Abstract
Objective
The aim of this prospective cohort study is to analyse the humoral and cellular vaccine responses in paediatric heart transplant recipients (HTR, n = 12), and compare it with the response in healthy controls (HC, n = 14). All participants were 5–18 years old and vaccinated with mRNA vaccine against SARS-CoV-2 between December 2021 and May 2022.
Methods
The humoral response was measured by quantifying antibody titers against SARS-CoV-2 spike protein (anti-S). The T-lymphocyte phenotype and SARS-CoV2-specific CD4+ and CD8+ T-cell response was studied by multiparametric flow cytometry through peripheral blood mononuclear cells by the quantification of degranulation markers (CD107a) and intracellular cytokines (IFN-γ, TNF-α and IL-2) after in vitro stimulation with SARS-CoV-2 peptides from structural proteins (S, M, N, E) and non-structural viral proteins.
Results
After vaccination, humoral response was found in all HTR, although they showed lower levels of anti-S IgG compared to HC (p = 0.003). However, in terms of cellular response, no significant differences were obtained in the prevalence of responders and magnitude of responses between groups. In addition, anti-S IgG levels directly correlated with a higher SARS-CoV-2 specific T-cell response (rho = 0.43; p = 0.027 and rho = 0.45; p = 0.02 for IFN-γ+ and TNF-α+ production of CD8+ T-cells, respectively). Activated T-cell phenotype in HTR was associated with a lower humoral response to SARS-CoV-2 vaccine.
Conclusion
HTR had humoral response after vaccination, although they showed lower levels of specific anti-S antibodies compared to HC. There were no significant differences in the SARS-CoV2-specific cellular response between the two groups. Obtaining satisfactory data on this type of response could potentially challenge the current vaccine guideline recommendations.
Keywords: SARS-CoV-2, Vaccines, Paediatric, Immunocompromised, Heart transplant recipients, COVID-19, Humoral immunity, Cell-mediated immunity
Graphical abstract
Highlights
-
•
Paediatric heart transplant recipients (HTR) had weak humoral response after SARS-CoV-2 mRNA vaccination.
-
•
HTR showed lower levels of SARS-CoV-2–specific IgG levels compared to healthy controls.
-
•
High SARS-CoV-2–specific IgG levels are associated with high T-cell response.
-
•
Activated T-cell phenotype in HTR correlated with low humoral response to SARS-CoV-2 vaccine.
Abbreviations
- Anit-N
antibody against SARS-CoV-2 nucleocapsid protein
- Anti-S
antibody against SARS-CoV-2 spike protein
- AU
arbitrary units
- BAU
standard binding antibody units
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- HC
healthy controls
- HGM
Gregorio Marañón Hospital
- HTR
heart transplant recipients
- IFN-γ
Interferon gamma
- IL-2
Interleukin-2
- IQR
interquartile ranges
- mRNA
Messenger ribonucleic acid
- PBMCs
peripheral blood mononuclear cells
- PFA
paraformaldehyde solution
- SEB
staphylococcal enterotoxin B
- SOTR
solid organ transplant recipients
- TNF
tumor necrosis factor
1. Introduction
Heart transplant recipients (HTR) have significant immunosuppression due to the medication required to maintain their graft [1]. For this reason, at the beginning of the COVID-19 pandemic, solid organ transplant recipients (SOTR) were included in the risk groups for severe COVID-19 disease. Currently, this association remains controversial, as some published studies were conducted in small populations and showed no differences in clinical manifestations between SOTR and healthy controls, particularly in the paediatric population [[2], [3], [4], [5], [6]]. In a case series report of paediatric SOTR with SARS-CoV-2 infection, no increased mortality was observed, in contrast to what has been published in adults [7,8]. However, SOTR remain at risk of severe SARS-CoV-2 infection and additional doses of vaccine are recommended to this group to ensure immunity [9,10].
Clinical trials conducted for the analysis of safety and efficacy of messenger RNA (mRNA) vaccine against SARS-CoV-2 in paediatric patients did not include immunosuppressed patients [[11], [12], [13], [14]]. Therefore, studies in these patient groups are crucial to better understand their vaccine response. Since the approval of the SARS-CoV-2 vaccine in the paediatric population, most studies in immunosuppressed patients have focused on the humoral vaccine response. These studies detected lower antibody levels against SARS-CoV-2 in immunosuppressed paediatric patients compared to levels produced by controls [[15], [16], [17]]. Hence, a booster dose is recommended for these patients [10,[18], [19], [20], [21]]. However, data on cell-mediated immunity are limited [22,23].
Classically, adequate vaccine response has been associated with the detection of humoral immunity by measurement of antibodies [24,25]. However, immunosuppressed individuals may experience a reduced humoral response due to their underlying disease or treatment regimen [26], the influence of which may vary depending on the combination of immunosuppressive drugs used [1]. Given the impairment of humoral immunity in these patients, several authors advocate the study of T cell-mediated immunity as a more accurate method to assess vaccine response in these patients [24]. Notably, these patients may develop cellular response in the absence of antibodies production [26,27].
The aim of this study is to analyse the humoral and cellular immune responses to the SARS-CoV-2 mRNA vaccine in immunocompromised paediatric HTR and compare it with a reference group of paediatric healthy controls.
2. Material and methods
2.1. Study design and participants
Patients who received a heart transplant between 2011 and 2020 and receiving transplant-related immunosuppressive drugs, aged 5–18 years, who are under follow-up in the Pediatric Cardiology Service of the Hospital Universitario Gregorio Marañón [(HGM), Madrid, Spain] and who received full vaccination against SARS-CoV-2 mRNA vaccine between December 2021 and May 2022 were included in this study [28]. Additionally, healthy controls (HC) who were aged 5–12 years and have received complete vaccination at Móstoles University Hospital (Madrid, Spain) during the same period, were also included as control group. All participants were vaccinated with the Pfizer-BioNTech (BNT162b2) mRNA vaccine. At the time of sampling, information about vaccination adverse events, symptoms in case of SARS-CoV-2 infection and the types and number of vaccine doses were collected.
