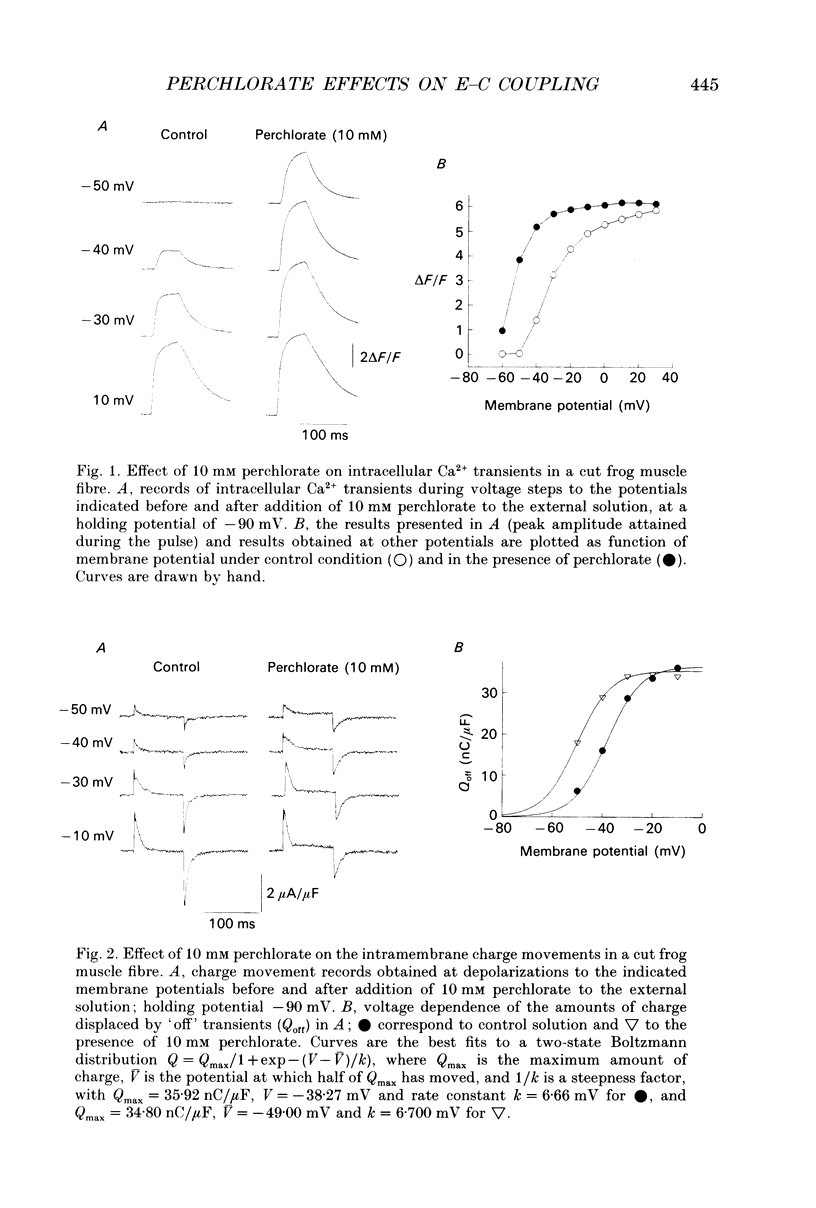
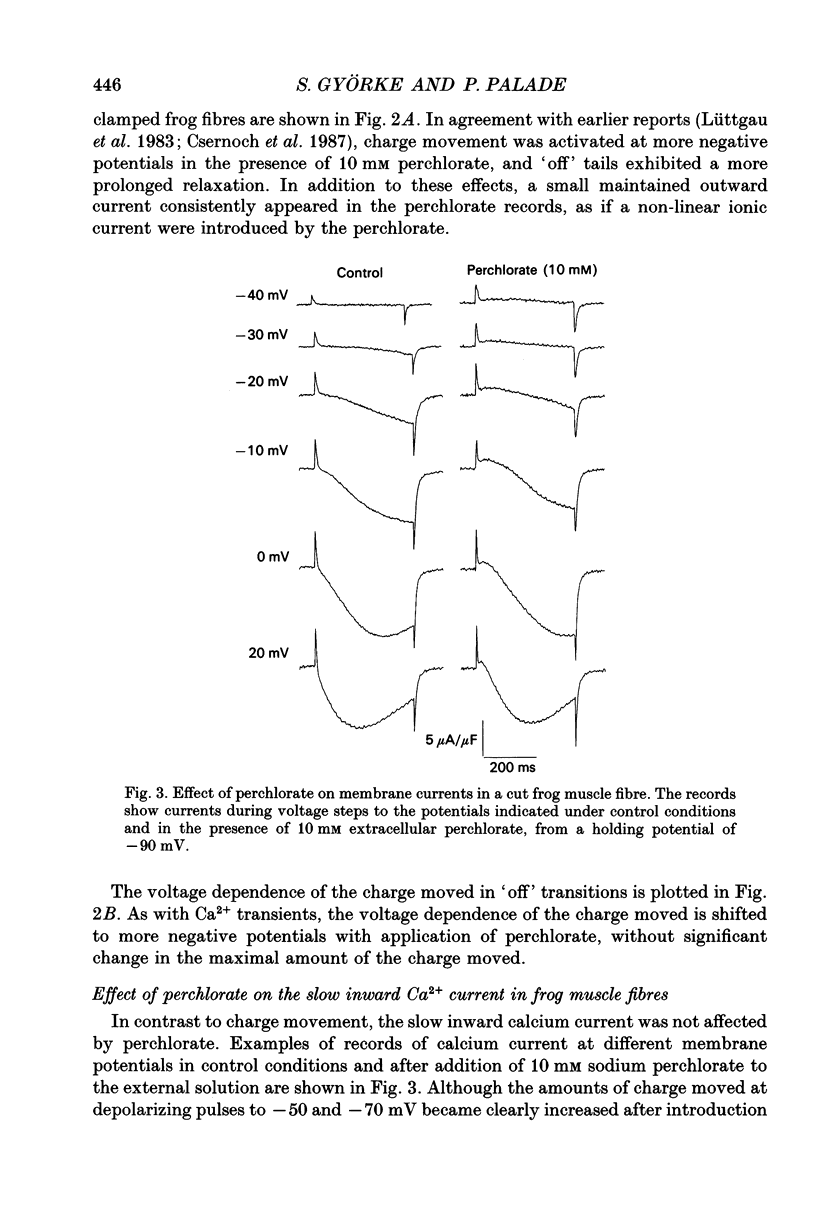
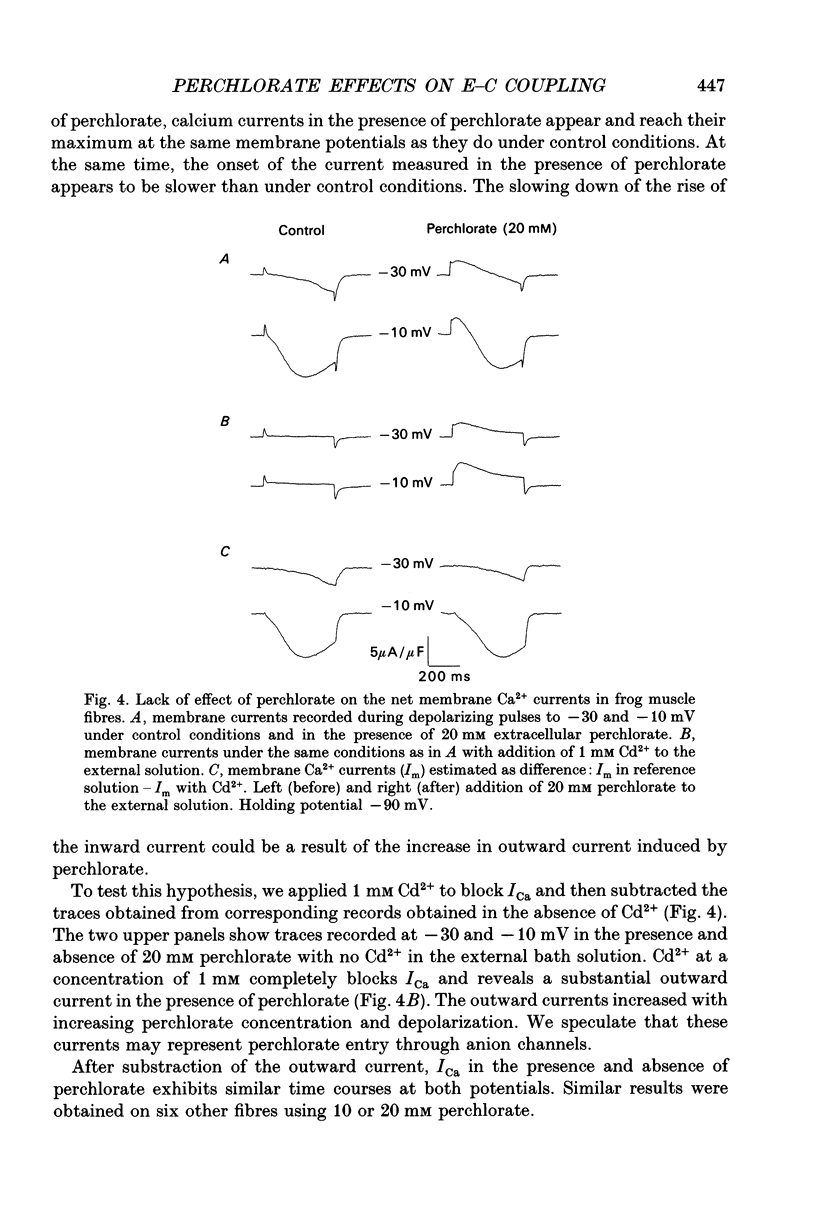
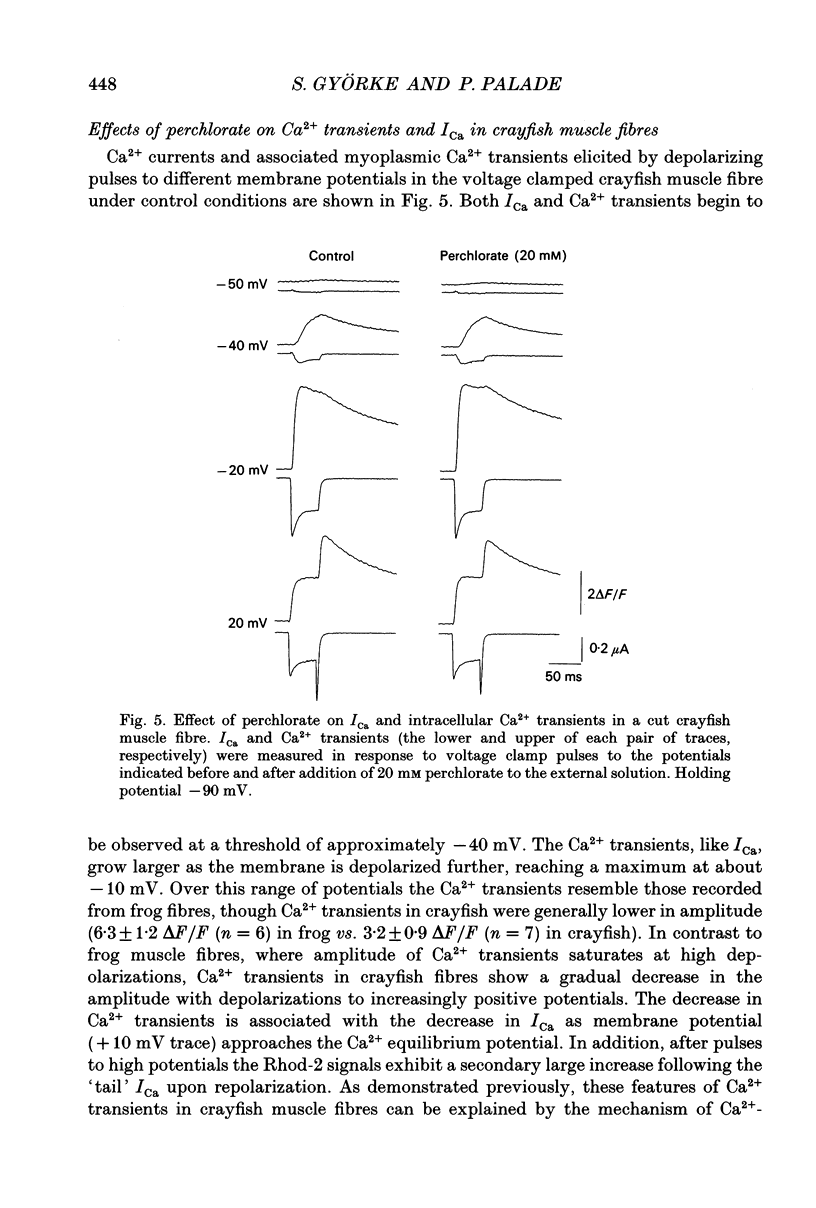
Abstract
1. The effects of perchlorate on various aspects of excitation-contraction coupling in frog and crayfish skeletal muscle have been examined in optical and electrophysiological experiments on voltage-clamped cut muscle fibres. 2. In the frog, perchlorate shifted the voltage dependence of charge movement and consequent sarcoplasmic reticulum (SR) Ca2+ release, but it had little effect on the slow inward calcium current. 3. In the crayfish, perchlorate had little effect on either calcium currents or the SR Ca2+ release that contributes to myoplasmic Ca2+ elevations. 