Abstract
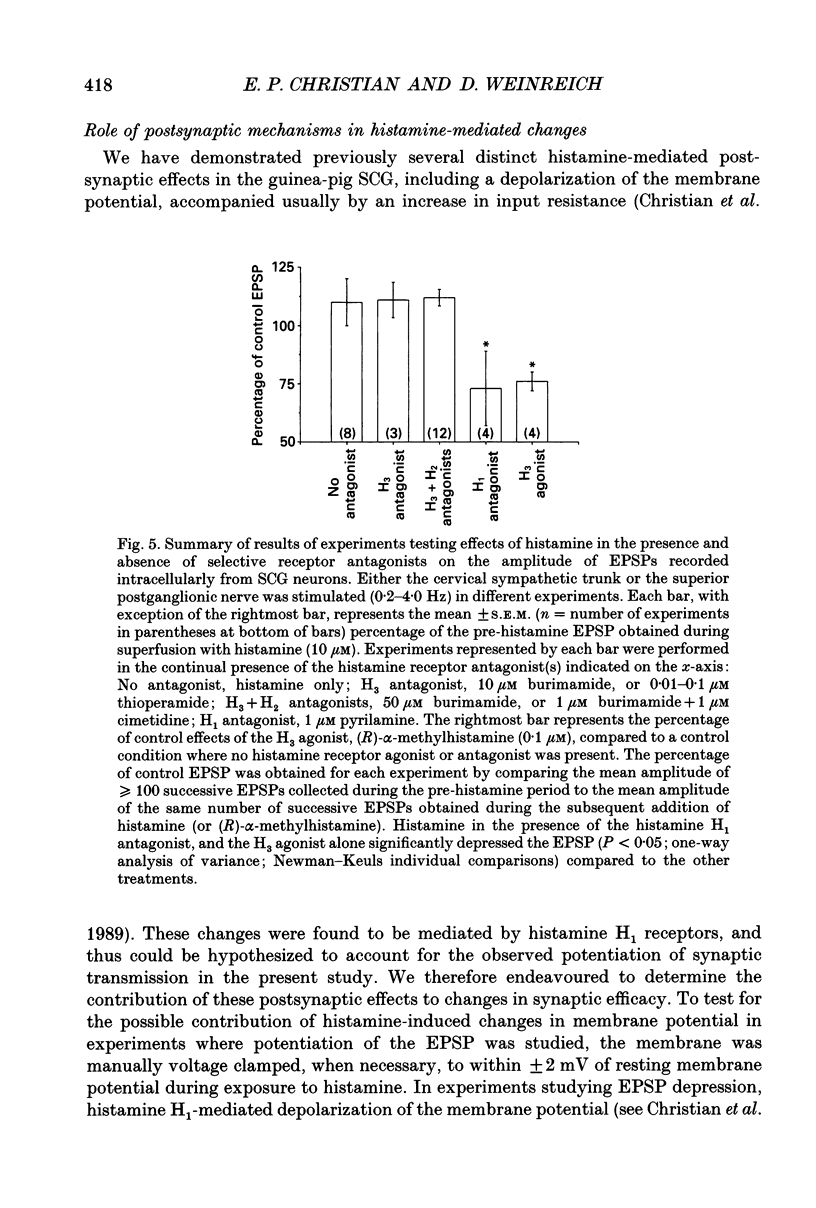
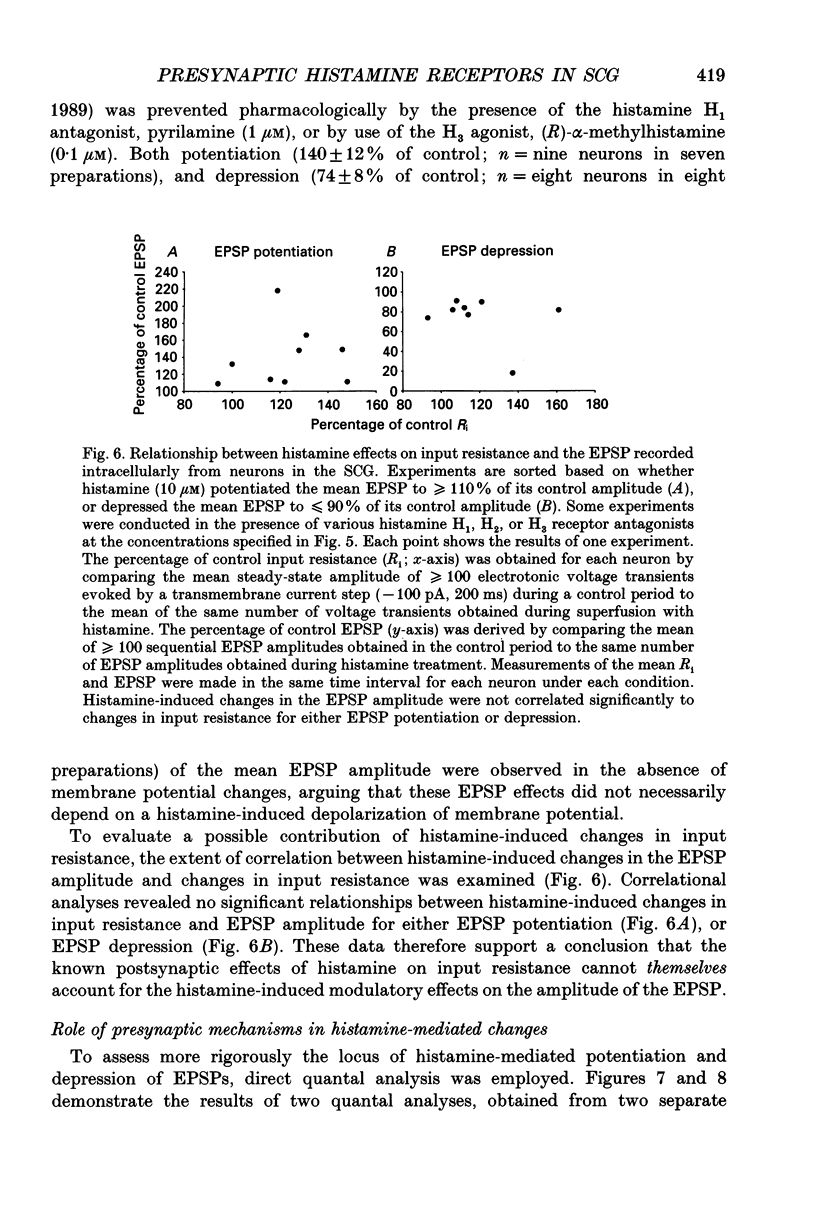
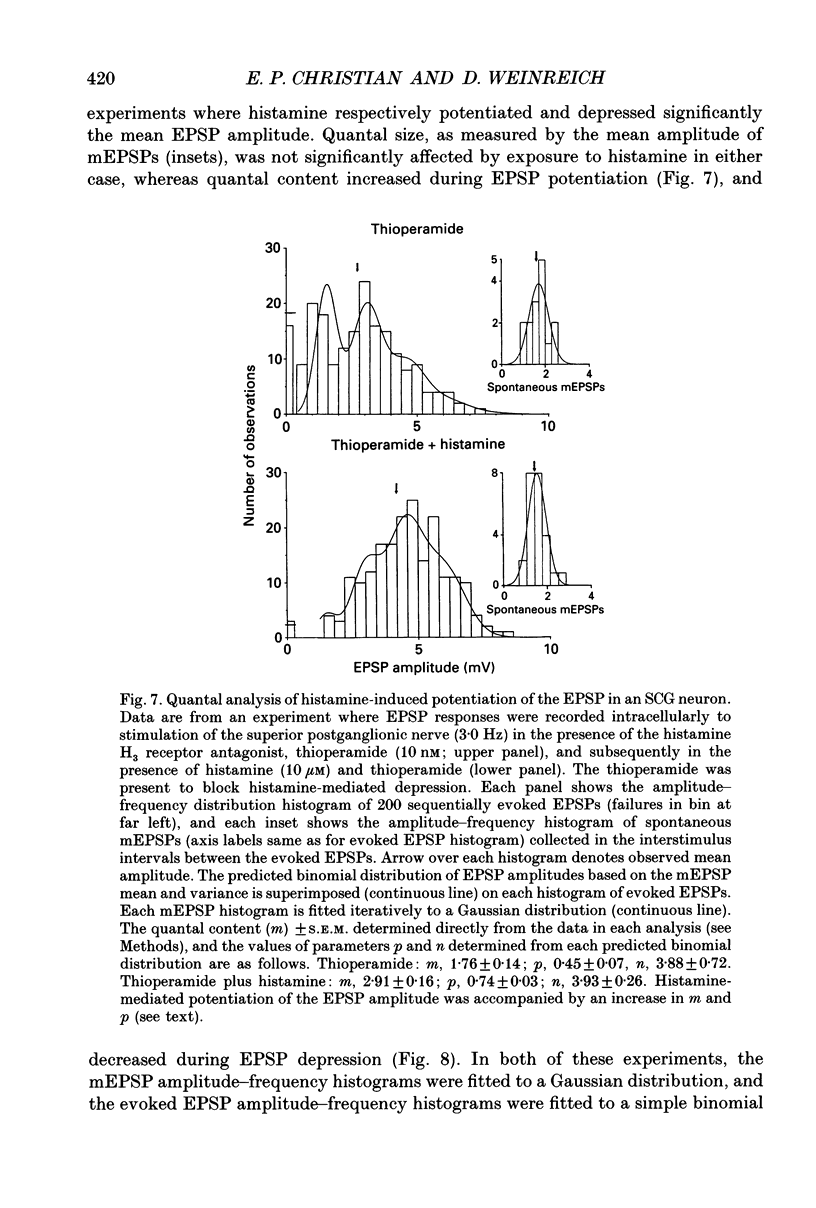
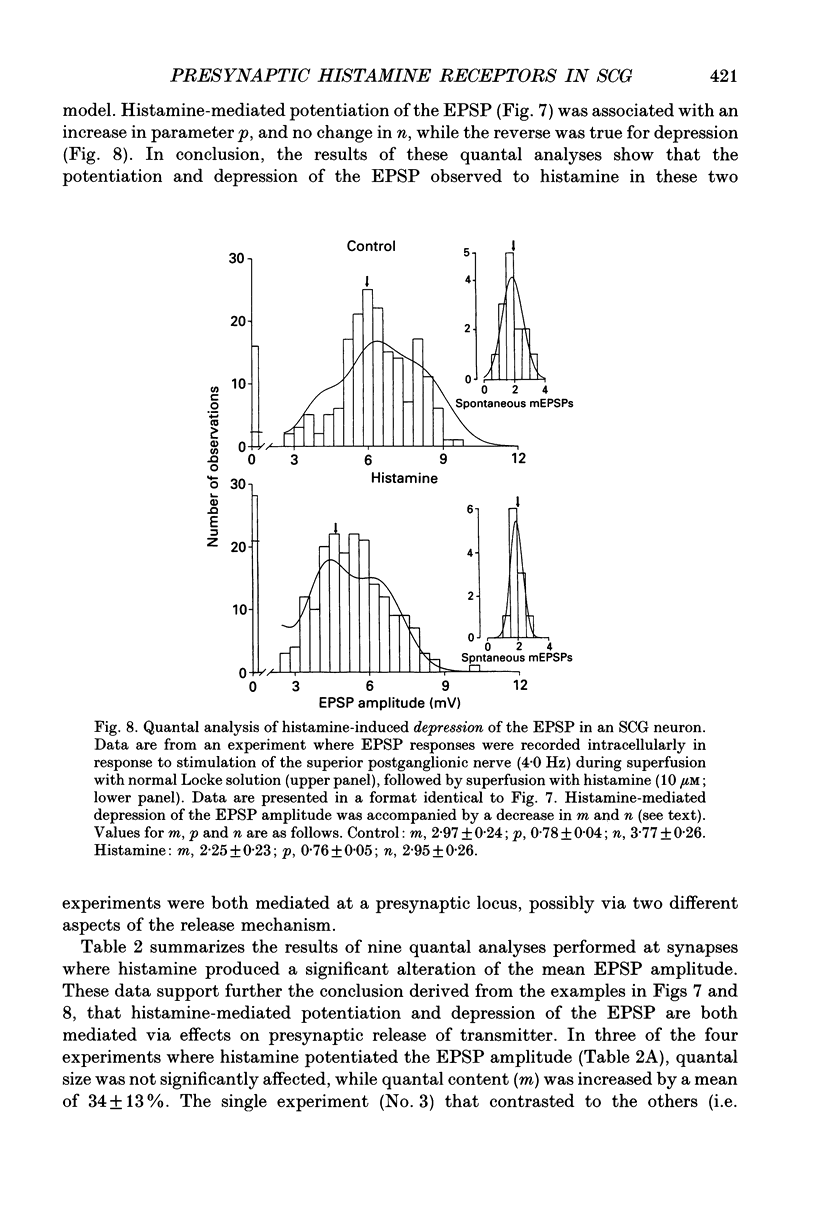
1. To study the effects of histamine on the efficacy of sympathetic ganglionic synaptic transmission, extracellular recordings of the postganglionic compound action potential (CAP) and intracellular recordings of excitatory postsynaptic potentials (EPSPs) elicited by preganglionic electrical stimulation were obtained from isolated guinea-pig superior cervical ganglia (SCG). 2. In different preparations, superfusion with histamine (0.1-100 microM) either potentiated or depressed the postganglionic CAP elicited by electrical stimulation of the cervical sympathetic trunk (0.2-3.0 Hz). The direction of response produced by histamine did not depend on stimulation frequency or histamine concentration; potentiation and depression both showed concentration dependence over the range of histamine concentrations tested. 