All patients were informed and an informed consent form prior to blood sampling was signed by the parents or legal guardians. This study was approved by the Ethics Committee of the Gregorio Marañón University Hospital (acta 21/2021) and the Ethics Committee of the Móstoles University Hospital (CEIC 2022/016).
2.2. Cell and plasma isolation
Blood samples from study participants were drawn from a peripheral vein, collected in BD Vacutainer™ PET ethylenediaminetetraacetic acid tubes (EDTA) and peripheral blood mononuclear cells (PBMCs) and plasma were isolated using a Ficoll density gradient. PBMCs were cryopreserved in freezing medium (fetal bovine serum [FBS; Thermo Fisher Scientific] + 7.5 % dimethyl sulfoxide [DMSO]) and stored in liquid nitrogen and plasma was stored at −20 °C, at the Spanish HGM BioBank until further use [29].
2.3. Quantification of anti-S and anti-N SARS-CoV-2 IgG
SARS-CoV-2 anti-spike IgG (Anti-S IgG) and anti-nucleocapsid protein (Anti-N IgG) were measured in plasma by employing the Architect SARS-CoV-2 anti-S IgG II and the Architect SARS-CoV-2 anti-N IgG reagents on an Architect-i autoanalyser for immunoassays (Abbott Laboratories, Chicago, IL, USA). The anti-S IgG result was quantitative, and reported in arbitrary units/milliliter (AU/mL); each arbitrary unit is equivalent to 0.142 standard binding antibody units (BAU)/mL. The result for anti-N IgG was qualitative (presence/absence). Positive result for anti-S and anti-N was considered convalescent. A positive result for a diagnostic test (SARS-CoV-2 RT-PCR or SARS-CoV-2 antigenic test) was also considered convalescent, whereas positive result for anti-S but negative for anti-N and no positive results for a diagnostic test were deemed vaccinated and naïve uninfected [30].
2.4. Cell stimulation and SARS-CoV-2–specific T-cell response
To analyse the specific T-cell response to SARS-CoV-2, PBMCs were thawed, washed using 10 % RPMI medium (R-10; RPMI 1640 supplemented with 10 % heat-inactivated FBS, 100 U/ml penicillin G, 100 μl/ml streptomycin sulphate and 1 % l-glutamine [Lonza, Gampel-Bratsch, Switzerland]), rested for 2 h in R-10 with 10 U/ml DNase I (Invitrogen) at 37 °C/5%CO2 and incubated for 6 h in R-10 at 37 °C/5 % CO2 with 1 μg/ml anti-CD28/CD49d, 0.7 μg/ml monensin (BD Biosciences), 10 μg/ml brefeldin A (Biolegend), anti–CD107a-FITC and presence or absence of 1 μg/ml of each peptide pool of SARS-CoV-2 peptides (PepTivator® SARS-CoV-2 Select premium grade; Miltenyi). These peptides derived from SARS-CoV-2 consisted of a pool of 88 peptides of 9–22 amino acids in length originated from structural proteins (S, M, N, E) as well as non-structural viral proteins.
2.5. Immunophenotyping and intracellular cytokine staining
For ex vivo phenotyping and functional assay, PBMCs were washed with phosphate-buffered saline (PBS; Thermo Fisher Scientific) with 3 % BSA (bovine serum albumin; Sigma-Aldrich), extracellularly stained for 30 min at room temperature with the viability marker LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen) and the extracellular antibodies. Then, PBMCs were washed and fixed and permeabilized with BD Cytofix/CytoPerm (BD Biosciences) at 4 °C for 30 min or with Fixation/Permeabilization Buffer Set (eBioscience) at 4 °C for 45 min, following the manufacturer's protocol. Then, cells were stained at 4 °C for 30 min with intracellular antibodies, washed and fixed with 4 % paraformaldehyde solution (PFA; Sigma-Aldrich).
To assay ex vivo T-cell phenotyping, PBMCs were extracellularly stained with LIVE/DEAD Fixable Aqua Dead Cell Stain, anti-DUMP-channel-BV510 (anti-CD56, anti-CD14 and anti-CD19), anti-CD3-PerCP-Cy5.5, anti-CD4-APC-R700, anti-CD38-FITC, anti-CD45RA-ECD, anti-HLA-DR-APC, anti-CD154-Pe-Cy7, anti-CD137-APC-Cy7 and anti-CD25-BV421; they were permeabilized and fixed with Fixation/Permeabilization Buffer Set (eBioscience) and intracellularly stained with anti-FoxP3-PE. Isotype controls were included for anti-CD154, anti-CD137, anti-CD25 and anti-FoxP3. Antibodies details can be found at Supplementary Table 1.
To assay specific T-cell response, after incubation PBMCs were extracellularly stained with LIVE/DEAD Fixable Aqua Dead Cell Stain, anti-DUMP-channel-BV510 (anti-CD56, anti-CD14 and anti-CD19), anti-CD3-PerCP-Cy5.5, anti-CD4-APC-R700, anti-CD45RA-ECD; permeabilized and fixed with BD Cytofix/CytoPerm and intracellularly stained with anti-IL-2-PE, anti-TNF-α-APC and anti-IFN-γ-BV421 (antibodies details at Supplementary Table 1).
Lymphocytes were defined as viable cells having low forward/side scatter and expressing CD3, and/or no CD4, but not CD19, CD14 and CD56 (Supplementary Fig. 2A). The specific T-cell response to each stimuli was determined by the sum of the expression of each cytokine (IFN-γ, IL-2 and TNF-α) in the different T-cell subsets after stimulation with SARS-CoV-2 peptides, minus the frequency of cells expressing the same biomarkers in the unstimulated condition (background subtraction). A third condition stimulated with staphylococcal enterotoxin B (SEB; Sigma Aldrich) was used as a positive control, and was included in each analysis (Supplementary Fig. 2B). In order to classify an individual as a responder, we considered a SARS-CoV-2 specific response threshold higher than 0.05 %, a threshold based on the distribution of T-cell cytokine frequencies in the unstimulated control conditions as previously described [31,32]. For this analysis, a minimum of 106 total events were recorded for each condition.
