Abstract
Background
The role of pulsatile versus non-pulsatile flow during cardiopulmonary bypass (CPB) is still in debate. This systematic review aimed to comprehensively assess the impact of pulsatile versus non-pulsatile flow on patients’ recovery.
Methods
We searched MEDLINE, EMBASE, and Cochrane Library databases for randomized controlled trials comparing pulsatile and non-pulsatile flow in cardiac surgeries with CPB. Data were analyzed using the random-effects model. Then, sensitive analysis and meta-regression were conducted.
Findings
32 studies including 2568 patients were considered in this meta-analysis. There is no difference in in-hospital mortality between the two groups (risk ratio [RR] = 0.74, 95 % confidence interval [CI] = 0.35–1.56, p = 0.43). The ICU stay for the pulsatile group was still significantly shorter than that for the non-pulsatile group (mean difference [MD] = -0.19, 95%CI = −0.35∼-0.03, p = 0.02). Patients in the pulsatile group experienced a shorted stay in hospital (MD = −0.68, 95%CI = −0.97∼-0.39, p < 0.01) and a lower risk for acute kidney injury (AKI) compared with non-pulsatile group (RR = 0.46, 95%CI 0.35–0.60, p < 0.01). There was no significant difference of the postoperative cognitive dysfunction (POCD) between the two groups no matter the roller pump or the intra-aortic balloon pump was used (RR = 0.98, 95%CI = 0.87–1.11, p = 0.78).
Conclusions
The use of pulsatile flow during CPB in heart surgery has a protective effect on patient recovery. It can reduce the incidence of AKI, shorten the ICU and hospital stays, but its positive effect on postoperative mortality and POCD is not yet apparent.
Keywords: Cardiopulmonary bypass, Pulsatile flow, Cardiac surgery, Recovery, Mortality
Highlights
-
•
The largest meta-analysis assesses the pulsatile flow on the recovery of patients undergoing cardiopulmonary bypass.
-
•
The use of pulsatile flow during cardiopulmonary bypass reduce the incidence of AKI, shorten the ICU and hospital stays.
-
•
The majority of benefit results originates the pulsatile flow generated by IABP.
1. Introduction
A critical component of cardiac surgery is the use of cardiopulmonary bypass (CPB), which temporarily takes over the function of the heart and lungs during the procedure. Traditionally, CPB has utilized non-pulsatile or steady flow to perfuse the body. Although non-pulsatile flow can maintain a certain level of perfusion pressure, an increasing body of research suggests that pulsatile blood flow is superior, which more closely mimics the body's physiological state. However, the role of pulsatile versus non-pulsatile flow during CPB is still in debate.
Pulsatile flow, characterized by its periodic fluctuations in pressure and volume, is thought to offer several advantages over non-pulsatile flow, including more energy, better tissue perfusion, and potentially reduced inflammatory responses [[1], [2], [3], [4], [5], [6]]. The contraction of the heart can generate pulsatile blood flow, thereby creating pulsatile shear stress (PSS). The PSS can promote homeostasis of endothelial cells and contribute to the health of the cardiovascular system [7]. A study of microvascular effects of pulsatility in sleeping piglets showed ocular surface capillary densities and flow patterns were better preserved with pulsatile versus continuous flow during 6 h of CPB [8]. It is consistent with the views of Marjolein P. Hoefeijzers, that is,there is a late effect of pulsatility on the microcirculation [9]. These factors are hypothesized to contribute to improved postoperative outcomes, such as reduced organ dysfunction and lower rates of complications [10].
Despite the theoretical benefits, the implementation of pulsatile flow in clinical practice has been limited due to technical challenges and the lack of robust evidence supporting its superiority over non-pulsatile flow [11]. Current research in the field is fragmented, with some studies suggesting improved outcomes with pulsatile flow, while others show no significant difference or even draw attention to potential drawbacks, such as increased hemolysis [[12], [13], [14]].
The reasons for the differences in the aforementioned research conclusions mainly include variations in sample size, surgical methods, pulsatile blood flow frequencies, the manner in which pulsatile blood flow is generated, and the different outcomes measured in the studies. Therefore, the purpose of this systematic review is to comprehensively assess the impact of pulsatile versus non-pulsatile flow on patients’ recovery.
2. Methods
2.1. Study design
The protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (PROSPERO; CRD42023341939). The explain for any amendments of the protocol was also provided on the PROSPERO register (CRD42023341939). This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15].The PRISMA checklist was listed in Supplemental Table 1.
2.2. Inclusion criteria
-
●
Patients:
Adult patients suffered cardiovascular surgery with cardiopulmonary bypass.
-
●
Intervention:
Cardiopulmonary bypass with pulsatile flow is the intervention. The pulsatile flow can be confirmed by pressure wave of pulsatile index.
-
●
Comparator:
Cardiopulmonary bypass without pulsatile flow is the comparator.
-
●
Outcomes:
Primary outcomes: in-hospital all-cause death.
Secondary outcomes: ICU stays, hospital stays, duration of mechanical ventilation, neurological complications, renal function, liver function and routine blood analysis results.
-
●
Inclusion criteria Study type:
Randomized control trails (RCT).
2.3. Exclusion criteria
-
●
Non-RCT article.
-
●
Literature reviews, systematic reviews, meta-analysis, conference or meeting abstracts, animal experiments, repeatedly published literature, etc.
-
●
Data cannot be transformed or the outcome indexes do not meet the requirements.
-
●
Non-English Literature
2.4. Search strategy
A systematic search was performed using MEDLINE, EMBASE, and Cochrane Library. All English articles published before 26 MARCH 2023 were selected. The search strategy is shown in Supplemental Table 2. The retrieval field is restricted to “topic” (the title, abstract, keywords and medical subject headings). Additionally, snowball search for reference lists of published systematic reviews and meta-analyses were reviewed.