3. Experiments employing a variety of histamine receptor agonists or antagonists revealed that histamine-induced potentiation of the postganglionic CAP could be attributed to histamine H1 receptor activation, and depression to H3 receptor activation. 4. Histamine similarly potentiated or depressed the intracellularly recorded EPSP. However, these opposite effects occurred at different synapses. In agreement with the studies on the postganglionic CAP, histamine H1 antagonists prevented histamine-induced potentiation of the EPSP and H3 receptor antagonists prevented histamine-induced depression. 5. Direct quantal analyses of histamine-induced synaptic potentiation and depression were implemented to determine the pre- and postsynaptic components of these effects. Quantal size was estimated by measuring the amplitude of spontaneous miniature EPSP amplitudes. Histamine-induced potentiation and depression of the evoked EPSP were found to be accompanied by increased or decreased quantal content respectively, and unchanged quantal size, providing evidence that presynaptic mechanisms were involved in mediating both effects. 6. Some guinea-pigs were actively sensitized to ovalbumin. Subsequent exposure of the isolated SCG from these animals to the sensitizing antigen produced changes in the EPSP amplitude that correlated significantly to the response produced by exogenously applied histamine at the same synapse. 7. The correspondence between the effects of specific antigen challenge and exogenous histamine on evoked EPSPs at a synapse provides evidence that endogenous histamine released during an immunological response to antigen challenge can activate histamine H1 and H3 receptors to modulate synaptic efficacy in sympathetic ganglia.