4. Two alternative explanations for these results are discussed. There may be two functional types of dihydropyridine receptors, those (perchlorate sensitive) that communicate with the ryanodine receptor via charge movement and those (perchlorate insensitive) that function solely as Ca2+ entry points. Alternatively, the results would be consistent with two separate voltage sensors on each dihydropyridine receptor, only one of which is perchlorate sensitive.
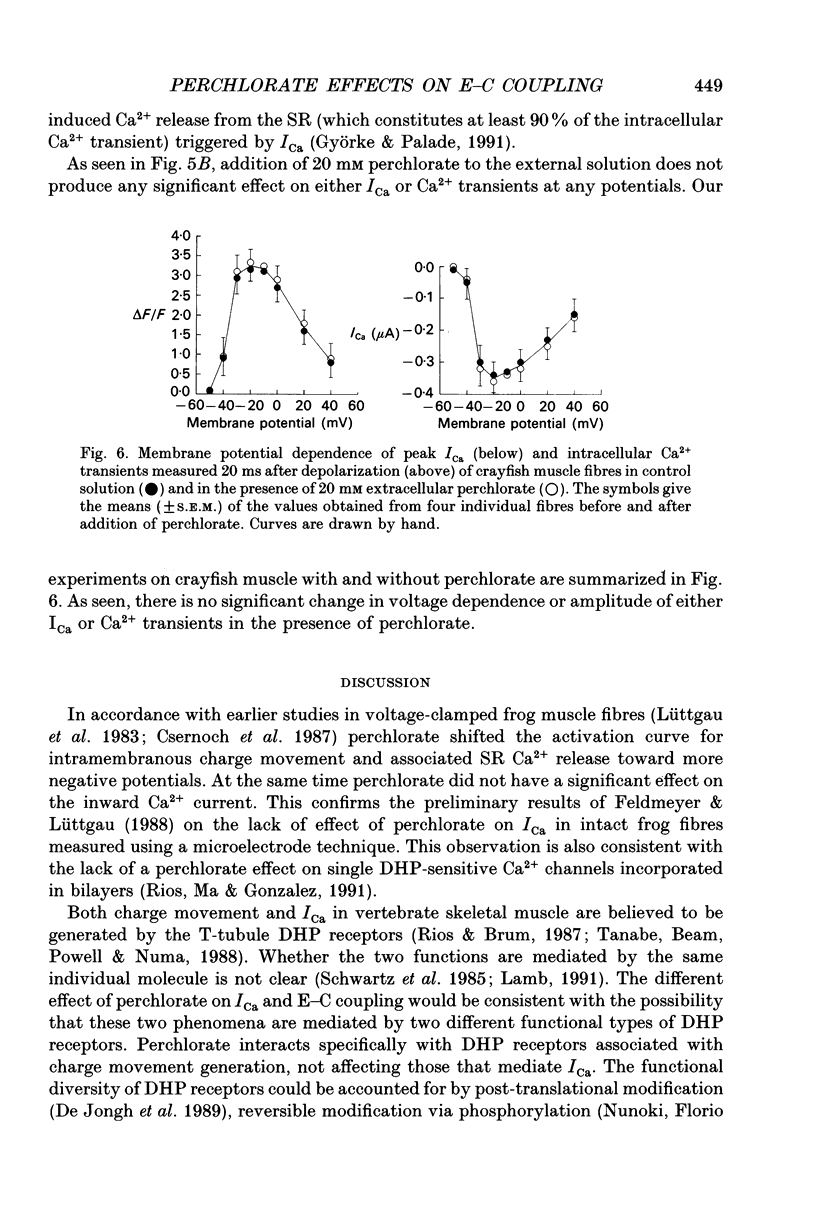
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Csernoch L., Kovács L., Szücs G. Perchlorate and the relationship between charge movement and contractile activation in frog skeletal muscle fibres. J Physiol. 1987 Sep;390:213–227. doi: 10.1113/jphysiol.1987.sp016695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. Fast gating kinetics of the slow Ca2+ current in cut skeletal muscle fibres of the frog. J Physiol. 