The literature search will be conducted by two researchers. Any disagreements regarding the inclusion of studies will be resolved through a process of discussion. In case of unresolved disagreements, a senior reviewer will be consulted for further input.
2.5. Data extraction
Two reviewers independently extracted the data. The standard data extraction form included the following general information.
-
●
Study characteristics: publication year, title, authors, country and sponsoring
-
●
Methods: study design and statistical analysis methods.
-
●
Patient characteristics: such as sex, age, BMI, surgery types and preoperative comorbidities.
-
●
Surgery characteristics: such as surgery type, surgery time, cardiopulmonary bypass time and aortic clamp time.
-
●
Intervention characteristics: methods of pulsatile perfusion.
-
●
Pre-defined primary and secondary outcomes. The first reported postoperative data of the postoperative biochemical parameters for organ functions and blood indices were extracted.
For categorical variables, percentages were extracted. For continuous outcomes, means with standard deviations or medians with interquartile ranges were extracted. Under the assumption of a normal distribution, we transformed the interquartile range into standard deviations according to the Cochrane Handbook for Systematic Reviews of Interventions (Part 2, Chapter 7.7.3.5).
2.6. Risk of bias
Methodological quality of each individual trial was assessed according to the Cochrane Risk of Bias Tool [16]. Two members of our team independently assessed the risk of bias of the trials based on six domains: allocation generation, allocation concealment, blinding, completeness of outcome data, possible selected outcome reporting, and any other potential sources of bias.
2.7. Quantification and statistical analysis
Data processing was conducted using Review Manager (version 5.3). Meta-analysis was performed using the package “meta” in R (version 4.2.2). Continuous and dichotomous outcomes were presented as mean differences (MD) with 95 % confidence intervals (CI) and risk ratios (RR) with 95 % CI, respectively. For continuous outcomes with different units or scales, the standardized mean difference (SMD) was used. For continuous data, the inverse variance model was used, whereas for dichotomous data, we used the Mantel-Haenszel model. Statistical heterogeneity was tested using the χ2 test and I2 statistic. The random-effects model was selected to generate final results. Subgroup analysis based on different ways to generate pulsatile flow was performed. Sensitivity analysis was conducted using the leave-one-out approach. Meta-regression was conducted to analyze the influence of the study-level variables with respect to estimates and variation in the final results. Additionally, funnel plots and Egger’s test were produced to assess reporting bias. P < 0.05 was considered statistically significant.
2.8. Certainty of evidence assessment
We used the Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology to rate the quality of evidence for each outcome [17]. The GRADEpro (http://ims.cochrane.org/gradepro) was applied to build these evidence profiles.
3. Result
3.1. Literature research and characteristics of the studies
A total of 839 studies were found during the initial search of the databases including Medline, Embase and Cochrane library. After removing the duplicates, there were 526 studies for the title and abstract screening. Two designated authors independently excluded studies. After reviewing the full texts, 32 studies including 2568 patients were considered in this meta-analysis. Details are shown in Supplemental Fig. 1.
The basic information of studies included in meta-analysis are shown in Table 1. Roller pump was used in most of the included studies, among which 10 studies used IABP combine with roller pump. 6 of the studies used the centrifugal pump. CABG is the most common surgical method included in the studies focusing on pulsatile blood flow perfusion, with only 6 studies involving valvular surgery. The baseline variables of the included studies are summarized in Supplemental Table 2. There was no significant difference among the included studies regarding on the body surface area, body mass index, ejection fraction, CPB time, aortic cross-clamping time and gender. 29 studies reported the age of their participants with a mean difference of 0.62 (p = 0.0327). The final risk of bias graph is shown in Supplemental Fig. 2. The bias in each included study is summarized in Supplemental Fig. 3.
Table 1.
Basic information of studies included in meta-analysis.
| Study | Publish year | Sample size |
Methods of Pulsatile perfusion | Surgery type | |
|---|---|---|---|---|---|
| Pulsatile | No-pulsatile | ||||
| Adademir 2012 [14] | 2012 | 42 | 43 | Roller pump (Jostra HL 20) | CABG |
