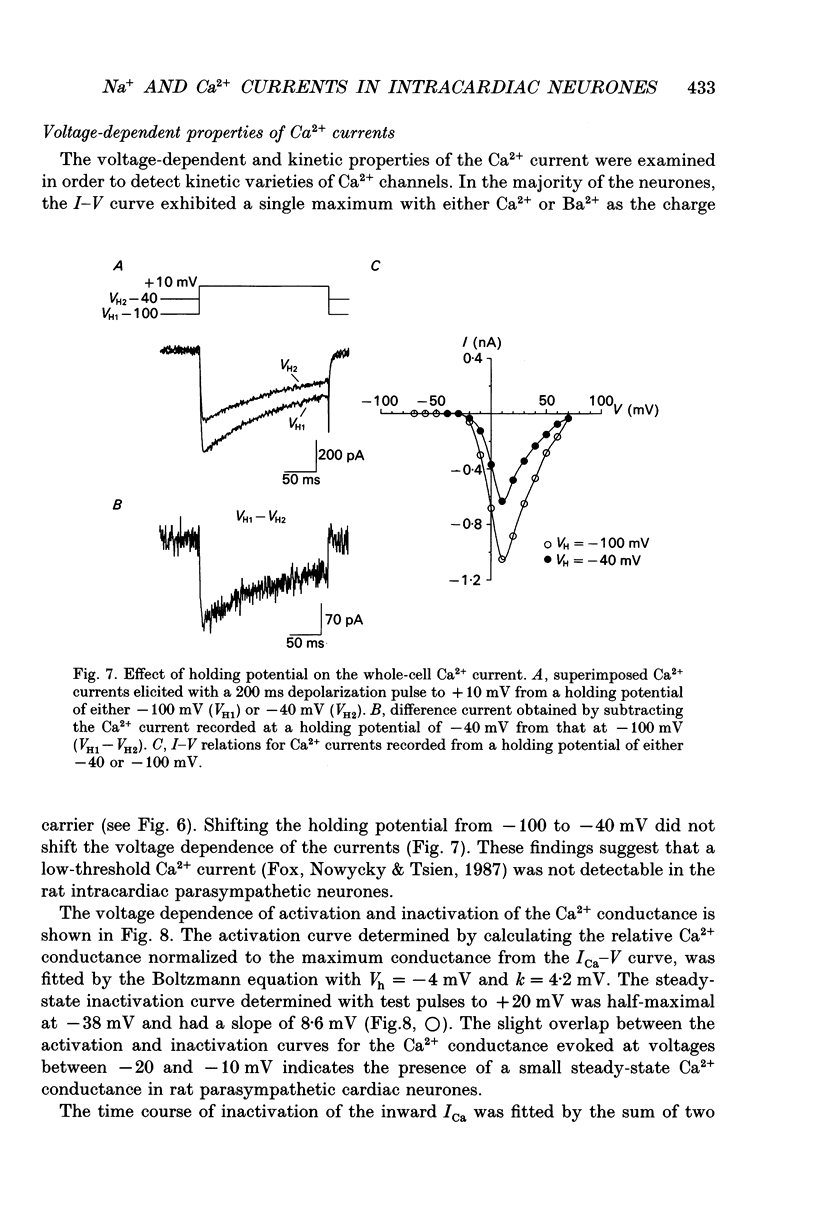
- Akasu T., Tsurusaki M., Tokimasa T. Reduction of the N-type calcium current by noradrenaline in neurones of rabbit vesical parasympathetic ganglia. J Physiol. 1990 Jul;426:439–452. doi: 10.1113/jphysiol.1990.sp018148. [DOI] [PMC free article] [PubMed] [Google Scholar]
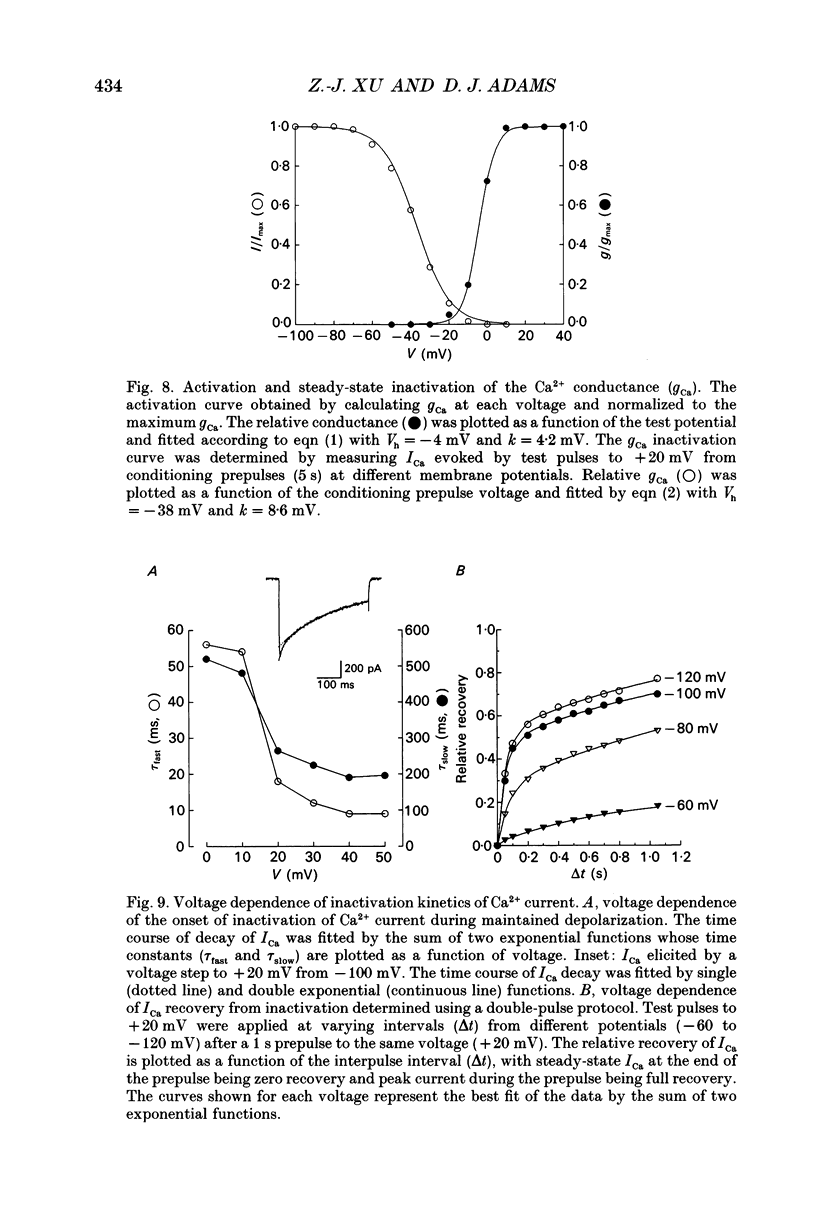
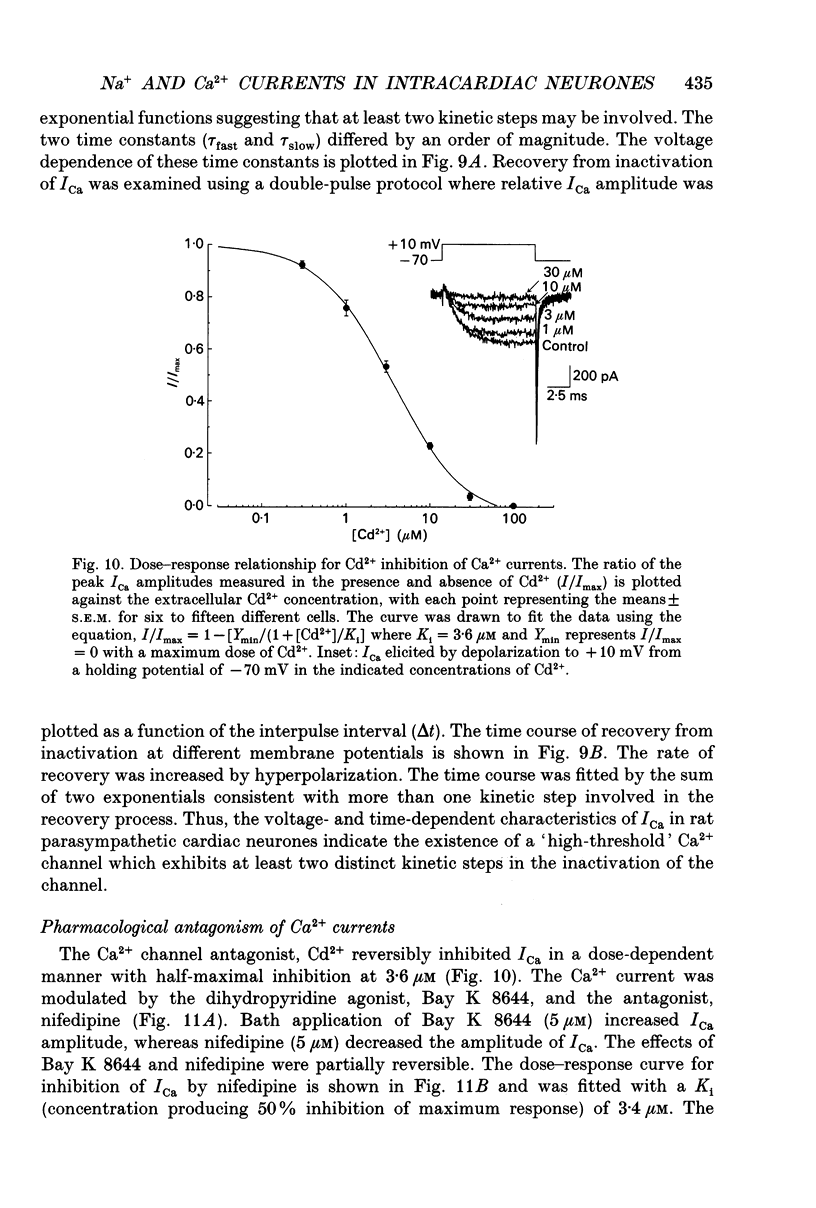
- Allen T. G., Burnstock G. Intracellular studies of the electrophysiological properties of cultured intracardiac neurones of the guinea-pig. J Physiol. 1987 Jul;388:349–366. doi: 10.1113/jphysiol.1987.sp016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. Calcium currents in the normal adult rat sympathetic neurone. J Physiol. 1989 May;412:493–512. doi: 10.1113/jphysiol.1989.sp017628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Inactivation of the low-threshold transient calcium current in rat sensory neurones: evidence for a dual process. J Physiol. 1986 Jul;376:341–357. doi: 10.1113/jphysiol.1986.sp016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Tse A., Giles W. R. Electrophysiology of parasympathetic neurones isolated from the interatrial septum of bull-frog heart. J Physiol. 