Abstract
1. The influence of postnatal hypoxia on regulation of breathing and turnover rate of carotid body dopamine was examined in newborn rats. The percentage change in frequency, tidal volume and ventilation elicited by transient hyperoxia was assessed by flow plethysmography in unanaesthetized pups. The alteration in ventilation was taken as an index of peripheral chemoreceptor activity. 2. The rats were born and reared in hypoxia. The inspired oxygen fraction (FI,O2) was 0.12-0.14 until 2 days after delivery when the rats were placed into room air and the ventilatory chemoreflex was tested. At 4 days of age, i.e. 2 days after termination of hypoxia, the rats were tested again. The ventilatory data were compared with those from a previous study in normoxic rats. 3. We found a smaller decrease in ventilation (8.8 +/- 3.9%, mean +/- S.D.) in the hypoxic rats at 2 days of age compared with normoxic rats (22.7 +/- 6.4%; P < 0.001). In contrast, at 4 days of age there was no difference in ventilatory response between the posthypoxic rats (19.2 +/- 4.6%) and normoxic pups (18.6 +/- 4.9%). 4. The turnover rates of dopamine in carotid bodies were determined at 0-6, 6-12, 12-24 h and 2 days after birth in hypoxic rats and in 2-day-old posthypoxic rat pups at different time intervals after termination of hypoxia. Postnatal hypoxia sustained a high turnover rate which decreased after termination of the hypoxia. 5. We propose that the weak chemoreflex in hypoxic rat pups is brought about by a high release of carotid body dopamine.
Full text
PDF


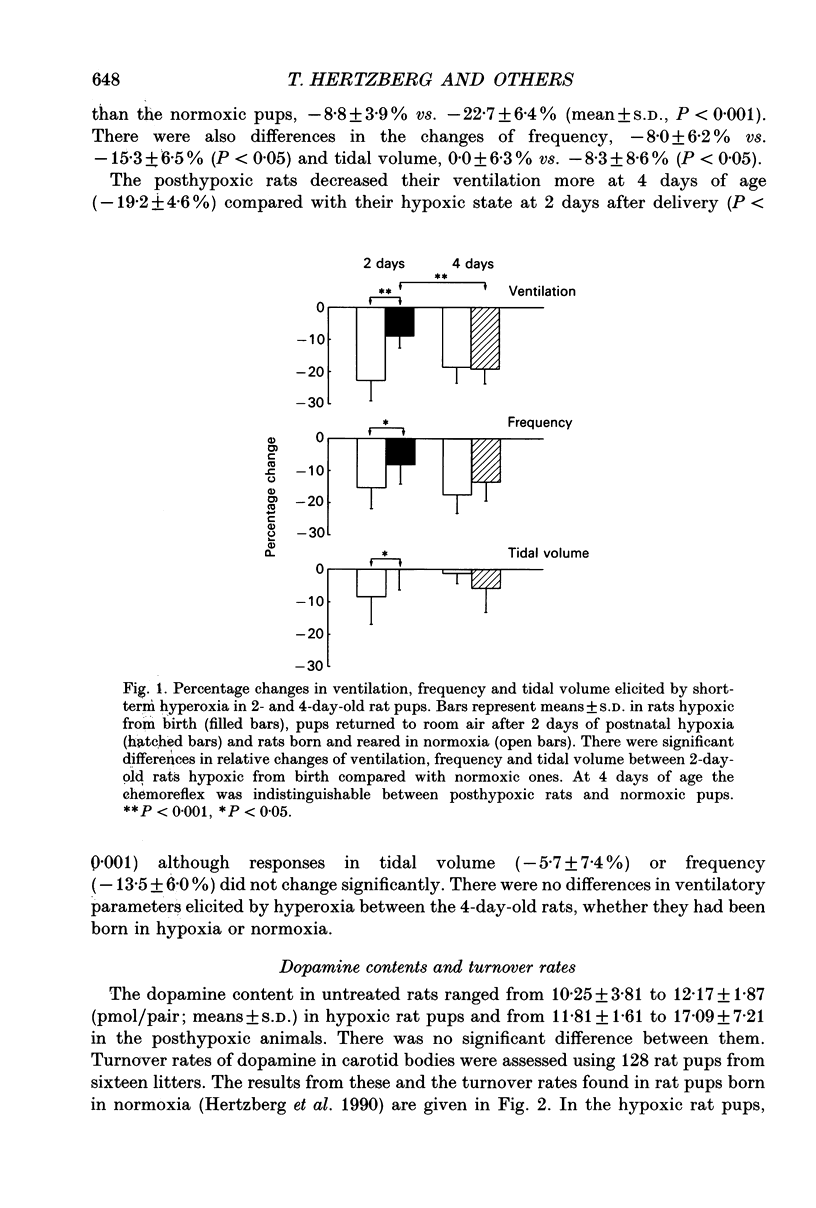
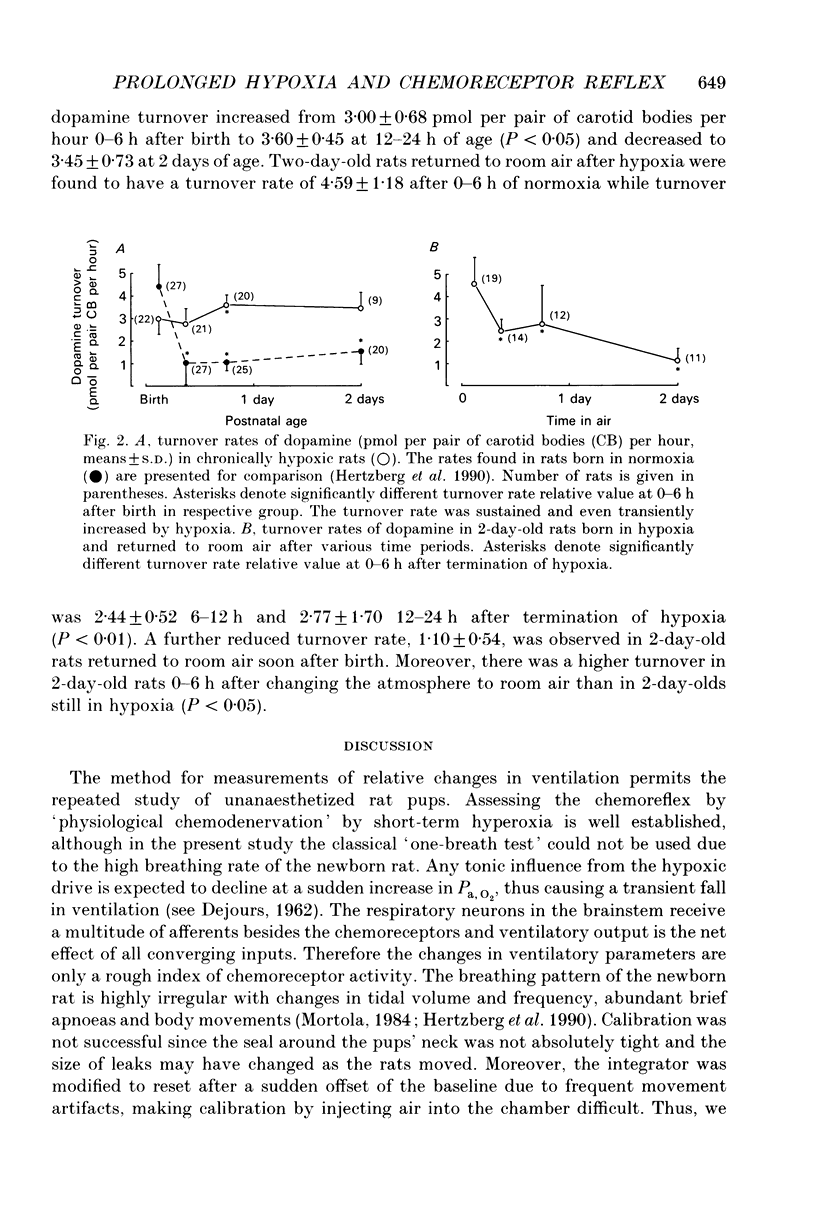





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almaraz L., Fidone S. Carotid sinus nerve C-fibers release catecholamines from the cat carotid body. Neurosci Lett. 1986 Jun 18;67(2):153–158. doi: 10.1016/0304-3940(86)90389-7. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Dawes G. S., Hanson M. A., McCooke H. B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984 Jun;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C. E., Hanson M. A., McCooke H. B. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol. 1988 Apr;10(2):167–174. [PubMed] [Google Scholar]