Cell acquisition was carried out in a Gallios flow cytometer (Beckman Coulter) and FlowJo 10.7.1 software (TreeStar) was used for data analysis.
2.6. Statistical analysis
Median and interquartile ranges [IQR] were used to describe continuous variables and numbers and percentages to describe categorical variables. Differences between HTR and HC groups, among participants with and without prior SARS-CoV-2 infection, were analysed by two-tailed Mann-Whitney U test and X2 test for continuous and categorical variables respectively. The Spearman test was used to analyse correlations between variables. All differences with a p-value of <0.05 were considered statistically significant. Statistical analyses were performed using Statistical Package for the Social Sciences software (SPSS 20.0; SPSS, Inc., Chicago, IL) and graphs were generated with GraphPad Prism version 9.0 (GraphPad Software, Inc.).
3. Results
3.1. Study participants
The median age of all children was 11.2 years [IQR: 8.6–12.6]. The clinical characteristics of HTR (n = 12) and HC (n = 14) are described and compared in Table 1.
Table 1.
Clinical Characteristics of study groups.
| HTR n = 12 | HC n = 14 | p-value | |
|---|---|---|---|
| Age (years) | 13.2 [[10], [11], [12], [13], [14], [15]] | 8.7 [[8], [9], [10], [11]] | 0.001 |
| Sex (male), n (%) | 6/12 (50) | 7/14 (50) | 0.999 |
| Immunosuppressive treatment, n (%) | |||
| Tacrolimus | 11 (91.7) | na | |
| Mycophenolate | 6 (50) | na | |
| Sirolimus | 1 (8.3) | na | |
| Corticosteroids | 3 (25) | na | |
| Doses of vaccine, n (%) | <0.05 | ||
| Two doses | 3/12 (25) | 14/14 (100) | |
| Three doses | 9/12 (75) | 0 | |
| Time from last dose of vaccine to follow-up sample collection, months | <0.001 | ||
| 4.3 [1.7–4.9] | 0.6 [0.6–0.6] | ||
| SARS-CoV-2 infection, yes (%) | 5/12 (41.6) | 11/14 (78.5) | 0.054 |
| Diagnosis test, n (%) | 0.999 | ||
| SARS-CoV-2 RT-PCR | 2/5 (40) | 4/11 (36.4) | |
| SARS-CoV-2 antigenic test | 3/5 (60) | 7/11 (63.6) | |
| Symptomatic infection, yes (%) | 2/5 (40) | 7/11 (63.6) | 0.596 |
| Fever | 1/2 (20) | 6/7 (54) | |
| Cough | 1/2 (20) | 2/7 (18.1) | |
| Abdominal pain | 0 | 1/7 (9.09) | |
| Headache | 0 | 2/7 (18.1) | |
| Otalgia | 1/2 (20) | 0 | |
| Pneumonia | 0 | 0 | |
| Admission to hospital | 0 | 0 | |
| Lapse of time between infection and vaccination (%) | |||
| Prior to vaccination | 1/5 | 10/11 | 0.005 |
| Between 1st y 2nd dose | 0 | 1/11 | 0.999 |
| Between 2nd y 3rd dose | 1/5 | na | |
| After full vaccination regimen (Oct-21) | 3/5 | No data | |
| Thymus total excision, yes (%) | 12/12 (100) | 0 (0) | |
Continuous variables are expressed as medians and interquartile ranges [IQR]. Categorical variables are expressed as numbers and percentages. Two-tailed Mann-Whitney U test and X2 test were used for continuous and categorical variables respectively. Abbreviations: HTR: heart transplant recipients; HC: healthy controls; RT-PCR, reverse transcription polymerase chain reaction; na, no applicable. p-value<0.05 are in bold and 0.05≤p-value<0.1 are in italics.
The most commonly used immunosuppressive drugs in HTR were tacrolimus in combination with mycophenolate, none were treated with Rituximab (Table 1). Seventy-five percent of the HTR received three-dose schedule of mRNA vaccine, while all HC received two-dose schedule.
Overall, 16 patients (61.5 %) had positive prior diagnosis of SARS-CoV-2 infection, corresponding 5 to HTR (42 %) and 11 to HC (78.5 %) groups (p = 0.013). For the HTR group, 2 of the infections occurred before completing the full vaccination regimen. (Table 1). All HTR were on immunosuppressive treatment at the time of infection and had undergone a complete excision of the thymus during surgery. However, anti-N antibodies were detected in only 8 participants (HTR = 2, and HC = 6), with no significant differences between groups. There was no difference in associated symptomatology between the two groups, as shown in Table 1. No patient required admission.
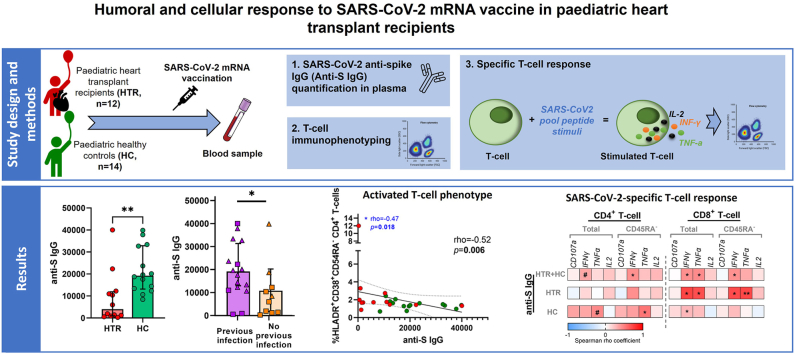
3.2. Paediatric heart transplant recipients showed lower levels of specific anti-S antibodies after vaccination compared to healthy controls
Anti-S IgG levels were significantly lower in HTR group compared to HC group after vaccination (Fig. 1 A). We found no significant correlations between age and anti-S IgG levels neither in the overall participant group nor segregating by studied groups (Supplementary Fig. 1). There are significant differences between the time since the last vaccine dose received and the follow-up sample between HTR and HC (Table 1).