Full text
PDF



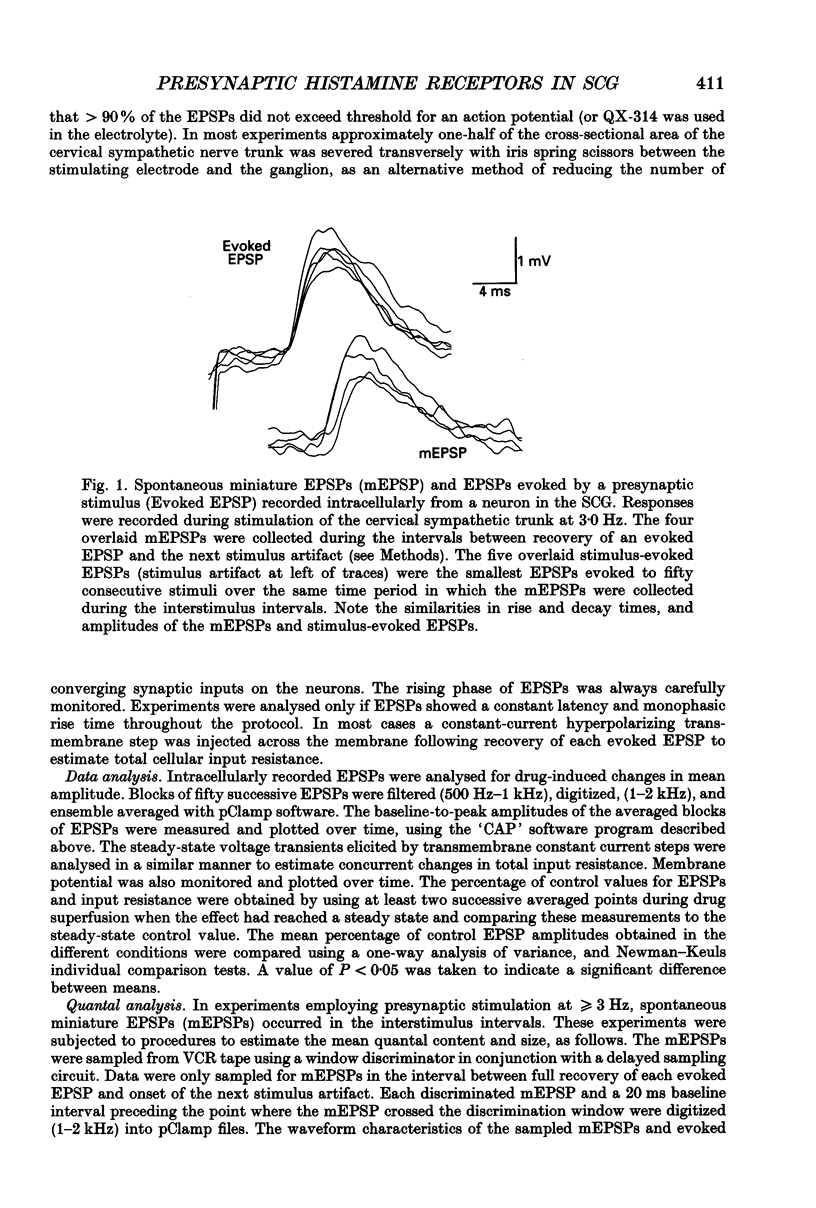

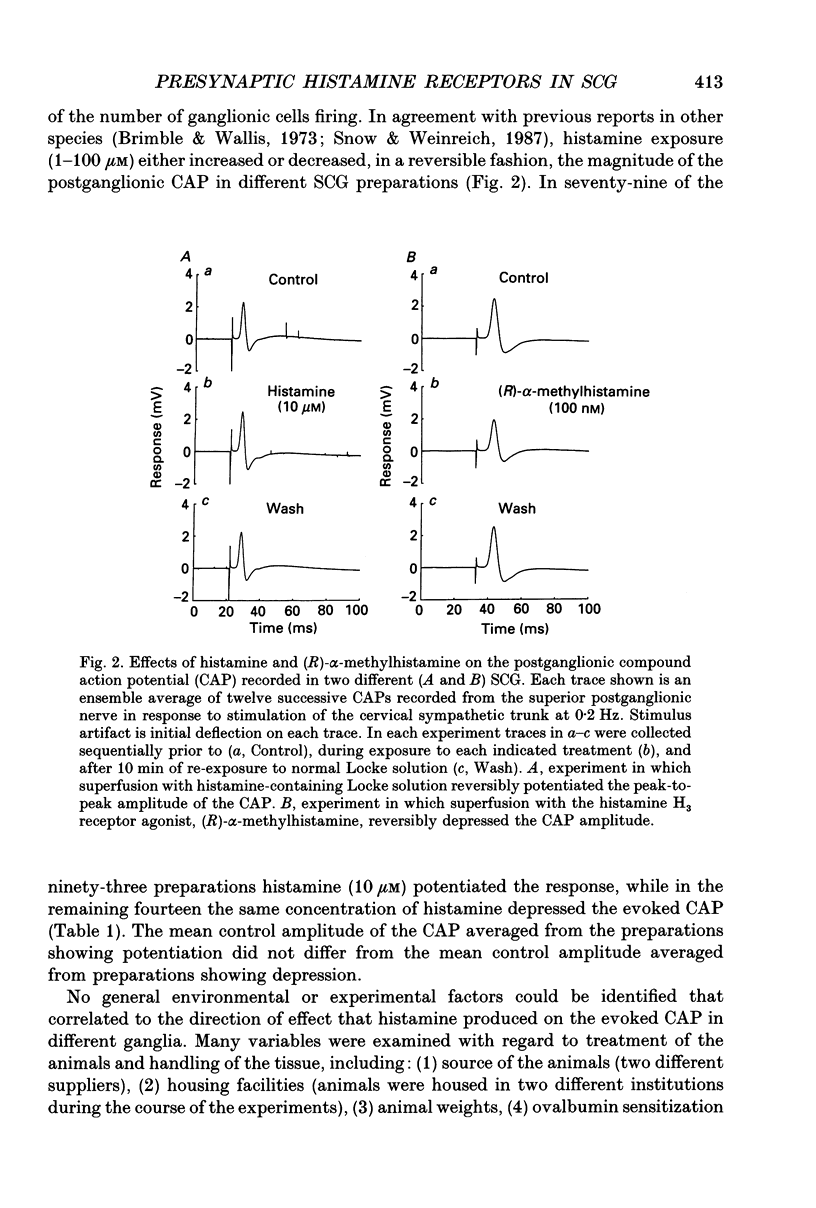