| Amouzegar 2017 [15] | 2017 | 36 | 36 | Controling tidal volume | CABG |
| Badner 1997 [16] | 1997 | 40 | 40 | Roller Pump flow interrupter (Pulsatile Flow Controller II, Cobe-Stöckert) | CABG |
| Borulu 2020 [17] | 2020 | 20 | 20 | Roller pump (Terumo® Advanced Perfusion System 1) | CABG |
| Dodonov 2021 [4] | 2021 | 27 | 25 | Centrifugal pump (DeltaStream DP3) | Valve |
| Driessen 1995 [18] | 1995 | 19 | 19 | Centrifugal pump (Sarns 7800) | CABG |
| Engels 2014 [19] | 2014 | 18 | 19 | Centrifugal pump (Deltastream DP3) | Valve |
| Graβler 2019 [20] | 2019 | 21 | 19 | Centrifugal pump (Deltastream DP3) | CABG |
| Henze 1990 [21] | 1990 | 8 | 14 | IABP + Roller pump | CABG |
| Kadoi 2013 [22] | 2013 | 49 | 48 | IABP + Roller pump | CABG |
| Kawahara 1999 [23] | 1999 | 11 | 11 | IABP + Roller pump | CABG |
| Kocakulak 2004 [24] | 2004 | 20 | 20 | Roller pump (Sarns 8000) | CABG |
| Koning 2012 [25] | 2012 | 16 | 17 | Centrifugal pump (Sarns Terumo) | CABG |
| Koning 2016 [26] | 2016 | 20 | 20 | Centrifugal pump (Sorin Mirandola) | CABG |
| Louagie 1992 [27] | 1992 | 50 | 50 | Roller pump pulsatile control unit (EC 26, Cobe-Stöckert pump system 10-00-00) | CABG |
| Mali 2021 [28] | 2021 | 25 | 25 | Roller pump (Stöckert) | Valve |
| Mirmohammadsadeghi 2020 [29] | 2020 | 26 | 26 | Roller pump (Stöckert S5) | CABG |
| Murkin 1995 [30] | 1995 | 158 | 158 | Roller Pump flow interrupter (Pulsatile Flow Controller II, Cobe-Stöckert) | CABG |
| O'Neil 2012 [2] | 2012 | 10 | 10 | Roller pump (Jostra HL 20) | CABG/Valve |
| O'Neil 2018 [3] | 2018 | 10 | 10 | Roller pump (Jostra HL 20) | CABG/Valve |
| Onorati 2007 | 2007 | 50 | 50 | IABP + Roller pump | CABG |
| Onorati 2008 [31] | 2008 | 48 | 48 | IABP + Roller pump | CABG |
| Onorati 2009.1 [32] | 2009 | 40 | 40 | IABP + Roller pump | CABG |
| Onorati 2009.2 [33] | 2009 | 87 | 71 | IABP + Roller pump | CABG |
| Onorati 2009.3 [34] | 2009 | 20 | 20 | IABP + Roller pump | CABG |
| Onorati 2010 [35] | 2010 | 20 | 20 | IABP + Roller pump | CABG |
| Öztürk 2015 [36] | 2015 | 20 | 20 | Roller pump (Jostra HL 20) | CABG |
| Poswal 2004 [37] | 2004 | 50 | 50 | Roller pump (Sarns 9000) | CABG |
| Santini 2011 [38] | 2011 | 15 | 15 | Roller pump (Jostra) | CABG |
| Serraino 2012 [5] | 2012 | 231 | 270 | IABP + Roller pump | CABG |
| Shahandashti 2023 [39] | 2023 | 28 | 29 | Roller pump (Stockert S5) | CABG |
| Song 1997 [40] | 1997 | 35 | 35 | Roller pump (Sarns 7400) | Valve/Congenital heart disease |
CABG: coronary artery bypass graft, IABP: intra-aortic balloon pump.
3.2. Primary outcome
3.2.1. Perioperative mortality
12 studies including 1532 patients reported the perioperative mortality [4,5,[18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. These studies were grouped based on the form of pulsatile blood flow generation, including roller pump group, the centrifugal pump group, and the IABP group. There are 4 studies in the roller pump group, 2 studies in the centrifugal group and 6 studies in the IABP group. The overall data results from 12 studies indicated that there is no difference in mortality rates between the two groups (RR = 0.74, 95%CI = 0.35–1.56, p = 0.43). Additionally, there was also no significant difference in mortality rates between the pulsatile blood flow group and the non-pulsatile blood flow group, regardless of whether roller pump, centrifugal pump or IABP was used (Fig. 1).
Fig. 1.
Forest plot of mortality for pulsatile flow versus non-pulsatile flow.
IABP: intra-aortic balloon pump, RR: relative risk, CI: confidence intervals.
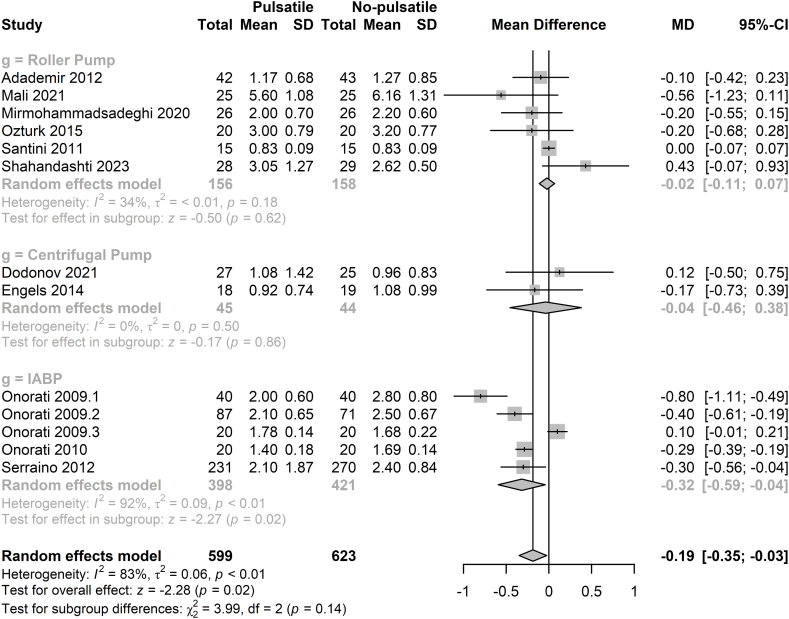
3.2.2. ICU stay and hospital stay
13 studies including 1222 patients reported the ICU stay postoperatively [4,5,18,20,[23], [24], [25], [26],[28], [29], [30], [32], [33]]. There was no significant difference in ICU stay between the pulsatile group and non-pulsatile group when patients were under the treatment of roller pump and centrifugal pump. However, when the pulsatile flow was generated by the IABP, the ICU stay in the pulsatile group was significantly decreased (MD = −0.32d, 95%CI = −0.59∼-0.04d, p = 0.02). Details are shown in Fig. 2. Furthermore, regardless of the method of pulsatile blood flow generation, the ICU stay for the pulsatile group was still significantly shorter than that for the non-pulsatile group (MD = −0.19d, 95%CI = −0.35∼-0.03d, p = 0.02). 12 studies with 1175 participants reported the data of hospital stay [4,5,18,20,[23], [24], [25], [26],28,29,31,32]. Patients in the pulsatile group experienced a shorted stay in hospital than the patients in the non-pulsatile group (MD = −0.68d, 95%CI = −0.97∼-0.39d, p < 0.01, Fig. 3).
Fig. 2.
Forest plot of ICU stay for pulsatile flow versus non-pulsatile flow.
IABP: intra-aortic balloon pump, MD: mean difference, CI: confidence intervals.
Fig. 3.
Forest plot of hospital stay for pulsatile flow versus non-pulsatile flow.
