Abstract
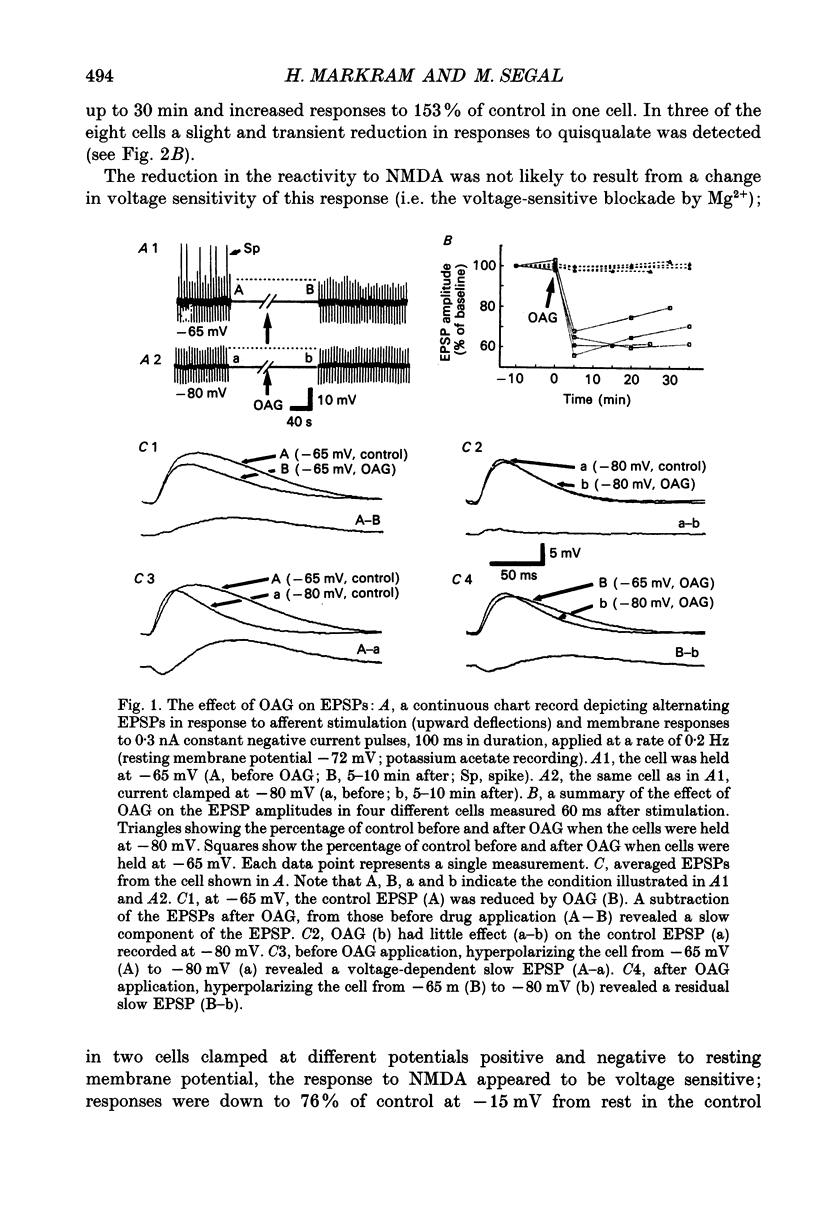
1. The effects of 1-oleoyl-2-acetylglycerol (OAG), an activator of protein kinase C (PKC), on NMDA receptor-mediated responses were investigated in CA1 neurones of hippocampal slices using current- and voltage-clamp techniques. 2. Topical application of OAG caused a suppression of the slow, voltage-sensitive, NMDA receptor-mediated component of excitatory postsynaptic potentials (EPSPs) evoked by stimulating the schaffer-collateral commissural afferents and had no effect on the fast, voltage-insensitive, quisqualate/kainate component. 3. OAG suppressed the amplitude of inward current responses to NMDA down to about one-third of control responses. OAG could also increase the duration of the responses to NMDA by up to twofold. The effect of OAG on the duration but not on the amplitude of the response to NMDA was blocked by pre-loading cells with the K+ channel blocker, Cs+. Topical application of OAG had no significant effect on current responses to quisqualate. 4. An OAG isomer, which does not activate PKC, had no effect on responses to NMDA. Intracellular application of the kinase inhibitor, H-7, completely blocked the effect of OAG on the amplitude and duration of responses to NMDA, as well as on the slow EPSP. Finally, topical application of another activator of PKC, phorbol 12-myristate 13-acetate (PMA), also suppressed responses to NMDA. PMA reduced the slow component of synaptic responses in about half of the cells tested. 5. We propose that activation of PKC in CA1 hippocampal neurones suppresses NMDA receptor-mediated responses.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991 Aug;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Colley P. A., Sheu F. S., Routtenberg A. Dose-dependent phorbol ester facilitation or blockade of hippocampal long-term potentiation: relation to membrane/cytosol distribution of protein kinase C activity. Brain Res. 1989 Aug 28;495(2):205–216. doi: 10.1016/0006-8993(89)90214-x. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Dildy J. E., Leslie S. W. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989 Oct 16;499(2):383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- Dreher M. L., Hanley M. R. Multiple modes of protein kinase C regulation and their significance in signalling. Trends Pharmacol Sci. 1988 Apr;9(4):114–115. doi: 10.1016/0165-6147(88)90184-8. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Lynch M. A., Bliss T. V. Long-term potentiation of synaptic transmission in the hippocampus of the rat; effect of calmodulin and oleoyl-acetyl-glycerol on release of [3H]glutamate. Neurosci Lett. 1986 Apr 11;65(2):171–176. doi: 10.1016/0304-3940(86)90299-5. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Andrade R., Nicoll R. A. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J Neurosci. 