Fig. 1.
Humoral and cellular specific SARS-CoV-2 response after vaccination. Bar graphs representing anti-S IgG levels after vaccination in paediatric and adolescent heart transplant recipients (red; n = 12) and healthy controls (green; n = 14) (A). Dot plots representing the percentage of SARS-CoV-2 specific T-cell response (left) and rate of responders (right) within total and memory (CD45RA−) CD4+ and CD8+ T-cell comparing heart transplant recipients and healthy controls after vaccination (B). Bar graphs representing anti-S IgG levels after vaccination in paediatric and adolescent participants with previous SARS-CoV-2 infection (purple; n = 16) and without previous infection (orange; n = 10) (C). Dot plots representing the percentage of SARS-CoV-2 specific T-cell response (left) and rate of responders (right) within total and memory (CD45RA−) CD4+ and CD8+ T-cell comparing participants with previous SARS-CoV-2 infection and without previous infection (D). For C and D HTR and HC have been represented by squares and triangles, respectively. Abbreviation: HTR, heart transplant recipients; HC, healthy controls. Continuous variables were compared using Mann-Whitney U test and rate of responders using X2 test. #0.05≤p-value<0.1; ∗p-value<0.05; ∗∗p-value<0.01. Only statistical p-value <0.1 is shown.
In addition to vaccine humoral response, we assayed SARS-CoV-2 specific T-cell response by intracellular cytokine staining. The response to SARS-CoV-2 peptides was determined by the expression of the degranulation surface marker CD107a+ and intracellular cytokines (IFN-γ+, TNF-α+ and IL-2+) in total and memory (CD45RA−) CD4+ and CD8+ T-cells. No significant differences were obtained in the magnitude of responses comparing HTR and HC groups (Supplementary Fig. 3A). In the analysis of the overall participants, there were no differences in the magnitude of the SARS-CoV-2-specific T-cell response nor in the proportion of responders (Fig. 1B). Among HTR, 9 (75 %) and 11 (92 %) had SARS-CoV-2 specific CD4+ and CD8+ T-cell response, respectively, while the rate of responders among HC was of 13 (93 %) and 12 (86 %) for SARS-CoV-2 specific CD4+ and CD8+ T-cells, respectively.
Trying to evaluate the influence of a previous SARS-CoV-2 infection on the humoral response after vaccination, participants with a infection by SARS-CoV-2 prior to study sample (n = 16) were compared with those uninfected (n = 10). Previously infected participants showed higher levels of anti-S IgG after vaccination (p = 0.03) (Fig. 1C) and increase SARS-CoV-2 specific T-cell response and rate of responders despite not reaching statistical significance (Fig. 1D). Comparing participants with prior infection or not by SARS-CoV-2, higher levels of IL-2 on CD8+ T-cells were observed on participants with previous infection (Supplementary Fig. 3B).
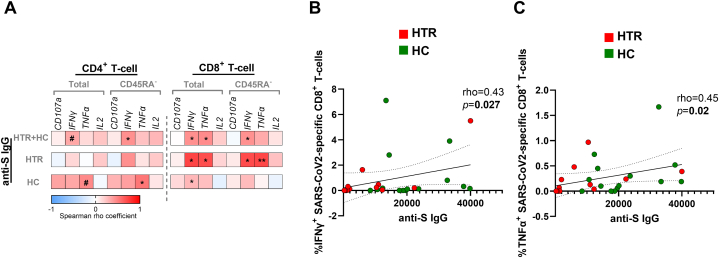
3.3. Higher SARS-CoV-2–specific IgG levels are associated with a higher SARS-CoV-2-specific T-cell response
Direct correlations were shown between anti-S IgG levels and SARS-CoV-2-specific IFN-γ+ and TNF-α+ production in total and memory CD8+ T-cells including all participants (Fig. 2A–C). In the same analysis segregated by HTR and HC, correlations remained significant only in the HTR group. No significant correlations were observed between SARS-CoV-2 cellular response and age in all participants or segregating by group (data not shown).
Fig. 2.
Association of SARS-CoV-2–specific anti-S IgG levels with T-cell response after vaccination. Correlation matrix presenting associations of anti-IgG levels with SARS-CoV-2-specific total and CD45RA− CD4+ and CD8+ T-cells expressing CD107a+ and producing the cytokines IFN-γ+, TNF-α+ and IL-2+ in all paediatric and adolescent participants (n = 26), heart transplant recipients (n = 12) and healthy controls (n = 14) (A). Dots plots representing correlations between anti-IgG levels and SARS-CoV-2-specific CD8+ T-cells producing IFN-γ+ (B) and TNF-α+ (C) in all participants. Abbreviations: HTR, heart transplant recipients; HC, healthy controls. The Spearman rho correlation coefficient test was used. #0.05≤p-value<0.1; ∗p-value<0.05; ∗∗p-value<0.01.
3.4. Activated T-cell phenotype found in heart transplant recipients is associated with a lower humoral response to SARS-CoV-2 vaccine
After describing SARS-CoV-2 response, we investigated the immune phenotype that might be involved in that specific response. HTR showed higher proportion of memory CD4+ T-cells (defined as CD45RA−) than HC, and the same trend was observed regarding CD8+ T-cell subset (Table 2). To determine the possible role of age on the memory and naïve T-cell distribution, correlations between age and frequency of CD45RA− CD4+ and CD8+ T-cells were performed. The frequency of CD45RA− CD4+ T-cells directly correlated with age when all participants were included; however, this significance was lost when the analysis was performed separately by groups (Supplementary Fig. 4A and B). Moreover, HTR showed increase proportion of activated CD4+ (HLA-DR+CD38+, HLA-DR+ and CD25+) and CD8+ (HLA-DR+CD38+ and HLA-DR+) T-cells; and a trend towards higher proportion of T regulatory cells, Tregs, (CD25+FoxP3+) was observed on CD4+ T-cells compared to HC (Table 2).