IABP: intra-aortic balloon pump, MD: mean difference, CI: confidence intervals.
3.2.3. Mechanical ventilation time
5 studies with 271 patients demonstrated the postoperative mechanical ventilation time [4,18,23,28,33]. Patients with pulsatile flow seemed have a shorter mechanical ventilation time than the patients with non-pulsatile flow (MD = −1.85h, 95%CI = −3.59∼-0.10h, p = 0.04, in random effects model, Supplemental Fig. 4).
3.2.4. Chest tube volume
Only 3 studies reported the postoperative 24h drainage volume of chest tube [18,31,34]. Among the 225 participants, there was no difference between the two groups (MD = −85.31 ml, 95%CI = −268.47–97.85 ml, p = 0.36). Details are shown in the Supplemental Fig. 5.
3.3. Secondary outcome
3.3.1. Renal function
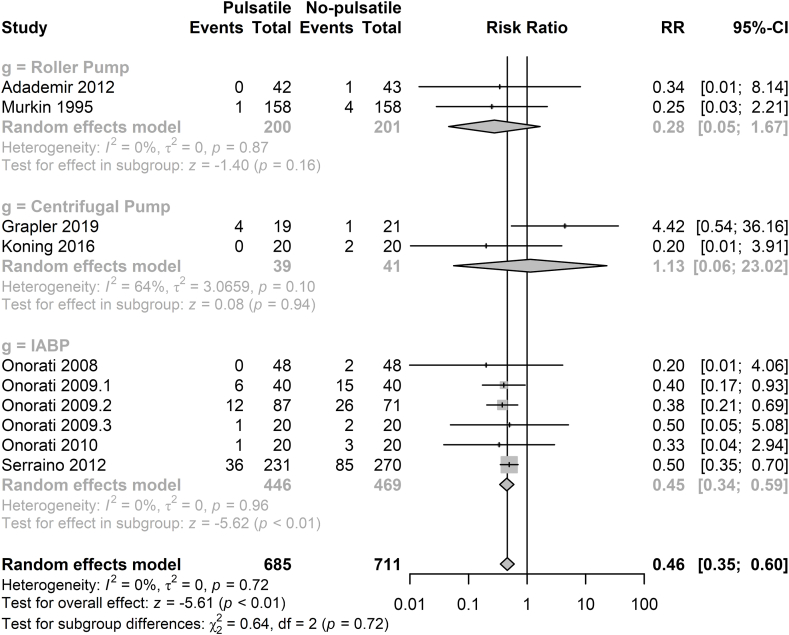
There were 10 studies including 1396 participants report whether AKI occurred postoperatively [5,18,19,21,[23], [24], [25], [26],31,35]. Patients in the pulsatile group had a lower risk for the development of AKI compared with non-pulsatile group (RR = 0.46, 95%CI 0.35–0.60, p < 0.01, Fig. 4). 7 studies demonstrated the blood urea nitrogen (BUN) data postoperatively. There was no significant difference between the two groups (Supplemental Fig. 6) [18,20,33,34,36,37]. 12 studies with 1336 participants reported the creatinine data [3,5,18,20,22,24,25,33,[36], [37], [38], [39], [40]]. The creatinine level in the pulsatile group was significantly lower than that of pulsatile group (MD = −0.10 mg/dl, 95%CI = −0.18∼-0.03 md/dl, p < 0.01, Supplemental Fig. 7) Meanwhile, there were 9 studies with 1214 participants collected the data of creatinine clearance postoperatively [5,18,20,22,24,25,34,36,37]. The creatinine clearance level in the pulsatile group was significantly higher than that in the non-pulsatile group (MD = 6.85 ml/min, 95%CI = 1.66–12.04 ml/min, p < 0.01, Supplemental Fig. 8).
Fig. 4.
Forest plot of the incidence of acute kidney injury.
IABP: intra-aortic balloon pump, RR: relative risk, CI: confidence intervals.
3.3.2. Blood analysis
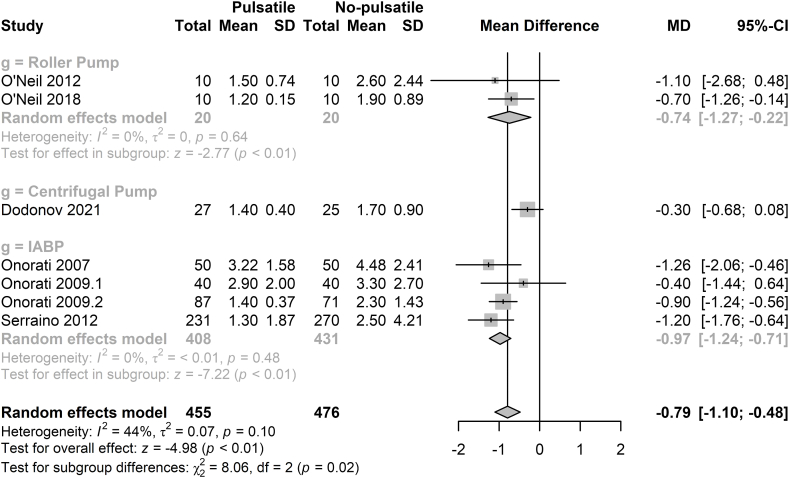
We also collected the data form the blood analysis records, including the concentration of hemoglobin, hematocrit, lactic acid, platelets and white blood cells. There was no significant difference on the concentration of hemoglobin and hematocrit level between the two groups (Supplemental Figs. 9 and 10). In the pulsatile group, the lactic acid level was significantly lower (MD = −0.79 mmol/L, 97%CI = −1.10∼-0.48 mmol/L, p < 0.01, Fig. 5) [[2], [3], [4], [5],22,24,25]. The level of platelets cells is similar in the two groups, but white blood cell counts was significant lower in the pulsatile group (Supplemental Figs. 11 and 12).
Fig. 5.
Forest plot of lactic acid level for pulsatile flow versus non-pulsatile flow.