- Blesa M. I., Lahiri S., Rashkind W. J., Fishman A. P. Normalization of the blunted ventilatory response to acute hypoxia in congenital cyanotic heart disease. N Engl J Med. 1977 Feb 3;296(5):237–241. doi: 10.1056/NEJM197702032960501. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966 Dec;154(3):493–498. [PubMed] [Google Scholar]
- Cardenas H., Zapata P. Dopamine-induced ventilatory depression in the rat, mediated by carotid nerve afferents. Neurosci Lett. 1981 Jun 12;24(1):29–33. doi: 10.1016/0304-3940(81)90354-2. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V. Mediation of Ventilatory Adaptations. Physiol Rev. 1982 Jan;62(1):262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol. 1978 Jun;279:425–436. doi: 10.1113/jphysiol.1978.sp012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. The effects of acetylcholine and dopamine on carotid chemosensory activity in the rabbit. J Physiol. 1979 Mar;288:411–423. [PMC free article] [PubMed] [Google Scholar]
- Edelman N. H., Lahiri S., Braudo L., Cherniack N. S., Fishman A. P. The blunted ventilatory response to hypoxia in cyanotic congenital heart disease. N Engl J Med. 1970 Feb 19;282(8):405–411. doi: 10.1056/NEJM197002192820801. [DOI] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD F., LACAISSE A., DEJOURS P. [Ventilatory O2 stimulus in the neonatal period in man]. J Physiol (Paris) 1960 Jan-Feb;52:108–109. [PubMed] [Google Scholar]
- Hanbauer I., Hellstrom S. The regulation of dopamine and noradrenaline in the rat carotid body and its modification by denervation and by hypoxia. J Physiol. 1978 Sep;282:21–34. doi: 10.1113/jphysiol.1978.sp012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I., Karoum F., Hellstrom S., Lahiri S. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience. 1981;6(1):81–86. doi: 10.1016/0306-4522(81)90245-1. [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Kumar P., Williams B. A. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J Physiol. 