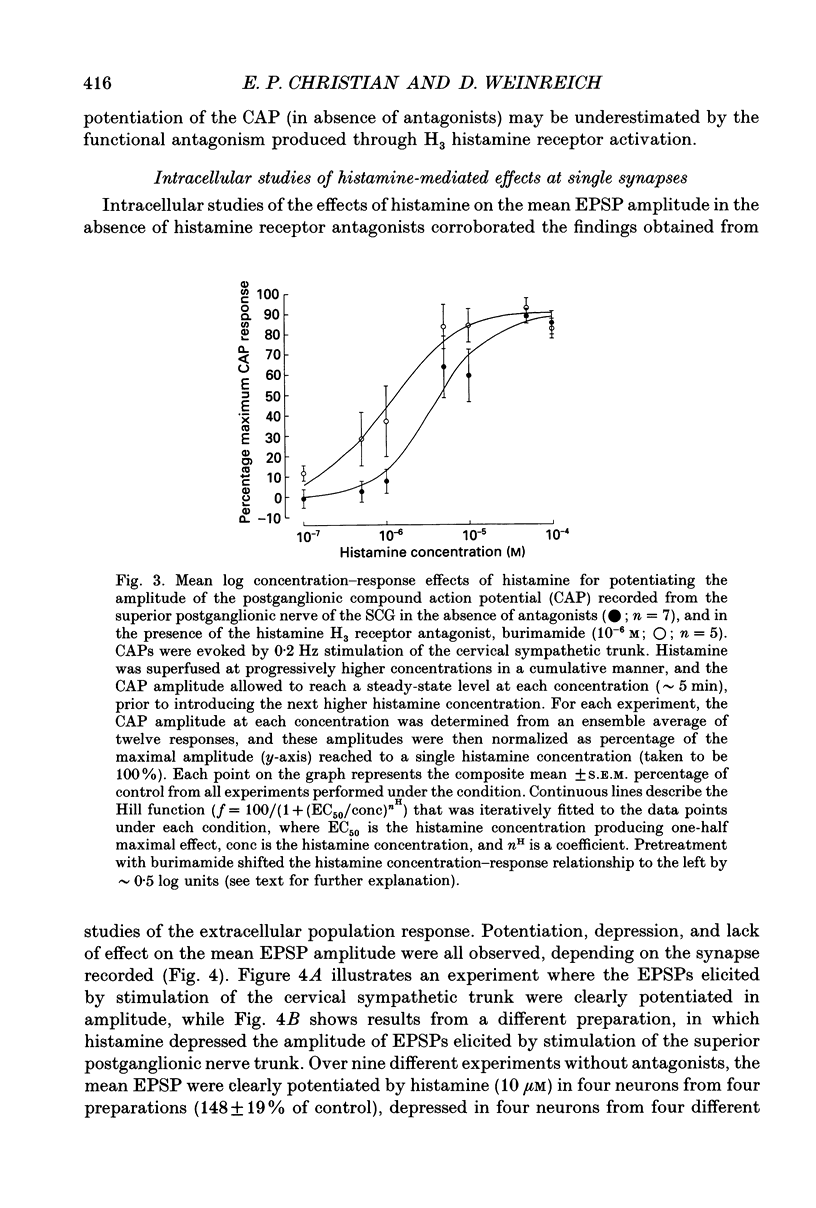
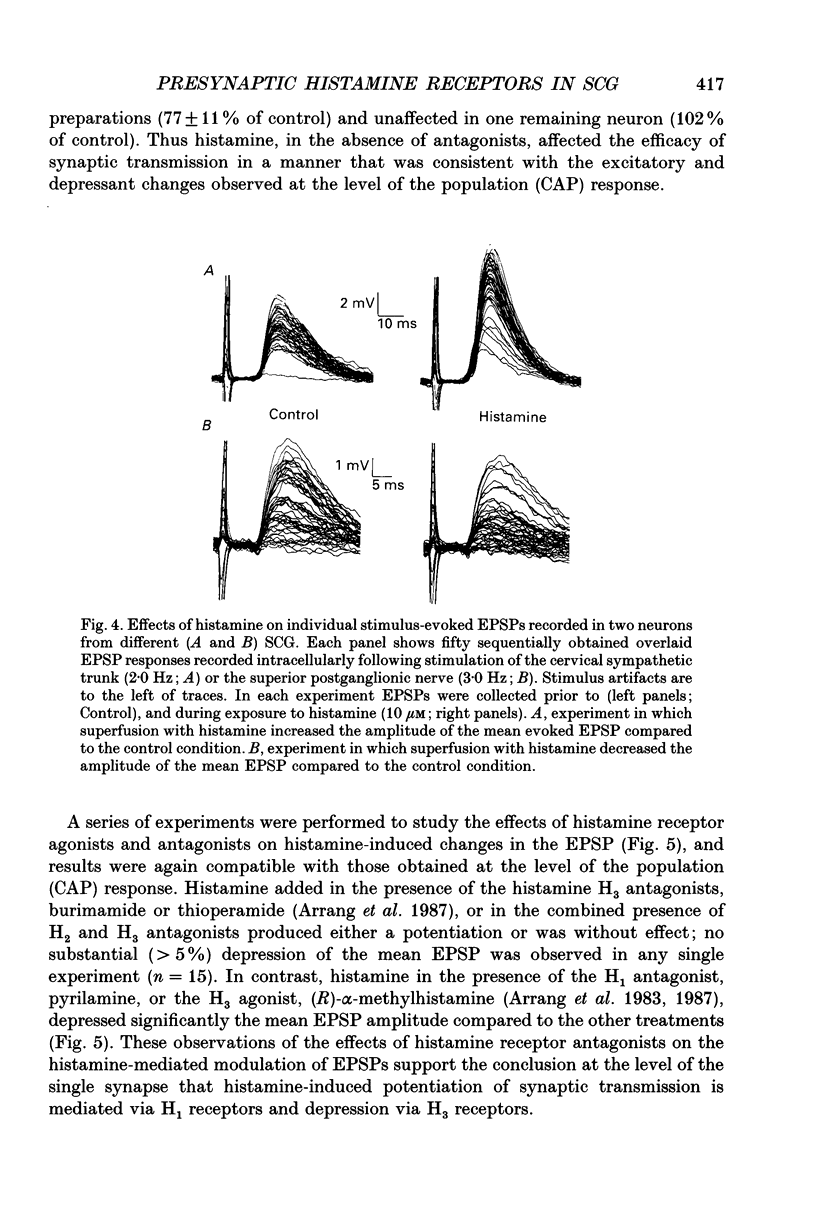






Selected References
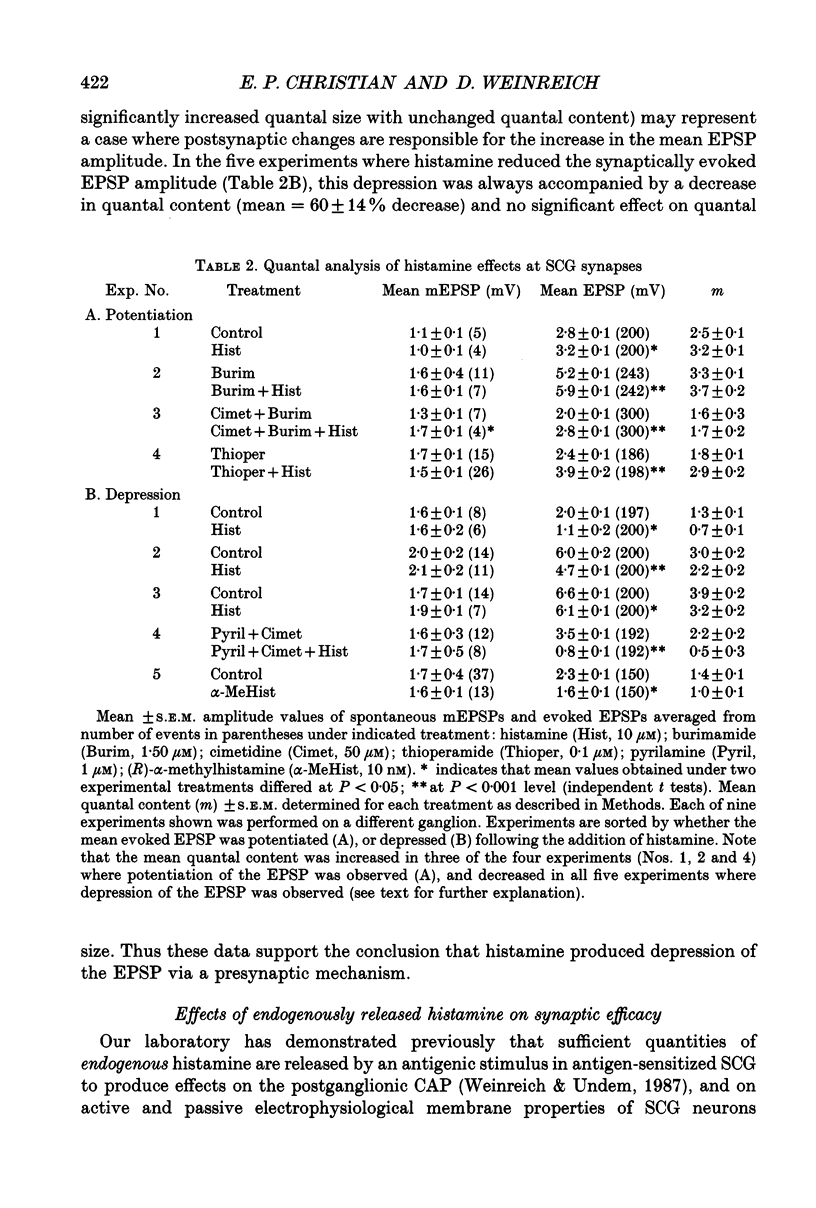
These references are in PubMed. This may not be the complete list of references from this article.