IABP: intra-aortic balloon pump, MD: mean difference, CI: confidence intervals.
3.3.3. Postoperative cognitive dysfunction (POCD)
4 studies with 475 participants reported the incidence of POCD on the 7th day postoperatively [21,32,41,42]. There was no significant difference between the pulsatile group and non-pulsatile group no matter the roller pump or the IABP was used (RR = 0.98, 95%CI = 0.87–1.11, p = 0.78, Fig. 6).
Fig. 6.
Forest plot of the incidence of postoperative cognitive dysfunction for pulsatile flow versus non pulsatile flow.
IABP: intra-aortic balloon pump, RR: relative risk, CI: confidence intervals.
3.3.4. Liver function
There were 4 studies reported the ALT and AST level after surgery [5,25,33,34]. In the pulsatile group, the level of ALT was significantly lower than that of non-pulsatile group (MD = −6.25U/L, 95%CI = −12.35∼-0.15U/L, p = 0.04, Supplemental Fig. 13). However, there was no significant difference in level of AST between the two groups (MD = −5.81U/L, 95%CI = −13.71–2.10U/L, p = 0.15, Supplemental Fig. 14). Notably, only studies with IABP-generated pulsatile flow achieved significantly improved ALT and AST.
3.4. Reporting bias
Funnel plots for meta-analysis are provided in Supplementary Fig. 15 and did not suggest small study bias.
3.5. Sensitivity analysis
The results of the sensitivity analyses are provided in the Supplementary Fig. 16. After conducting the leave-one-out method sensitivity analysis, the results showed that the most outcomes with statistically homogeneous, including hospital stays, AKI, postoperative creatinine level, creatinine clearance and lactic acid level, were not impacted by individual studies. Notably, some outcomes with significant statistical difference, such as ICU stays and ALT, were observed no statistical difference after excluding studies with IABP- generated pulsatile flow.
3.6. Meta-regression analysis
3 important study-level factors including year, sample size and pulsatile type were investigated for meta-regression analysis to explore the potential heterogeneity sources of the meta-analysis results. Supplemental Table 4 presented the meta-regression results. For the primary outcome, the above 3 factors did not have impact on the mortality, hospital stay, ICU stay. Pulsatile type may influence the results of mechanical ventilation time and chest drainage. The AKI results, POCD results were not influenced by the year, sample size and pulsatile type.
3.7. Certainty of evidence assessment
The certainty of evidence assessment according to the GRADE method is shown in Supplemental Table 5. Most outcomes were assessed as low. Studies were mainly downgraded because of limitations in allocation concealment, lack of blinding of personnel and high heterogeneity of the results.
4. Discussion
In order to comprehensively evaluate the impact of pulsatile flow on patients during the CPB period from multiple aspects, we conducted this meta-analysis. Among the 32 studies included, a total of 2568 patients were involved. In terms of the primary outcomes, we found that pulsatile flow does not affect the mortality in the perioperative period, but it has advantages in ICU stay, postoperative mechanical ventilation time, chest tube drainage volume, and hospital stay. In the secondary results, pulsatile flow can significantly reduce the incidence of postoperative AKI, which also includes reducing the patient's serum creatinine level by improving creatinine clearance. Besides, the blood lactate level of patients in the pulsatile flow group was significantly lower than that of the non-pulsatile blood flow group after the surgery. Meanwhile, pulsatile flow also has an impact on the liver function. However, pulsatile blood flow does not affect the occurrence of POCD.
In our study, we found that pulsatile flow did not influence the mortality after CPB. This result is in line with clinical logic. The pulsatility of blood flow during the CPB period is merely a characteristic of maintaining blood pressure perfusion, and more importantly, both pulsatile and non-pulsatile blood flows maintain a certain perfusion pressure, ensuring the perfusion of vital organs, and therefore, it does not have a significant impact on the patient's outcome of death. A retrospective study including 134 adult patients underwent cardiac surgery also did not demonstrate the difference on the postoperative mortality [13]. To better assess the impact of pulsatile flow, we further observed the postoperative recovery of patients. The pulsatile flow group showed certain advantages in overall aspects such as ICU stay, mechanical ventilation time, and hospital stay, which indicating that the postoperative recovery of patients in the pulsatile flow group is significantly better than that of the non-pulsatile blood flow group. A meta-analysis conducted in 2015 also found the use of pulsatile flow during CPB had a shorter ICU and hospital stays [43]. Taken together, we can confirm that pulsatile flow during CPB has a certain positive significance for the recovery of patients. However, the precise mechanism of its action still requires further exploration.
Furthermore, we aim to uncover which complications occurred in the non-pulsatile flow group after surgery that led to their postoperative recovery being inferior to that of the pulsatile blood flow group. Therefore, we analyzed conditions such as AKI and POCD. In addition to our study, there are many clinical or basic studies that have focused on the impact of pulsatile flow on the structure and function of the kidneys. In 2015, a meta-analysis was conducted by Myung Ji Nam et al. to determine the effects of pulsatile flow on the postoperative renal functions, which included 9 studies with 674 patients [44]. They also found that patients in the pulsatile flow group had a higher creatinine clearance and lower creatinine level. In the piglets CPB model, Aida Salameh et al. found that laminar flow produced significant cellular edema in the kidney and the markers for hypoxia were elevated predominately in the proximal tubules of the kidney [45]. In addition, the mode of blood delivery in extracorporeal membrane oxygenation (ECMO), which is an extended application of CPB in the intensive care unit, is also frequently mentioned when studying pulsatile blood flow. Recent research has indicated that the loss of pulsatile flow in the early stage of ECMO was associated with higher rate of acute brain injury and limb ischemia [46,47].
