Abstract
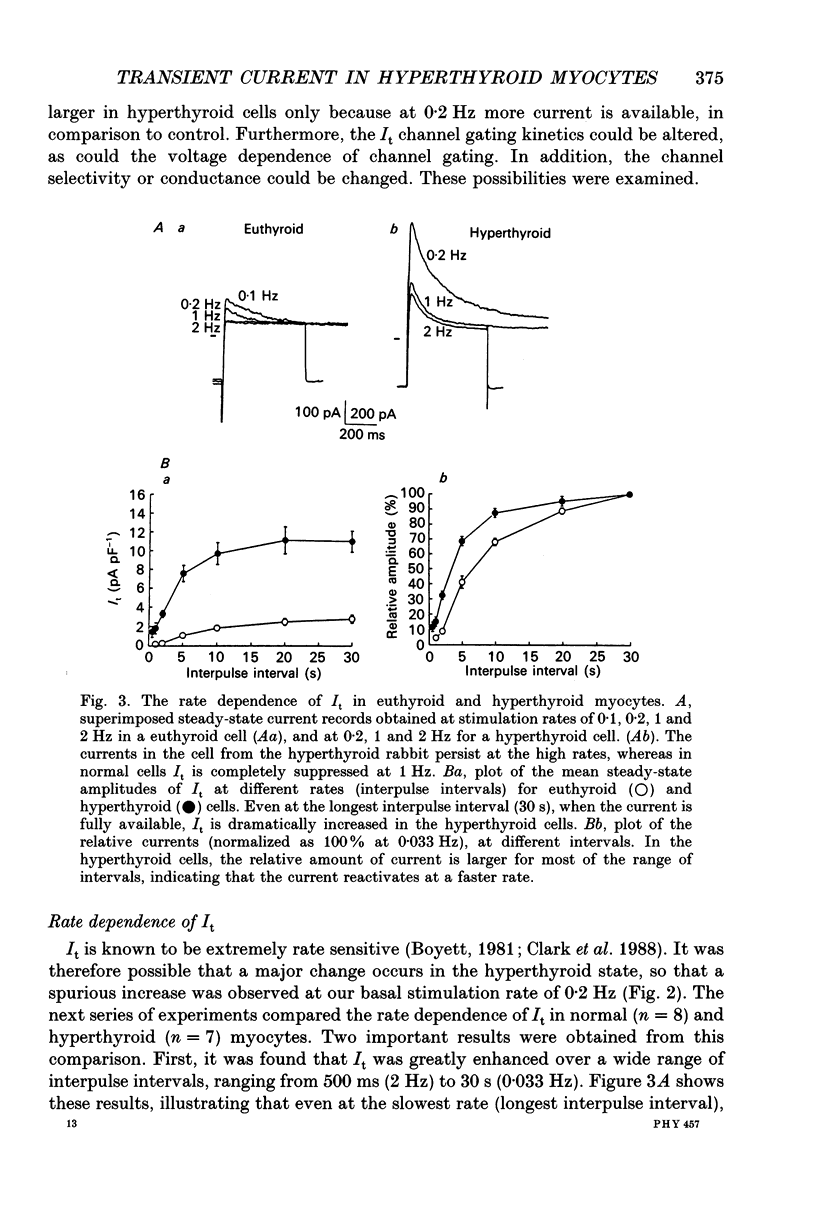
1. Transient outward potassium currents (I(t)) were compared in single cardiac myocytes obtained from normal and hyperthyroid rabbits. Currents were recorded using the suction electrode whole-cell voltage clamp technique. 2. In ventricular myocytes from hyperthyroid animals (at 22 degrees C and a stimulation rate of 0.2 Hz), I(t) was 4- to 5-fold larger than in normal myocytes, in a potential range of -20 to +60 mV. As in normal myocytes, I(t) in hyperthyroid myocytes was calcium insensitive, and was more than 90% suppressed by 2 mM 4-aminopyridine. 3. The increase in I(t) was observed over a wide range of stimulation rates, even at rates sufficiently slow to enable complete reactivation of the I(t) channels. However, there was a major change in the rate dependence of I(t) in hyperthyroid myocytes, with significant I(t) current still present at rates (e.g. 1-2 Hz) at which it is normally completely suppressed. 4. The augmentation of I(t) in the hyperthyroid myocytes could not be accounted for by changes in the voltage dependence or the kinetics of channel activation or inactivation. There was no change in the reversal potential of I(t), implying no change in the selectivity of the channel. 