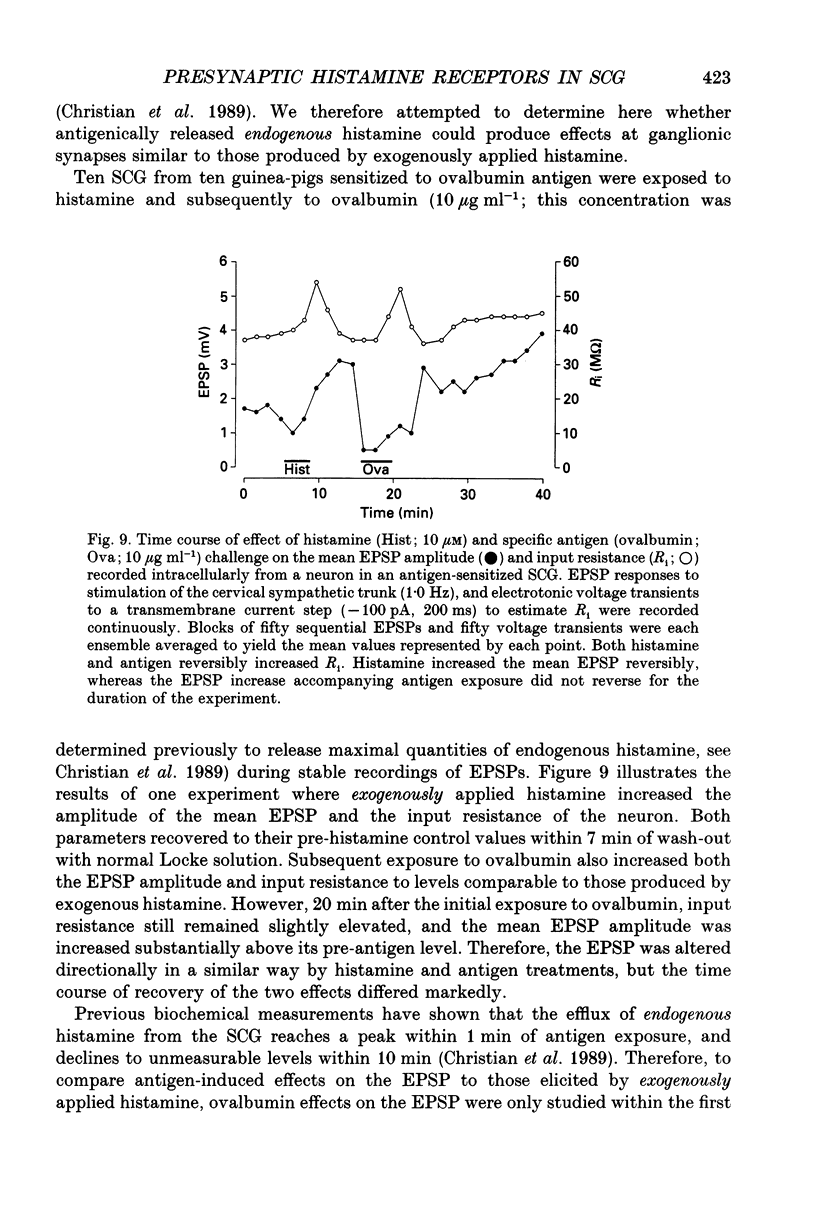
- Arrang J. M., Garbarg M., Lancelot J. C., Lecomte J. M., Pollard H., Robba M., Schunack W., Schwartz J. C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987 May 14;327(6118):117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
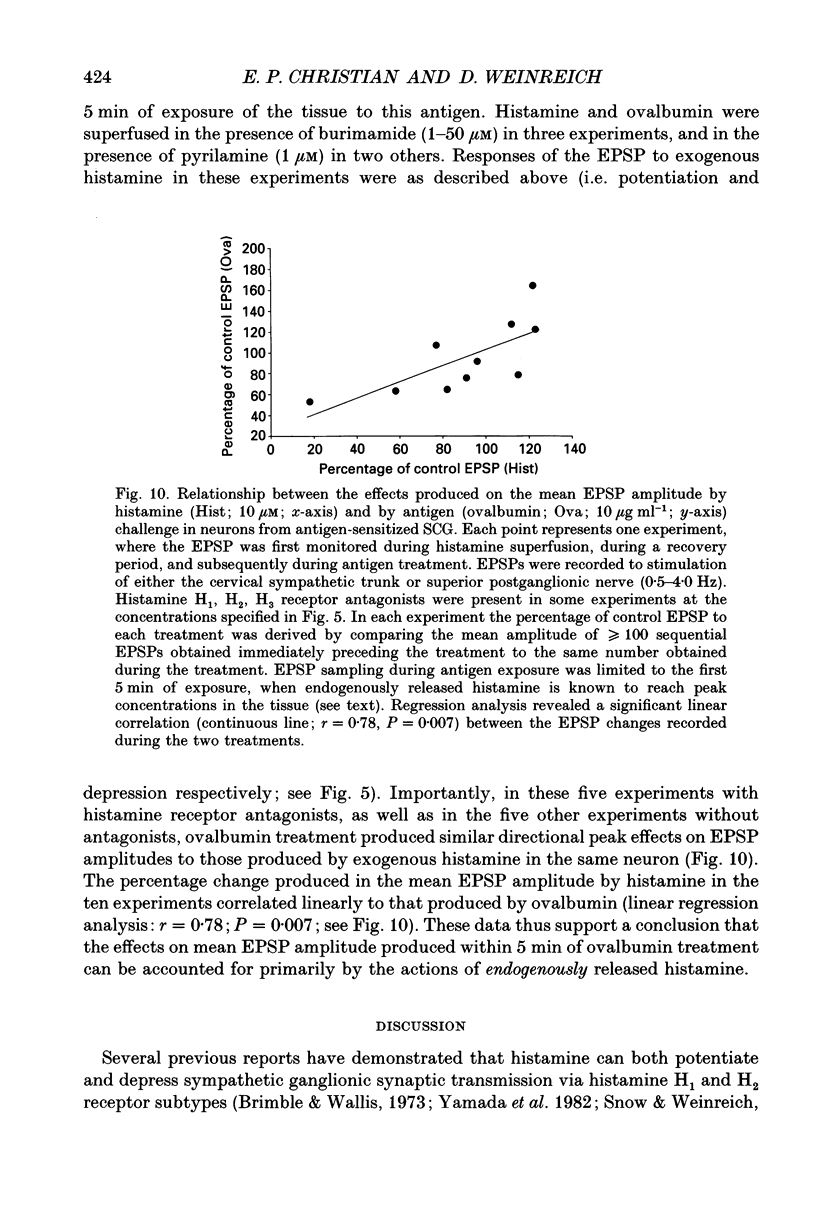
- Arrang J. M., Garbarg M., Schwartz J. C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983 Apr 28;302(5911):832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arrang J. M., Schwartz J. C., Schunack W. Stereoselectivity of the histamine H3-presynaptic autoreceptor. Eur J Pharmacol. 1985 Oct 29;117(1):109–114. doi: 10.1016/0014-2999(85)90478-9. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Isacoff E. Y. Burst-patterned stimulation promotes nicotinic transmission in isolated perfused rat sympathetic ganglia. J Physiol. 1988 Aug;402:515–532. doi: 10.1113/jphysiol.1988.sp017218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezenoff H. E., Gertner S. B. The actions of polymyxin B and histamine on ganglionic transmission. Can J Physiol Pharmacol. 1972 Aug;50(8):824–831. doi: 10.1139/y72-119. [DOI] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Wallis D. I. Histamine H1 and H2-receptors at a ganglionic synapse. Nature. 1973 Nov 16;246(5429):156–158. doi: 10.1038/246156a0. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Undem B. J., Weinreich D. Endogenous histamine excites neurones in the guinea-pig superior cervical ganglion in vitro. J Physiol. 1989 Feb;409:297–312. doi: 10.1113/jphysiol.1989.sp017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Ichinose M., Belvisi M. G., Barnes P. J. Histamine H3-receptors inhibit neurogenic microvascular leakage in airways. J Appl Physiol (1985) 1990 Jan;68(1):21–25. doi: 10.1152/jappl.1990.68.1.21. [DOI] [PubMed] [Google Scholar]
- Ichinose M., Stretton C. D., Schwartz J. C., Barnes P. J. Histamine H3-receptors inhibit cholinergic neurotransmission in guinea-pig airways. Br J Pharmacol. 1989 May;97(1):13–15. doi: 10.1111/j.1476-5381.1989.tb11917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S., Sperelakis N. A novel class (H3) of histamine receptors on perivascular nerve terminals. Nature. 1987 May 14;327(6118):158–160. doi: 10.1038/327158a0. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski H. Histamine in nervous tissue. J Physiol. 1943 Jun 30;102(1):32–41. doi: 10.1113/jphysiol.1943.sp004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees G. M., Nishi S. Analysis of the mechanism of action of some ganglion-blocking drugs in the rabbit superior cervical ganglion. Br J Pharmacol. 1972 Sep;46(1):78–88. doi: 10.1111/j.1476-5381.1972.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl T. Effects of histamine agonists and antagonists (H1 and H2) on ganglionic transmission and on accumulation of cyclic nucleotides (cAMP and cGMP) in rat superior cervical ganglion in vitro. Neuropharmacology. 1983 Feb;22(2):203–211. doi: 10.1016/0028-3908(83)90010-2. [DOI] [PubMed] [Google Scholar]