Current research results seem to lean towards the phenomenon that pulsatile flow can improve organ perfusion and alleviate ischemic and hypoxic damage to organs. Throughout the lengthy process of species evolution, a circulatory system that relies on cardiac contraction to generate pulsatile blood flow has been developed. This type of circulatory system is not only present in humans but is also common in the animal kingdom. Evolution has favored pulsatile blood flow, suggesting that it must possess certain physiological functions that have yet to be fully elucidated by humans. It is highly likely that vascular endothelial cells serve as the vehicle for the physiological effects of pulsatile flow. The PSS can effectively transmit signals to vascular endothelial cells [7]. It has been found that the pulsatile flow could maintain normal perfusion of sublingual mucosal microcirculation and improved the tissue oxygen saturation, while the non-pulsatile group exhibited deterioration in perfusion during CPB [3]. Additionally, between 3 and 6 h of CPB, the quality of ocular microvascular perfusion deteriorated with non-pulsatile flow and improved with pulsatile flow in a pig model [8].
In clinical, the pulsatile flow generated by IABP also could decrease the postoperative lactate levels [48]. Patients with pulsatile flow during CPB may have a better regional cerebral oxygen saturation value despite there was no difference on the neurocognitive function [49]. Besides, there also a meta-analysis found patients in the pulsatile group had a better postoperative pulmonary function regarding the data of PaO2/FiO2 [43]. Researchers have demonstrated that when the aortic endothelial cells exposed to the continuous flow, the pro-inflammatory signaling would be activated and the pulsatile flow could reduce the undesirable sequalae [6].
However, clinical studies around the world on pulsatile and non-pulsatile perfusion do not reach the same conclusions for the same endpoint. The method to generate the pulsatile flow during CPB may have a great impact on the prognosis of the patients. Furthermore, the different pulse rate during the pulsatile flow perfusion may also contribute to the negative treatment effect compared with non-pulsatile flow perfusion [33]. Additionally, different ways to generate pulsatile flow can also impact the clinical prognosis of patients. The pulsatile flow can be achieved during CPB by the use of pulsator devices within the circuit or by adjusting the arterial pump to create rapid fluctuations in flow [50,51]. Another approach involves generating pulsatility outside of the extracorporeal circuit using an IABP [52]. Previous study and meta-analyses have indicated that the majority of supportive evidence for pulsatile flow originates from studies utilizing an IABP for pulsatility generation [53]. Interestingly, the subgroup and sensitivity analyses results of this meta-analysis also demonstrated favorable clinical outcomes, including reduced ICU and hospital stays, lower postoperative lactic acid levels, and improved renal and hepatic function. More research is needed to determine if only IABP can produce enough pulsatile flow for noticeable clinical effects.
This meta-analysis has several limitations. 1. The purpose of this study is to evaluate the impact of pulsatile flow on patients hence a large number of studies relevant to the topic were included. However, the wide time span of the included studies and the different sample sizes of each study may introduce certain biases to the research findings. 2. The results of our meta-analysis showed high study heterogeneity, which decreased the quality level of evidence of this study. However, sensitivity and meta-meta-regression analyses revealed that different methods to generate pulsatile flow could be the most important source of heterogeneity. Further subgroup meta-analysis showed that majority of supportive evidence for pulsatile flow originates from studies utilizing an IABP for pulsatility generation, which indirectly supports the above speculation. 3. Since we only included English publications, language bias may occur. 4. Pulsatile flow can be generated by various methods, such as IABP, roller pumps, and centrifugal pumps. However, the quality of pulsatility produced by these methods is inferior to that generated by the rhythmic contractions of the heart. Further effort is warranted to explore the techniques for generating physiological pulsatile flow and its true efficacy.
5. Conclusion
The use of pulsatile flow during CPB in heart surgery has a protective effect on patient recovery. It can reduce the postoperative blood lactate levels and the incidence of AKI, shorten the ICU and hospital stays, but its positive effect on postoperative mortality and POCD is not yet apparent.
CRediT authorship contribution statement
Weidong Yan: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Tianlong Wang: Writing – original draft, Software, Project administration, Methodology, Conceptualization. Jing Wang: Validation, Supervision. RuiNing Yang: Investigation, Conceptualization. Han Zhang: Methodology, Conceptualization. Mingru Zhang: Writing – review & editing. Bingyang Ji: Methodology, Funding acquisition, Formal analysis, Data curation.
Availability of data and materials
The data has been entirely included in the manuscript and supplementary files. Code for the analyses can be obtained from the corresponding author.
Ethical statement
Not applied.