1989 Apr;411:563–574. doi: 10.1113/jphysiol.1989.sp017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg T., Hellström S., Lagercrantz H., Pequignot J. M. Development of the arterial chemoreflex and turnover of carotid body catecholamines in the newborn rat. J Physiol. 1990 Jun;425:211–225. doi: 10.1113/jphysiol.1990.sp018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg T., Lagercrantz H. Postnatal sensitivity of the peripheral chemoreceptors in newborn infants. Arch Dis Child. 1987 Dec;62(12):1238–1241. doi: 10.1136/adc.62.12.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz J., Rudolph A. M. Cardiorespiratory response to cyanide of arterial chemoreceptors in fetal lambs. Am J Physiol. 1987 May;252(5 Pt 2):H916–H922. doi: 10.1152/ajpheart.1987.252.5.H916. [DOI] [PubMed] [Google Scholar]
- Itskovitz J., Rudolph A. M. Denervation of arterial chemoreceptors and baroreceptors in fetal lambs in utero. Am J Physiol. 1982 May;242(5):H916–H920. doi: 10.1152/ajpheart.1982.242.5.H916. [DOI] [PubMed] [Google Scholar]
- Kressin N. A., Nielsen A. M., Laravuso R., Bisgard G. E. Domperidone-induced potentiation of ventilatory responses in awake goats. Respir Physiol. 1986 Aug;65(2):169–180. doi: 10.1016/0034-5687(86)90048-4. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Brody J. S., Motoyama E. K., Velasquez T. M. Regulation of breathing in newborns at high altitude. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):673–678. doi: 10.1152/jappl.1978.44.5.673. [DOI] [PubMed] [Google Scholar]
- Llados F., Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol. 1978 Jan;274:487–499. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayock D. E., Standaert T. A., Guthrie R. D., Woodrum D. E. Dopamine and carotid body function in the newborn lamb. J Appl Physiol Respir Environ Exerc Physiol. 1983 Mar;54(3):814–820. doi: 10.1152/jappl.1983.54.3.814. [DOI] [PubMed] [Google Scholar]
- Mortola J. P. Breathing pattern in newborns. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jun;56(6):1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Morgan C. A., Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol (1985) 1986 Oct;61(4):1329–1336. doi: 10.1152/jappl.1986.61.4.1329. [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Tenney S. M. Effects of hyperoxia on ventilatory and metabolic rates of newborn mice. Respir Physiol. 1986 Mar;63(3):267–274. doi: 10.1016/0034-5687(86)90094-0. [DOI] [PubMed] [Google Scholar]