1990 Jun;425:347–367. doi: 10.1113/jphysiol.1990.sp018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Best P. M. Effect of perchlorate on calcium release in skinned fibres stimulated by ionic substitution and caffeine. Pflugers Arch. 1990 Mar;415(6):688–692. doi: 10.1007/BF02584006. [DOI] [PubMed] [Google Scholar]
- Formelová J., Hurnák O., Novotová M., Zachar J. Ryanodine receptor purified from crayfish skeletal muscle. Gen Physiol Biophys. 1990 Oct;9(5):445–453. [PubMed] [Google Scholar]
- Foulks J. G., Perry F. A. The effects of temperature, local anaesthetics, pH, divalent cations, and group-specific reagents on repriming and repolarization-induced contractures in frog skeletal muscle. Can J Physiol Pharmacol. 1979 Jun;57(6):619–630. doi: 10.1139/y79-095. [DOI] [PubMed] [Google Scholar]
- Gomolla M., Gottschalk G., Lüttgau H. C. Perchlorate-induced alterations in electrical and mechanical parameters of frog skeletal muscle fibres. J Physiol. 1983 Oct;343:197–214. doi: 10.1113/jphysiol.1983.sp014888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurnák O., Proks P., Krizanová O., Zachar J. DHP-sensitive Ca2+ channels from crayfish skeletal muscle T-tubules incorporated into planar lipid bilayers. Gen Physiol Biophys. 1990 Dec;9(6):643–646. [PubMed] [Google Scholar]
- Krizanova O., Novotova M., Zachar J. Characterization of DHP binding protein in crayfish striated muscle. FEBS Lett. 1990 Jul 16;267(2):311–315. doi: 10.1016/0014-5793(90)80951-e. [DOI] [PubMed] [Google Scholar]
- Lamb G. D. Ca2+ channels or voltage sensors? Nature. 1991 Jul 11;352(6331):113–113. doi: 10.1038/352113b0. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Gottschalk G., Kovács L., Fuxreiter M. How perchlorate improves excitation-contraction coupling in skeletal muscle fibers. Biophys J. 1983 Aug;43(2):247–249. doi: 10.1016/S0006-3495(83)84346-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoki K., Florio V., Catterall W. A. Activation of purified calcium channels by stoichiometric protein phosphorylation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6816–6820. doi: 10.1073/pnas.86.17.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Ríos E., Ma J. J., González A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991 Apr;12(2):127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]