Funding
Not applied.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2025.e41630.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jiang Q., et al. Frequency domain analysis and clinical outcomes of pulsatile and non-pulsatile blood flow energy during cardiopulmonary bypass. Perfusion. 2021;36(8):788–797. doi: 10.1177/02676591211012216. [DOI] [PubMed] [Google Scholar]
- 2.O'Neil M.P., et al. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann. Thorac. Surg. 2012;94(6):2046–2053. doi: 10.1016/j.athoracsur.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 3.O'Neil M.P., et al. Microvascular responsiveness to pulsatile and nonpulsatile flow during cardiopulmonary bypass. Ann. Thorac. Surg. 2018;105(6):1745–1753. doi: 10.1016/j.athoracsur.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Dodonov M., et al. Efficacy of pulsatile flow perfusion in adult cardiac surgery: hemodynamic energy and vascular reactivity. J. Clin. Med. 2021;10(24) doi: 10.3390/jcm10245934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serraino G.F., et al. Pulsatile cardiopulmonary bypass with intra-aortic balloon pump improves organ function and reduces endothelial activation. Circ. J. 2012;76(5):1121–1129. doi: 10.1253/circj.cj-11-1027. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen K.T., et al. Acute response of human aortic endothelial cells to loss of pulsatility as seen during cardiopulmonary bypass. Cells Tissues Organs. 2022;211(3):324–334. doi: 10.1159/000512558. [DOI] [PubMed] [Google Scholar]
- 7.Adams J.A., Uryash A., Lopez J.R. Non-Invasive pulsatile shear stress modifies endothelial activation; A narrative review. Biomedicines. 2022;10(12) doi: 10.3390/biomedicines10123050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvernebo A.K., et al. Ocular surface microcirculation is better preserved with pulsatile versus continuous flow during cardiopulmonary bypass-An experimental pilot. Artif. Organs. 2022;46(5):786–793. doi: 10.1111/aor.14137. [DOI] [PubMed] [Google Scholar]
- 9.Hoefeijzers M.P., et al. The pulsatile perfusion debate in cardiac surgery: answers from the microcirculation? J. Cardiothorac. Vasc. Anesth. 2015;29(3):761–767. doi: 10.1053/j.jvca.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Li G., et al. The outcome of pediatric patients undergoing congenital cardiac surgery under pulsatile cardiopulmonary bypass in different frequencies. Ther Clin Risk Manag. 2018;14:1553–1561. doi: 10.2147/tcrm.S170642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulson T.G., et al. The association between pulsatile cardiopulmonary bypass and acute kidney injury after cardiac surgery: a before-and-after study. J. Cardiothorac. Vasc. Anesth. 2020;34(1):108–113. doi: 10.1053/j.jvca.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Shahandashti F.J., et al. Pulsatile versus non-pulsatile perfusion in coronary artery bypass operation: the comparison of laboratory and clinical outcomes. Perfusion. 2023;38(5):1053–1061. doi: 10.1177/02676591221096224. [DOI] [PubMed] [Google Scholar]
- 13.Kadiroğulları E., et al. Effect of pulsatile cardiopulmonary bypass in adult heart surgery. Heart Surg. Forum. 2020;23(3):E258–e263. doi: 10.1532/hsf.2857. [DOI] [PubMed] [Google Scholar]
- 14.Tan Z., et al. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: extent of hemolysis and clinical significance. Asaio j. 2020;66(9):1025–1030. doi: 10.1097/mat.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teirlinck C.H., et al. Responders to exercise therapy in patients with osteoarthritis of the hip: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(20) doi: 10.3390/ijerph17207380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt G.H., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347. AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adademir T., et al. The effects of pulsatile cardiopulmonary bypass on acute kidney injury. Int. J. Artif. Organs. 2012;35(7):511–519. doi: 10.5301/ijao.5000097. [DOI] [PubMed] [Google Scholar]
- 19.Koning N.J., et al. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br. J. Anaesth. 2016;116(2):223–232. doi: 10.1093/bja/aev411. [DOI] [PubMed] [Google Scholar]
- 20.Mali S., et al. Comparison of renal function in coronary artery bypass graft surgery with pulsatile versus non-pulsatile perfusion: a randomized clinical trial. International cardiovascular research journal. 2021;15(3):105–110. [Google Scholar]
- 21.Murkin J.M., et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J. Thorac. Cardiovasc. Surg. 1995;110(2):340–348. doi: 10.1016/s0022-5223(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 22.Onorati F., et al. A randomized trial of pulsatile perfusion using an intra-aortic balloon pump versus nonpulsatile perfusion on short-term changes in kidney function during cardiopulmonary bypass during myocardial reperfusion. Am. J. Kidney Dis. 2007;50(2):229–238. doi: 10.1053/j.ajkd.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Onorati F., et al. Off-pump coronary artery bypass surgery versus standard linear or pulsatile cardiopulmonary bypass: endothelial activation and inflammatory response. Eur. J. Cardio. Thorac. Surg. 2010;37(4):897–904. doi: 10.1016/j.ejcts.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Onorati F., et al. Pulsatile perfusion with intra-aortic balloon pumping ameliorates whole body response to cardiopulmonary bypass in the elderly. Crit. Care Med. 2009;37(3):902–911. doi: 10.1097/CCM.0b013e3181962aa9. [DOI] [PubMed] [Google Scholar]
- 25.Onorati F., et al. Body perfusion during adult cardiopulmonary bypass is improved by pulsatile flow with intra-aortic balloon pump. Int. J. Artif. Organs. 2009;32(1):50–61. doi: 10.1177/039139880903200107. [DOI] [PubMed] [Google Scholar]
- 26.Onorati F., et al. Intra-aortic balloon pump induced pulsatile perfusion reduces endothelial activation and inflammatory response following cardiopulmonary bypass. Eur. J. Cardio. Thorac. Surg. 2009;35(6):1012–1019. doi: 10.1016/j.ejcts.2008.12.037. ; discussion 1019. [DOI] [PubMed] [Google Scholar]
- 27.Song Z., Wang C., Stammers A.H. Clinical comparison of pulsatile and nonpulsatile perfusion during cardiopulmonary bypass. J. Extra Corpor. Technol. 1997;29(4):170–175. [PubMed] [Google Scholar]
- 28.Engels G.E., et al. The effect of pulsatile cardiopulmonary bypass on lung function in elderly patients. Int. J. Artif. Organs. 2014;37(9):679–687. doi: 10.5301/ijao.5000352. [DOI] [PubMed] [Google Scholar]