Table 2.
T-cell phenotyping of the studied groups.
| HTR (n = 12) | HC (n = 14) | p-value | |||
|---|---|---|---|---|---|
| CD4+T-cell | Total | %HLA-DR+CD38+ | 1.77 [1.19–2.04] | 1.31 [1.07–1.70] | 0.136 |
| %HLA-DR+ | 6.47 [3.51–7.5] | 3.25 [2.49–3.82] | 0.022 | ||
| %CD38+ | 25.7 [23.17–34.1] | 43.7 [36.1–49.5] | 0.010 | ||
| %CD25+ | 21.55 [18.45–33.5] | 11.2 [9.27–15] | 0.002 | ||
| %CD40L+ | 1.59 [0.61–2.98] | 1.15 [0.5–11.32] | 0.797 | ||
| %Treg | 1.1 [0.71–4.51] | 0.65 [0.39–1.11] | 0.088 | ||
| %CD45RA- | 77.3 [57.7–89.1] | 31 [25.25–35.8] | 0.001 | ||
| CD45RA- | %HLA-DR+CD38+ | 1.7 [1.43–2.55] | 1.39 [1.20–1.67] | 0.057 | |
| %HLA-DR+ | 6.31 [4.88–8.16] | 5.25 [4.34–5.49] | 0.324 | ||
| %CD38+ | 20.85 [12.65–26.12] | 19[14.12-23-67] | 0.758 | ||
| %CD25+ | 28.7 [23.55–36.37] | 20.9 [17.1–37.4] | 0.247 | ||
| %CD40L+ | 1.41 [0.24–2.58] | 0.94 [0.24–2.74] | 0.959 | ||
| %Treg | 1.27 [0.7–4.22] | 1.41 [0.97–2.19] | 0.818 | ||
| CD8+T-cell | Total | %HLA-DR+CD38+ | 3.95 [2.34–5.32] | 2.23 [1.32–3.16] | 0.007 |
| %HLA-DR+ | 7.08 [5.05–11.87] | 5.53 [3.76–6.24] | 0.045 | ||
| %CD38+ | 20.95 [13–28.1] | 15 [13.35–22.75] | 0.341 | ||
| %CD137+ | 1.75 [0.62–2.88] | 1.44 [1.16–1.76] | 0.537 | ||
| %CD45RA- | 55.4 [32.4–67.3] | 36.85 [22.87–42.57] | 0.060 | ||
| CD45RA- | %HLA-DR+CD38+ | 4.49 [2.64–7.48] | 2.58 [1.87–5.58] | 0.700 | |
| %HLA-DR+ | 8.48 [5.54–17.22] | 8.92 [6.42–12.42] | 0.643 | ||
| %CD38+ | 13.9 [8.44–18.42] | 11.85 [7.86–16.97] | 0.681 | ||
| %CD137+ | 2.25 [0.87–5.09] | 2.39 [1.79–3.23] | 0.877 |
Variables are expressed as medians and interquartile ranges [IQR]. Abbreviation: HTR, heart transplant recipients; HC, healthy controls. Mann-Whitney U test was used. P-value<0.05 are in bold and 0.05≤p-value<0.1 are in italics.
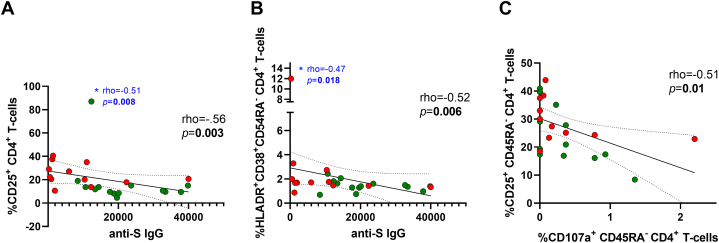
Thus, we studied if that activated phenotype could be associated with a SARS-CoV-2 vaccine response. Correlations between humoral and cellular SARS-CoV-2 specific response and T-cell phenotype markers are shown at Supplementary Fig. 4. Notably, anti-S IgG levels inversely correlated with CD25+ expression on total CD4+ T-cells and HLA-DR+CD38+ co-expression on CD4+ memory T-cells (Fig. 3A and B). Concerning SARS-CoV-2 specific response, the expression of degranulation marker, CD107a+, inversely correlated with the expression of CD25+ on CD4+ memory T-cells (Fig. 3C).
Fig. 3.
Association of SARS-CoV-2–specific anti-S IgG levels and SARS-CoV-2-specific T-cell degranulation with phenotype expression markers on T-cells after vaccination. Dot plots representing correlations between anti-IgG levels and CD25+ expression on total CD4+ T-cells (A) and HLA-DR+CD38+ co-expression on CD45RA− CD4+ T-cells (B). Dot plots representing correlation between SARS-CoV-2 specific degranulation marker CD107a+ on CD45RA− CD4+ T-cells and CD25+ expression on CD45RA− CD4+ T-cells (C) (n = 26). Correlations remained after excluding the outlier value (∗in blue) in each case (A–B). Heart transplant recipients are indicated in red and healthy controls in green. The Spearman rho correlation coefficient test was used.
4. Discussion
In this study, we described the anti-S IgG levels and SARS-CoV-2 specific T-cell response in a paediatric Spanish cohort of immunosuppressed HTR following SARS-CoV-2 mRNA vaccination compared to a reference group of paediatric HC that received the vaccines during the same period.