1990 Aug;427:89–125. doi: 10.1113/jphysiol.1990.sp018163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Oxford G. S. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 1985 May;85(5):743–763. doi: 10.1085/jgp.85.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Tetrodotoxin-resistant sodium current of rat nodose neurones: monovalent cation selectivity and divalent cation block. J Physiol. 1987 Aug;389:255–270. doi: 10.1113/jphysiol.1987.sp016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Shuba YaM, Savchenko A. N. Three types of calcium channels in the membrane of mouse sensory neurons. Pflugers Arch. 1988 Jun;411(6):661–669. doi: 10.1007/BF00580863. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Brown D. A. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987 Jun 1;77(1):55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Adams D. J. Inhibition of neurally-evoked transmitter release by calcium channel antagonists in rat parasympathetic ganglia. Br J Pharmacol. 1989 Aug;97(4):1125–1136. doi: 10.1111/j.1476-5381.1989.tb12570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook G. R., Fieber L. A., Adams D. J. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990 Oct;259(4 Pt 2):H997–1005. doi: 10.1152/ajpheart.1990.259.4.H997. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988 Aug;92(2):197–218. doi: 10.1085/jgp.92.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Hess P., McCleskey E. W., Rosenberg R. L. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Tsurusaki M., Nishimura T., Akasu T. Properties of voltage-dependent barium currents in neurons of pelvic parasympathetic ganglia of rabbit. Jpn J Physiol. 1990;40(3):423–427. doi: 10.2170/jjphysiol.40.423. [DOI] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X. H., Thomas J. X., Jr, Randall W. C., Wurster R. D. Intracellular recordings from canine intracardiac ganglion cells. J Auton Nerv Syst. 1991 Feb;32(2):177–182. doi: 10.1016/0165-1838(91)90068-e. [DOI] [PubMed] [Google Scholar]
- Xu Z. J., Adams D. J. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol. 1992 Oct;456:405–424. doi: 10.1113/jphysiol.1992.sp019343. [DOI] [PMC free article] [PubMed] [Google Scholar]