- 29.Mirmohammadsadeghi A., Jahannama N., Mirmohammadsadeghi M. Sleep quality after coronary artery bypass graft surgery: comparing pulsatile and nonpulsatile pump flow. J. Extra Corpor. Technol. 2020;52(4):314–318. doi: 10.1182/ject-2000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santini F., et al. Pulsatile pulmonary perfusion with oxygenated blood ameliorates pulmonary hemodynamic and respiratory indices in low-risk coronary artery bypass patients. Eur. J. Cardio. Thorac. Surg. 2011;40(4):794–803. doi: 10.1016/j.ejcts.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 31.Graßler A., et al. Effects of pulsatile minimal invasive extracorporeal circulation on fibrinolysis and organ protection in adult cardiac surgery-a prospective randomized trial. J. Thorac. Dis. 2019;11(Suppl 10):S1453–s1463. doi: 10.21037/jtd.2019.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Öztürk S., et al. Effect of the type of cardiopulmonary bypass pump flow on postoperative cognitive function in patients undergoing isolated coronary artery surgery. Anatol. J. Cardiol. 2016;16(11):875–880. doi: 10.14744/AnatolJCardiol.2015.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahandashti F.J., et al. Pulsatile versus non-pulsatile perfusion in coronary artery bypass operation: the comparison of laboratory and clinical outcomes. Perfusion. 2022 doi: 10.1177/02676591221096224. [DOI] [PubMed] [Google Scholar]
- 34.Louagie Y.A., et al. Does flow character of cardiopulmonary bypass make a difference? J. Thorac. Cardiovasc. Surg. 1992;104(6):1628–1638. [PubMed] [Google Scholar]
- 35.Onorati F., et al. Intra-aortic balloon pump-induced pulsatile flow reduces coagulative and fibrinolytic response to cardiopulmonary bypass. Artif. Organs. 2008;32(6):433–441. doi: 10.1111/j.1525-1594.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- 36.Borulu F., et al. Investigation of the effect of pulsatile and nonpulsatile flow on kidney in coronary surgery with NIRS. Heart Surg. Forum. 2020;23(4):E401–E406. doi: 10.1532/hsf.2341. [DOI] [PubMed] [Google Scholar]
- 37.Poswal P., et al. Comparative study of pulsatile and nonpulsatile flow during cardio-pulmonary bypass. Ann. Card Anaesth. 2004;7(1):44–50. [PubMed] [Google Scholar]
- 38.Amouzegar S.M., Lak M. An assessment of renal function with pulsatile perfusion during proximal graft using cardiac contraction in coronary artery bypass graft surgery. Nephro-Urol. Mon. 2017;9(5) doi: 10.5812/numonthly.14324. [DOI] [Google Scholar]
- 39.Kocakulak M., Küçükaksu S., Pişkin E. Pulsatile roller pump perfusion is safe in high risk patients. Int. J. Artif. Organs. 2004;27(5):433–439. doi: 10.1177/039139880402700514. [DOI] [PubMed] [Google Scholar]
- 40.Koning N.J., et al. Pulsatile flow during cardiopulmonary bypass preserves postoperative microcirculatory perfusion irrespective of systemic hemodynamics. J. Appl. Physiol. 2012;112(10):1727–1734. doi: 10.1152/japplphysiol.01191.2011. 1985. [DOI] [PubMed] [Google Scholar]
- 41.Henze T., Stephan H., Sonntag H. Cerebral dysfunction following extracorporeal circulation for aortocoronary bypass surgery: no differences in neuropsychological outcome after pulsatile versus nonpulsatile flow. Thorac. Cardiovasc. Surg. 1990;38(2):65–68. doi: 10.1055/s-2007-1013995. [DOI] [PubMed] [Google Scholar]
- 42.Kadoi Y., et al. Effects of balloon-induced pulsatile perfusion on postoperative short- and long-term cognitive dysfunction in diabetic patients with impaired cerebrovascular carbon dioxide reactivity. J. Cardiothorac. Vasc. Anesth. 2013;27(2):238–244. doi: 10.1053/j.jvca.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Lim C.H., et al. A meta-analysis of pulmonary function with pulsatile perfusion in cardiac surgery. Artif. Organs. 2015;39(2):110–117. doi: 10.1111/aor.12312. [DOI] [PubMed] [Google Scholar]
- 44.Nam M.J., et al. A meta-analysis of renal function after adult cardiac surgery with pulsatile perfusion. Artif. Organs. 2015;39(9):788–794. doi: 10.1111/aor.12452. [DOI] [PubMed] [Google Scholar]
- 45.Salameh A., et al. Protective effects of pulsatile flow during cardiopulmonary bypass. Ann. Thorac. Surg. 2015;99(1):192–199. doi: 10.1016/j.athoracsur.2014.07.070. [DOI] [PubMed] [Google Scholar]
- 46.Hart J.P., Davies M.G. Vascular complications in extracorporeal membrane oxygenation-A narrative review. J. Clin. Med. 2024;13(17) doi: 10.3390/jcm13175170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalra A., et al. Pulse pressure and acute brain injury in venoarterial extracorporeal membrane oxygenation: an extracorporeal life support organization registry analysis. ASAIO J. 2024 doi: 10.1097/MAT.0000000000002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James L., et al. Intraoperative use of intra-aortic balloon pump to generate pulsatile flow during heart transplantation: a single-center experience. Asaio j. 2024 doi: 10.1097/mat.0000000000002199. [DOI] [PubMed] [Google Scholar]
- 49.Bostancı İ., et al. Effects of pulsatile and non-pulsatile cardiopulmonary bypass techniques in coronary artery bypass grafting surgeries on cerebral perfusion. Turk J Anaesthesiol Reanim. 2024;52(1):22–29. doi: 10.4274/tjar.2024.231331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voss B., et al. Cardiopulmonary bypass with physiological flow and pressure curves: pulse is unnecessary. Eur. J. Cardio. Thorac. Surg. 2010;37(1):223–232. doi: 10.1016/j.ejcts.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 51.Bertini P., Guarracino F. Pro: pulsatile flow during cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2023;37(11):2370–2373. doi: 10.1053/j.jvca.2023.04.034. [DOI] [PubMed] [Google Scholar]
- 52.Hosmane S.R., Dawson A.G. In patients coming to theatre with an intra aortic balloon pump, is it better to turn it off or keep it on while on bypass? Interact. Cardiovasc. Thorac. Surg. 2010;11(3):314–321. doi: 10.1510/icvts.2010.237255. [DOI] [PubMed] [Google Scholar]
- 53.Tan A., Newey C., Falter F. Pulsatile perfusion during cardiopulmonary bypass: a literature review. J. Extra Corpor. Technol. 2022;54(1):50–60. doi: 10.1182/ject-50-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data has been entirely included in the manuscript and supplementary files. Code for the analyses can be obtained from the corresponding author.