5. Single-channel activity was recorded using the cell-attached mode of recording. In myocytes from hyperthyroid rabbit we observed the following: (a) active patches (often containing two channels) were obtained more frequently in comparison to control; (b) the unitary conductance of the channel was the same; (c) single-channel openings persisted at high stimulation rates. 6. In contrast to hyperthyroid ventricular cells, I(t) in atrial cells from the same hearts was not substantially changed. 7. The rate dependence of I(t) in atrial cells was also unaffected by hyperthyroidism, in contrast to the large changes observed in ventricular cells. Thus, in atrial cells from hyperthyroid hearts the current was totally suppressed at rates of 1-2 Hz, as in euthyroid conditions. 8. Single-channel recordings in the cell-attached mode showed a unitary conductance similar to that found in normal atrial cells. Channel activity was suppressed at 2 Hz, in contrast to hyperthyroid ventricular cells. 9. In conclusion, I(t) is drastically changed in hyperthyroid rabbit ventricle cells. The changes are in the magnitude of the macroscopic current and its rate dependence. Since the unitary conductance is unchanged (and the peak open probabilities are normally high at positive membrane potential(s) the number of active channels in the membrane must be increased. In atrial cells from the same hyperthyroid hearts no changes are apparent.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





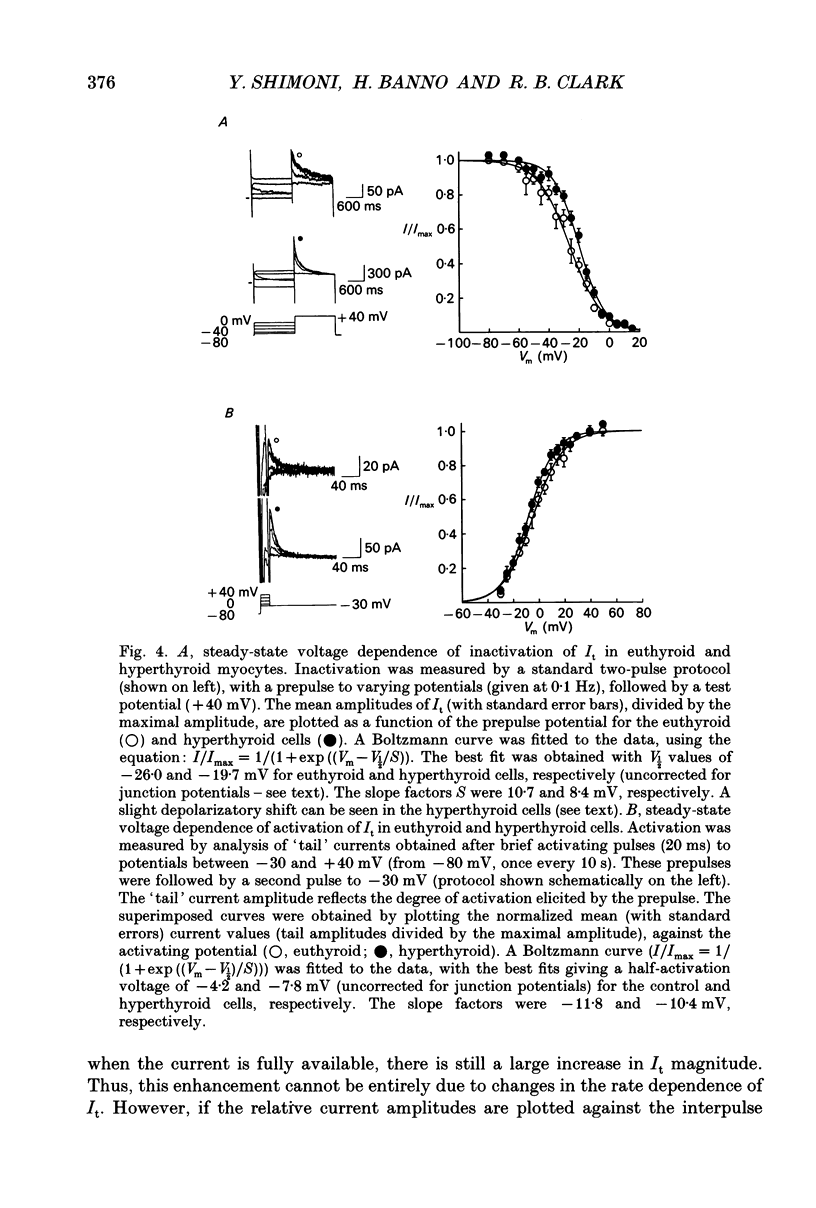

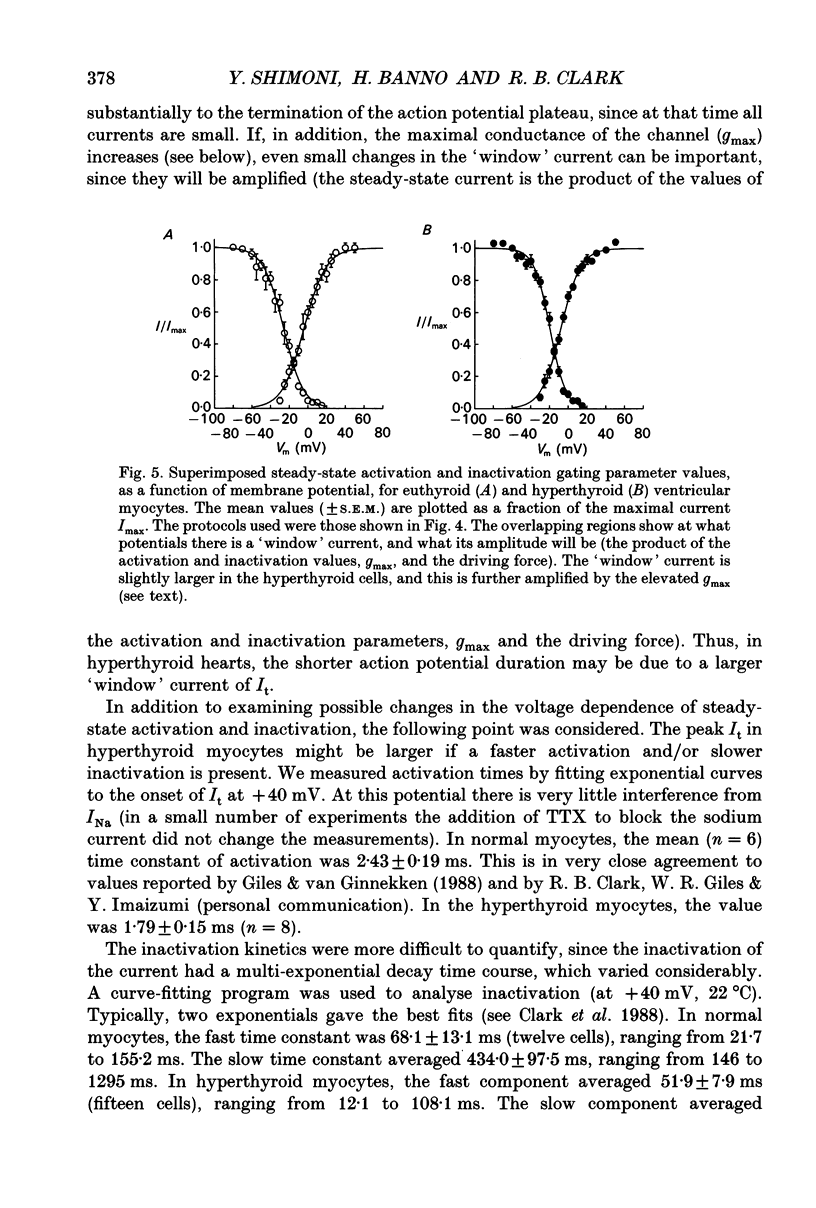
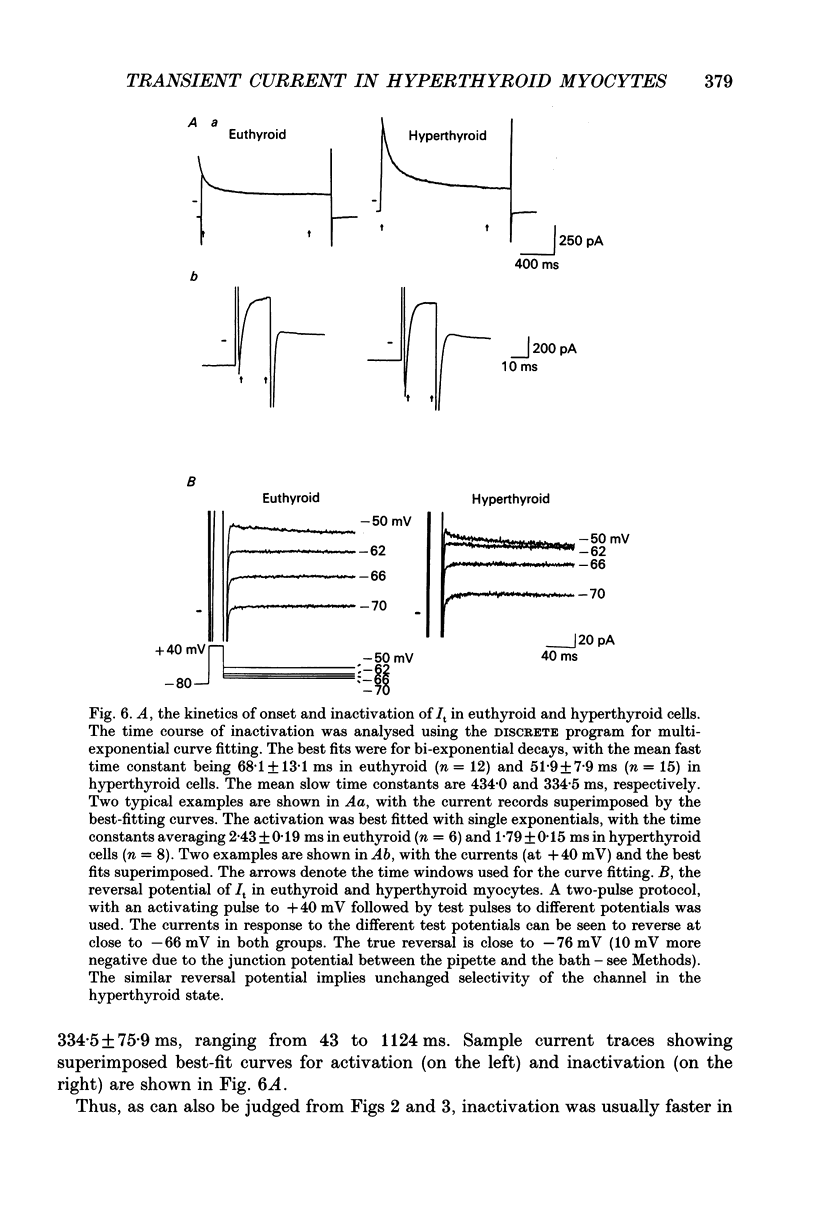



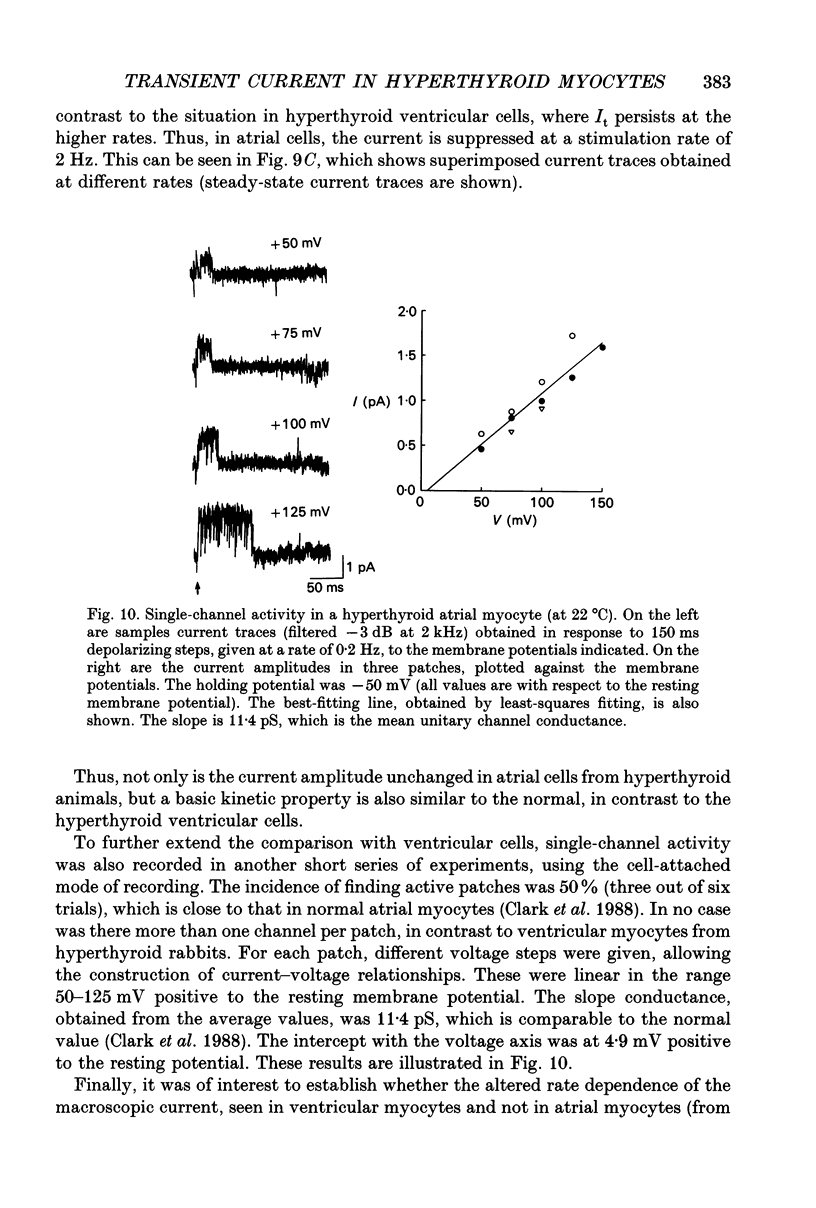
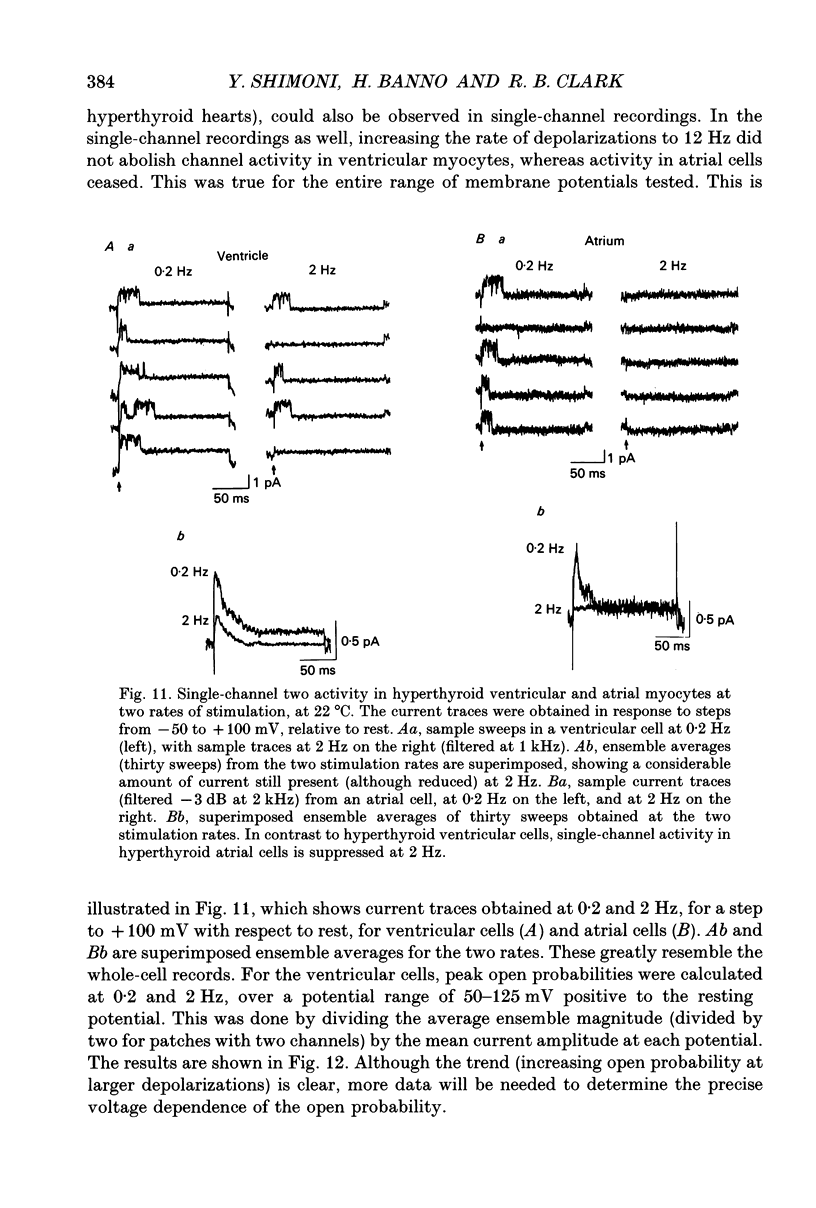
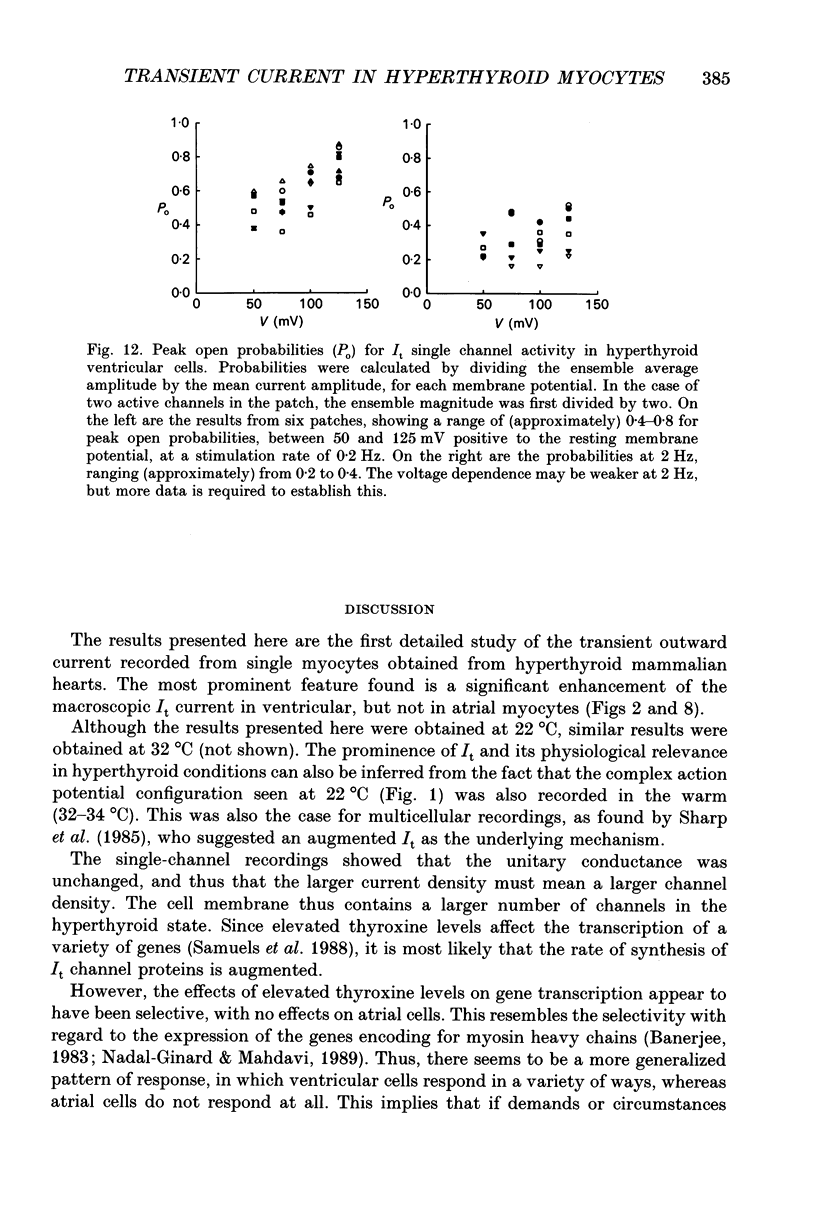




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K. Comparative studies of atrial and ventricular myosin from normal, thyrotoxic, and thyroidectomized rabbits. Circ Res. 1983 Feb;52(2):131–136. doi: 10.1161/01.res.52.2.131. [DOI] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Wier W. G. Voltage dependence of intracellular [Ca2+]i transients in guinea pig ventricular myocytes. Circ Res. 1987 Jul;61(1):148–154. doi: 10.1161/01.res.61.1.148. [DOI] [PubMed] [Google Scholar]