- Marshall I. Histamine modulation of neurotransmission in the sympathetic nervous system. J Auton Pharmacol. 1981 Jun;1(3):235–250. doi: 10.1111/j.1474-8673.1981.tb00452.x. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. An analysis of the release of acetylcholine from preganglionic nerve terminals. J Physiol. 1975 Feb;245(2):447–466. doi: 10.1113/jphysiol.1975.sp010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Snow R. W., Weinreich D. Presynaptic and postsynaptic effects of histamine and histamine agonists in the superior cervical ganglion of the rat. Neuropharmacology. 1987 Jul;26(7A):743–752. doi: 10.1016/0028-3908(87)90237-1. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of histamine, pilocarpine and 5-hydroxytryptamine on transmission through the superior cervical ganglion. J Physiol. 1957 Jan 23;135(1):66–72. doi: 10.1113/jphysiol.1957.sp005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Palmer J. M., Wood J. D. Presynaptic inhibition produced by histamine at nicotinic synapses in enteric ganglia. Neuroscience. 1988 Apr;25(1):171–179. doi: 10.1016/0306-4522(88)90016-4. [DOI] [PubMed] [Google Scholar]
- Timmerman H. Histamine H3 ligands: just pharmacological tools or potential therapeutic agents? J Med Chem. 1990 Jan;33(1):4–11. doi: 10.1021/jm00163a001. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Histamine H2 receptor mediates postsynaptic excitation and presynaptic inhibition in submucous plexus neurons of the guinea-pig. Neuroscience. 1989;28(3):735–744. doi: 10.1016/0306-4522(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Undem B. J., Hubbard W. C., Christian E. P., Weinreich D. Mast cells in the guinea pig superior cervical ganglion: a functional and histological assessment. J Auton Nerv Syst. 1990 Apr;30(1):75–87. doi: 10.1016/0165-1838(90)90164-e. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Undem B. J. Immunological regulation of synaptic transmission in isolated guinea pig autonomic ganglia. J Clin Invest. 1987 May;79(5):1529–1532. doi: 10.1172/JCI112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Tokimasa T., Koketsu K. Effects of histamine on acetylcholine release in bullfrog sympathetic ganglia. Eur J Pharmacol. 1982 Aug 13;82(1-2):15–20. doi: 10.1016/0014-2999(82)90547-7. [DOI] [PubMed] [Google Scholar]