1986 Feb;6(2):475–480. doi: 10.1523/JNEUROSCI.06-02-00475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Madison D. V., Nicoll R. A. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986 May 8;321(6066):175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Markram H., Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990 Aug;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992 Feb;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mistry D. K., Hablitz J. J. Nystatin-perforated patch recordings disclose NMDA-induced outward currents in cultured neocortical neurons. Brain Res. 1990 Dec 10;535(2):318–322. doi: 10.1016/0006-8993(90)91616-o. [DOI] [PubMed] [Google Scholar]
- Monnet F. P., Debonnel G., de Montigny C. Neuropeptide Y selectively potentiates N-methyl-D-aspartate-induced neuronal activation. Eur J Pharmacol. 1990 Jun 21;182(1):207–208. doi: 10.1016/0014-2999(90)90516-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nomura H., Ase K., Sekiguchi K., Kikkawa U., Nishizuka Y., Nakano Y., Satoh T. Stereospecificity of diacylglycerol for stimulus-response coupling in platelets. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1143–1151. doi: 10.1016/0006-291x(86)90754-0. [DOI] [PubMed] [Google Scholar]
- Ransom R. W., Stec N. L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem. 1988 Sep;51(3):830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Rush E. A., Aizenman E. Reduction of NMDA receptors with dithiothreitol increases [3H]-MK-801 binding and NMDA-induced Ca2+ fluxes. Br J Pharmacol. 1990 Sep;101(1):178–182. doi: 10.1111/j.1476-5381.1990.tb12109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. N., Baskys A., Carlen P. L. The effects of serotonin on N-methyl-D-aspartate and synaptically evoked depolarizations in rat neocortical neurons. Brain Res. 1988 Jul 26;456(2):286–292. doi: 10.1016/0006-8993(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Segal M., Markram H., Richter-Levin G. Actions of norepinephrine in the rat hippocampus. Prog Brain Res. 1991;88:323–330. doi: 10.1016/s0079-6123(08)63819-4. [DOI] [PubMed] [Google Scholar]
- Segal M. Synaptic transmission between cultured rat hippocampal neurons is enhanced by activation of protein kinase-C. Neurosci Lett. 1989 Jun 19;101(2):169–174. doi: 10.1016/0304-3940(89)90525-9. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Smith S. S. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res. 1989 Dec 4;503(2):354–357. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Vlachová V., Krůsek J. The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol. 1990 Nov;430:497–517. doi: 10.1113/jphysiol.1990.sp018304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden J., Speckmann E. J., Bingmann D., Straub H. Augmentation of N-methyl-D-aspartate induced depolarizations by GABA in neocortical and archicortical neurons. Brain Res. 1990 Feb 26;510(1):127–129. doi: 10.1016/0006-8993(90)90737-v. [DOI] [PubMed] [Google Scholar]