- Olson E. B., Jr, Vidruk E. H., McCrimmon D. R., Dempsey J. A. Monoamine neurotransmitter metabolism during acclimatization to hypoxia in rats. Respir Physiol. 1983 Oct;54(1):79–96. doi: 10.1016/0034-5687(83)90115-9. [DOI] [PubMed] [Google Scholar]
- Olson L. G., Hensley M. J., Saunders N. A. Ventilatory responsiveness to hypercapnic hypoxia during dopamine infusion in humans. Am Rev Respir Dis. 1982 Nov;126(5):783–787. doi: 10.1164/arrd.1982.126.5.783. [DOI] [PubMed] [Google Scholar]
- Pequignot J. M., Cottet-Emard J. M., Dalmaz Y., De Haut de Sigy M., Peyrin L. Biochemical evidence for norepinephrine stores outside the sympathetic nerves in rat carotid body. Brain Res. 1986 Mar 5;367(1-2):238–243. doi: 10.1016/0006-8993(86)91597-0. [DOI] [PubMed] [Google Scholar]
- Pequignot J. M., Cottet-Emard J. M., Dalmaz Y., Peyrin L. Dopamine and norepinephrine dynamics in rat carotid body during long-term hypoxia. J Auton Nerv Syst. 1987 Nov;21(1):9–14. doi: 10.1016/0165-1838(87)90087-7. [DOI] [PubMed] [Google Scholar]
- Piazza T., Lauzon A. M., Mortola J. P. Time course of adaptation to hypoxia in newborn rats. Can J Physiol Pharmacol. 1988 Feb;66(2):152–158. doi: 10.1139/y88-027. [DOI] [PubMed] [Google Scholar]
- Santolaya R. B., Lahiri S., Alfaro R. T., Schoene R. B. Respiratory adaptation in the highest inhabitants and highest Sherpa mountaineers. Respir Physiol. 1989 Aug;77(2):253–262. doi: 10.1016/0034-5687(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Irreversible respiratory insensitivity to acute hypoxia in man born at high altitude. J Appl Physiol. 1968 Sep;25(3):217–220. doi: 10.1152/jappl.1968.25.3.217. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C., Severinghaus J. W. Respiratory insensitivity to acute hypoxia persisting after correction of tetralogy of Fallot. J Appl Physiol. 1968 Sep;25(3):221–223. doi: 10.1152/jappl.1968.25.3.221. [DOI] [PubMed] [Google Scholar]