- Binah O., Rubinstein I., Gilat E. Effects of thyroid hormone on the action potential and membrane currents of guinea pig ventricular myocytes. Pflugers Arch. 1987 Jun;409(1-2):214–216. doi: 10.1007/BF00584774. [DOI] [PubMed] [Google Scholar]
- Boyett M. R. A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac Purkinje fibres. J Physiol. 1981;319:1–22. doi: 10.1113/jphysiol.1981.sp013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino R. A., Spann J. F., Jr, Pool P. E., Sonnenblick E. H., Braunwald E. Influence of the thyroid state on the intrinsic contractile properties and energy stores of the myocardium. J Clin Invest. 1967 Oct;46(10):1669–1682. doi: 10.1172/JCI105658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R. A., Everett A. W., Prior G., Zak R. Comparison of myosin heavy chains in atria and ventricles from hyperthyroid, hypothyroid, and euthyroid rabbits. J Biol Chem. 1984 Dec 25;259(24):15564–15571. [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H. Hormonal influences on cardiac myosin ATPase activity and myosin isoenzyme distribution. Mol Cell Endocrinol. 1984 Mar;34(3):169–181. doi: 10.1016/0303-7207(84)90173-4. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Sinha A. M., Umeda P. K., Jakovcic S., Rabinowitz M., Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984 Apr 10;23(8):1596–1599. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- Fedida D., Giles W. R. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol. 1991 Oct;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawthorn M. H., Gengo P., Wei X. Y., Rutledge A., Moran J. F., Gallant S., Triggle D. J. Effect of thyroid status on beta-adrenoceptors and calcium channels in rat cardiac and vascular tissue. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):539–544. doi: 10.1007/BF00182728. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Josephson R. A., Spurgeon H. A., Lakatta E. G. The hyperthyroid heart. An analysis of systolic and diastolic properties in single rat ventricular myocytes. Circ Res. 1990 Mar;66(3):773–781. doi: 10.1161/01.res.66.3.773. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Smith T. W. Effects of thyroid hormone on sodium pump sites, sodium content, and contractile responses to cardiac glycosides in cultured chick ventricular cells. J Clin Invest. 