Most studies in immunocompromised adults concluded that immunocompromised patients respond less to the vaccine and therefore require one additional dose than healthy patients [15,17,21,22]. Spinner et al. [17] analysed the post-vaccination humoral response, with a maximum of two vaccine doses, in forty HTR with a median age of 17.1 years [IQR 15.7–18.4], detecting seroconversion in only 28 (70 %). In the case of the cohort described by Feingold et al. [20], 17 (61 %) adolescents and young adults HTR (median age of 17.9 years, [IQR 15.2–15.9]) had humoral response at a median of 3 months after second dose vaccination. In our study, 75 % of HTR had received 3 doses and all patients showed antibodies anti-S after vaccination. This difference in the number of vaccine doses might explain the discrepancy in the results and, also supports the current recommendations for immunocompromised paediatric patients that include the administration of three primary doses [18]. However, analysing the magnitude of the response, anti-S levels after vaccination were lower than HC, accordingly to the literature [33].
While this diminished humoral immune response to vaccination is well documented in immunocompromised populations, less is known regarding the effect of vaccination on the cellular arm of immunity. Impressively, the majority of participants in our study had a detectable cellular response after receiving the vaccine (75 % for CD4+ T-cell and 91.7 % for CD8+ T-cell mediated responses). This is consistent with some studies that have shown detectable cellular immune responses despite diminished vaccine induced humoral responses in immunocompromised children and adolescents [34,35]. It is worth noting that, while the humoral response is considered an important indicator of immunity in the short term, the cellular immune response plays a more critical role in evaluating the enduring protection against SARS-CoV-2 [36]. In a previous cohort [19] of 33 SOTR aged 5–17 years, the proportion of individuals with measurable cell-mediated immune responses after a second dose of mRNA vaccine was the 73.9 % and this rate significantly increased after the third dose to 94.1 %, similarly to our results. Despite the presence of adequate immune response, three paediatric HTR developed COVID-19 after vaccination while no infection was documented for the control group. Importantly, during infection after vaccination, all HTR had mild disease.
The correlation of humoral and cellular immunogenicity remains to be elucidated but it can be explained by the interconnected nature of the immune response. One previous report suggested that low antibody levels generally were associated with limited cellular immunogenicity in the general population [[37], [38], [39]]. This observation agrees with our results, in which there is a positive strong correlation between anti-S IgG levels and IFN-γ SARS-CoV-2 specific production.
Classically, immunocompromised patients have been described as being at higher risk of SARS-CoV-2 infection and having more severe clinical manifestations with fatal consequences [40]. In our study, the symptomatology after infection before vaccination was mild in all subjects, with no differences between the two groups studied, which suggests the effectiveness of the vaccine in the HTR group (Table 1). This observation agrees with the recent studies that described no severe infections despite the immunosuppressed status [7,23]. Interestingly, a history of SARS-CoV-2 infection prior to vaccination has an impact on the robustness of the immune response among SOTR. Lemieux et al. [41] demonstrated that adult HTR with a previous infection showed an immune response to vaccine doses comparable to that of healthy controls. Our results coincide with it.
Thymic function has an important implication in viral infections, including COVID-19 disease [42,43]. Paediatric heart transplantation involves not only ongoing maintenance immunosuppression but also total thymus excision during the intervention. Decreased thymic function has been related to the phenomenon called memory inflation, which includes the alteration of the naive and memory T-cell proportions in the periphery skewing toward memory T-cells [44]. This phenomenon could be reflected in CD4+ and CD8+ T-cell memory subset distribution of HTR group, which displayed higher percentages of memory T-cells compared to HC. Due to the use of Tacrolimus, we expected lower T-cell activation in HTR [45], contrary to our results. Thymic dysfunction in elderly subjects had related defects in the homeostasis of T-cell compartment that include higher T-cell activation, proliferation and memory inflation [46]. Interestingly, we found that similar T-cell profile in the HTR group and it correlated with a lower SARS-CoV-2-specific CD4+ T-cell response in agreement with previous observations [46,47], where a lower activity of the thymus has been previously associated with diminished responsiveness to viral vaccines. In our study, we worked with children who have undergone total thymectomy which may also result in altered or nullifying thymic function.
The study limitations include that it is only a two-center study, a small size and a variety of immunosuppressive medication regimens (tacrolimus, used in 91.7 % of cases) and vaccine doses that precluded further meaningful subgroup analysis regarding the effects of the underlying diagnosis and types of immunosuppressive medications on the response to the vaccine. The study analysed binding but not neutralizing antibodies, but previous data have shown that the information provided by both determinations is highly similar and follows a similar pattern [48,49] and our definition of responders may be limited by the fact that our threshold for defining a positive T-cell response was based on the distribution of T-cell frequencies in the unstimulated control conditions as previously described [31]. It remains to be determined for both antibodies and T-cells, whether the chosen thresholds represent clinically relevant indicators for protection. It is worth noting that this study concentrated on a particular epidemiological period (when the Alpha and Delta variants were predominant). A crucial question for future pandemic control is whether mRNA vaccines tailored to new variants or other vaccine types can elicit a humoral and cellular response to manage the new variants effectively. The variability found in the response rate to SARS-CoV-2 vaccine among paediatric HTR could be, in part, explained to the different time interval from the latest dose of the vaccine to the time of testing. The time from last dose of vaccine to follow-up sample collection was different between groups. However, it is interesting to note that previous studies on paediatric HTR showed no differences in the magnitude of humoral and cellular vaccine response by immunosuppressant regimen and other modifying factors such as age at vaccination or time since transplant [19,20]. We observed a lack of concordance between the detection of anti-N IgG and the previous positive diagnosis of SARS-CoV-2 infection. This loss of anti-N IgG may be attributed to the extended period since the onset of the pandemic and vaccination in this population (between December 2021 and May 2022) [50]. Furthermore, since only 5 participants in the HTR group had a previous infection, the analysis of variables associated with prior infection was conducted considering all participants and was not performed separately for each group due to low statistical power. As a strength of our study, we comprehensively assessed both the humoral immune response and the cellular immune response that included both CD4+ and CD8+ T-cells and their cytokine profiles although in paediatric HTR may be affected by a heightened immune surveillance state, potentially related to anti-engraftment responses. The inclusion of a control group can be considered an additional asset to provide comparative data on immunogenicity of immunocompetent children and adolescents in a real-world setting. In addition, the study was conducted in an epidemic period with high SARS-CoV-2 virus circulation, allowing us to test vaccine efficacy more accurately.
5. Conclusions
All paediatric heart transplant recipients had humoral response after SARS-CoV-2 vaccination, although they showed lower levels of specific anti-S antibodies compared to healthy controls. However, there was no significant differences in the cellular response to the vaccine produced between the two groups. Therefore, we believe that more studies analysing the cellular response in the immunocompromised paediatric population are needed because these data might impact the currently established vaccination regimens.
CRediT authorship contribution statement
Amanda Bermejo-Gómez: Writing – original draft, Visualization, Investigation, Formal analysis, Conceptualization. Laura Tarancon-Diez: Writing – original draft, Visualization, Investigation, Formal analysis, Conceptualization. Beatriz Lazaro-Martin: Writing – review & editing, Resources, Data curation. Begoña Santiago-Garcia: Writing – review & editing, Resources. Nuria Gil Villanueva: Writing – review & editing, Resources. Roberto Alonso: Writing – review & editing, Resources, Investigation. Mª Ángeles Muñoz-Fernández: Writing – review & editing, Resources. Manuela Camino López: Writing – review & editing, Resources. Alicia Hernanz-Lobo: Supervision, Project administration, Funding acquisition, Conceptualization. María Luisa Navarro Gómez: Supervision, Project administration, Funding acquisition, Conceptualization.
Ethics statement
This study was approved by the Ethics Committee of the Gregorio Marañón University Hospital, Madrid, Spain (acta 21/2021) and the Ethics Committee of the Móstoles University Hospital, Madrid, Spain (CEIC 2022/016).
Data availability statement
Data will be made available on request.
Fundings
This work was supported by the Centro de Investigación Biomédica en Red de Enfermedades Infecciosas-ISCIII (CIBERINFEC) [CB21/13/00077] y de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN) [CB22/01/00041], Madrid, Spain. AB-G is funded by Instituto de investigación Sanitaria Gregorio Marañon (IiSGM) through the “Programa intramural de impulse a la I + D + i 2021 Contratos Post Formación Sanitaria Especializada”. LT-D is supported by the Instituto de Salud Carlos III (ISCIII) under grant agreement “CP23/00009” through the Miguel Servet Program, by GeSIDA through the “IV Premio para Jóvenes Investigadores 2021”, by Small Grant Award from ESPID and “Proyectos de I+D+i Programa Intramural IiSGM” (2023-II-PI-NOV-01). AH-L is funded by the Spanish Ministry of Science and Innovation-Instituto de Salud Carlos III and Fondos FEDER (Contrato Río Hortega CM20/00128). MNG was funded by Sociedad de Infectología Pediátrica through the “Beca de investigación José María Corretger 2020, X Congreso nacional SEIP”.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank HIV-HGM BioBank for the sample processing and storage and all patients and healthy volunteers, as well as their families for their participation in this study. We particularly acknowledge Laura Díaz, of the Flow Cytometry Unit from Instituto de Investigación Sanitaria Gregorio Marañón, for her technical assistance as flow cytometry technician.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e41584.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Velleca A., Shullo M.A., Dhital K., Azeka E., Colvin M., DePasquale E., Farrero M., García-Guereta L., Jamero G., Khush K., Lavee J., Pouch S., Patel J., Michaud C., Shullo M.A., Schubert S., Angelini A., Carlos L., Mirabet S., Patel J., Pham M., Urschel S., Kim K.-H., Miyamoto S., Chih S., Daly K., Grossi P., Jennings D.L., Kim I., Lim H.S., Miller T., Potena L., Velleca A., Eisen H., Bellumkonda L., Danziger-Isakov L., Dobbels F., Harkess M., Kim D., Lyster H., Peled Y., Reinhardt Z. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023;42:e1–e141. doi: 10.1016/j.healun.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Danziger-Isakov L., Blumberg E.A., Manuel O., Sester M. Impact of COVID-19 in solid organ transplant recipients. Am. J. Transplant. 2021;21:925–937. doi: 10.1111/ajt.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal N., Ovchinsky N., Foca M., Lamour J.M., Kogan‐Liberman D., Hsu D.T., Beddows K., Abraham L., Coburn M., Cunningham R., Nguyen T., Hayde N. COVID‐19 infection in pediatric solid organ transplant patients. Pediatr. Transplant. 2022;26 doi: 10.1111/petr.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry Z.S., Williams J.D., Vahia A., Fadel R., Parraga Acosta T., Prashar R., Shrivastava P., Khoury N., Pinto Corrales J., Williams C., Nagai S., Abouljoud M., Samaniego-Picota M., Abreu-Lanfranco O., Del Busto R., Ramesh M.S., Patel A., Alangaden G.J. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am. J. Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschopp J., L'Huillier A.G., Mombelli M., Mueller N.J., Khanna N., Garzoni C., Meloni D., Papadimitriou-Olivgeris M., Neofytos D., Hirsch H.H., Schuurmans M.M., Müller T., Berney T., Steiger J., Pascual M., Manuel O., Van Delden C. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am. J. Transplant. 2020;20:2876–2882. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhvi A., Barghash M., Lala A., Mitter S.S., Parikh A., Oliveros E., Rollins B.M., Brunjes D.L., Alvarez-Garcia J., Johnston E., Ryan K., Itagaki S., Moss N., Pinney S.P., Anyanwu A., Mancini D. Challenges in heart transplantation during COVID-19: a single-center experience. J. Heart Lung Transplant. 2020;39:894–903. doi: 10.1016/j.healun.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goss M.B., Galván N.T.N., Ruan W., Munoz F.M., Brewer E.D., O'Mahony C.A., Melicoff‐Portillo E., Dreyer W.J., Miloh T.A., Cigarroa F.G., Ranch D., Yoeli D., Adams M.A., Koohmaraie S., Harter D.M., Rana A., Cotton R.T., Carter B., Patel S., Moreno N.F., Leung D.H., Goss J.A. The pediatric solid organ transplant experience with COVID‐19: an initial multi‐center, multi‐organ case series. Pediatr. Transplant. 2021;25 doi: 10.1111/petr.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talgam-Horshi E., Mozer-Glassberg Y., Waisbourd-Zinman O., Ashkenazi-Hoffnung L., Haskin O., Levi S., Hamdani G., Landau D., Alfandary H. Clinical outcomes and antibody response in COVID-19–positive pediatric solid organ transplant recipients, pediatric infectious disease. Journal. 2021;40:e514–e516. doi: 10.1097/INF.0000000000003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration COVID-19 vaccines authorized for emergency use or FDA-approved. Silver spring, MD: US department of health and human services. Food and Drug Administration. 2021 https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines n.d. [Google Scholar]