1984 Oct;74(4):1481–1488. doi: 10.1172/JCI111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Smith T. W., Marsh J. D. Effect of thyroid hormone on slow calcium channel function in cultured chick ventricular cells. J Clin Invest. 1987 Jul;80(1):88–94. doi: 10.1172/JCI113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling G., Rupp H., Jacob R. Implications of myocardial transformation for cardiac energetics. Basic Res Cardiol. 1987;82 (Suppl 2):191–200. doi: 10.1007/978-3-662-11289-2_19. [DOI] [PubMed] [Google Scholar]
- Kosinski C., Gross G., Hanft G. Effect of hypo- and hyperthyroidism on binding of [3H]-nitrendipine to myocardial and brain membranes. Br J Clin Pharmacol. 1990;30 (Suppl 1):128S–130S. doi: 10.1111/j.1365-2125.1990.tb05483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- MacKinnon R., Morgan J. P. Influence of the thyroid state on the calcium transient in ventricular muscle. Pflugers Arch. 1986 Aug;407(2):142–144. doi: 10.1007/BF00580665. [DOI] [PubMed] [Google Scholar]
- Morkin E., Flink I. L., Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Prog Cardiovasc Dis. 1983 Mar-Apr;25(5):435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B., Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989 Dec;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Rubinstein I., Binah O. Thyroid hormone modulates membrane currents in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6):705–711. doi: 10.1007/BF00717748. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988 Apr;81(4):957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour A. M., Eldar H., Radda G. K. Hyperthyroidism results in increased glycolytic capacity in the rat heart. A 31P-NMR study. Biochim Biophys Acta. 1990 Nov 12;1055(2):107–116. doi: 10.1016/0167-4889(90)90110-y. [DOI] [PubMed] [Google Scholar]
- Sharp N. A., Neel D. S., Parsons R. L. Influence of thyroid hormone levels on the electrical and mechanical properties of rabbit papillary muscle. J Mol Cell Cardiol. 1985 Feb;17(2):119–132. doi: 10.1016/s0022-2828(85)80015-8. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Clark R. B., Giles W. R. Role of an inwardly rectifying potassium current in rabbit ventricular action potential. J Physiol. 1992 Mar;448:709–727. doi: 10.1113/jphysiol.1992.sp019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons C. Thyroid heart disease. Br Heart J. 1979 Mar;41(3):257–262. doi: 10.1136/hrt.41.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