- 10.Mbaeyi S., Oliver S.E., Collins J.P., Godfrey M., Goswami N.D., Hadler S.C., Jones J., Moline H., Moulia D., Reddy S., Schmit K., Wallace M., Chamberland M., Campos-Outcalt D., Morgan R.L., Bell B.P., Brooks O., Kotton C., Talbot H.K., Lee G., Daley M.F., Dooling K. The advisory committee on immunization practices' interim recommendations for additional primary and booster doses of COVID-19 vaccines — United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv M., Luo X., Shen Q., Lei R., Liu X., Liu E., Li Q., Chen Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: a systematic review. Vaccines. 2021;9:1102. doi: 10.3390/vaccines9101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R., Swanson K.A., Ma H., Xu X., Koury K., Kalina W.V., Cooper D., Jennings T., Brandon D.M., Thomas S.J., Türeci Ö., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., Safety Immunogenicity. Efficacy of the BNT162b2 covid-19 vaccine in adolescents. N. Engl. J. Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., Barnett E.D., Muñoz F.M., Maldonado Y., Pahud B.A., Domachowske J.B., Simões E.A.F., Sarwar U.N., Kitchin N., Cunliffe L., Rojo P., Kuchar E., Rämet M., Munjal I., Perez J.L., Frenck R.W., Lagkadinou E., Swanson K.A., Ma H., Xu X., Koury K., Mather S., Belanger T.J., Cooper D., Türeci Ö., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Evaluation of the BNT162b2 covid-19 vaccine in children 5 to 11 Years of age. N. Engl. J. Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hause A.M., Baggs J., Marquez P., Myers T.R., Gee J., Su J.R., Zhang B., Thompson D., Shimabukuro T.T., Shay D.K. COVID-19 vaccine safety in children aged 5–11 Years — United States, November 3–December 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1755–1760. doi: 10.15585/mmwr.mm705152a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haskin O., Ashkenazi-Hoffnung L., Ziv N., Borovitz Y., Dagan A., Levi S., Koren G., Hamdani G., Levi-Erez D., Landau D., Alfandary H. Serological response to the BNT162b2 COVID-19 mRNA vaccine in adolescent and young adult kidney transplant recipients. Transplantation. 2021;105:e226–e233. doi: 10.1097/TP.0000000000003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamez A.-C., Pradier A., Giannotti F., Petitpas A., Urdiola M.F., Vu D.-L., Masouridi-Levrat S., Morin S., Dantin C., Clerc-Renaud D., Eberhardt C.S., Kaiser L., Simonetta F., Chalandon Y. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56:3094–3096. doi: 10.1038/s41409-021-01466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinner J.A., Julien C.L., Olayinka L., Dreyer W.J., Bocchini C.E., Munoz F.M., Devaraj S. SARS-CoV-2 anti–spike antibodies after vaccination in pediatric heart transplantation: a first report. J. Heart Lung Transplant. 2022;41:133–136. doi: 10.1016/j.healun.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulek D.E., Ardura M.I., Green M., Michaels M.G., Chaudhuri A., Vasquez L., Danziger‐Isakov L., Posfay‐Barbe K.M., McCulloch M.I., L'Huillier A.G., Benden C. Update on COVID‐19 vaccination in pediatric solid organ transplant recipients. Pediatr. Transplant. 2022;26 doi: 10.1111/petr.14235. [DOI] [PubMed] [Google Scholar]
- 19.Bratic J.S., Gans H.A., Chen S.F., Banaei N., Johnston E.M., Sear K., Samreth S., Nadimpalli S.S. Pediatric solid organ transplant recipients demonstrate robust cell-mediated and humoral responses to three doses of mRNA SARS-CoV-2 vaccine. Am. J. Transplant. 2022;22:3047–3052. doi: 10.1111/ajt.17195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feingold B., Berman P., Moninger A., Huston A., Stinner B., West S.C., Rose‐Felker K., Zinn M.D., Miller S.A., Michaels M.G. Responsiveness to second and third dose of mRNA COVID‐19 vaccination in adolescent and young adult heart transplant recipients. Pediatr. Transplant. 2022;26 doi: 10.1111/petr.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., Wehmeyer M., Jahnke-Triankowski J., Mayer L., Hoffmann A., Fischer L., Addo M.M., Lütgehetmann M., Lohse A.W., Schulze Zur Wiesch J., Sterneck M. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin. Gastroenterol. Hepatol. 2022;20:162–172.e9. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udaondo C., Cámara C., Miguel Berenguel L., Alcobendas Rueda R., Muñoz Gómez C., Millán Longo C., Díaz – Delgado B., Falces-Romero I., Díaz Almirón M., Ochando J., Méndez – Echevarría A., Remesal Camba A., Calvo C. Humoral and cellular immune response to mRNA SARS-CoV-2 BNT162b2 vaccine in adolescents with rheumatic diseases. Pediatr. Rheumatol. 2022;20:64. doi: 10.1186/s12969-022-00724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahrs C., Harrison N. Vaccine response in the immunocompromised patient with focus on cellular immunity. Vaccines. 2022;10:882. doi: 10.3390/vaccines10060882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paramithiotis E., Sugden S., Papp E., Bonhomme M., Chermak T., Crawford S.Y., Demetriades S.Z., Galdos G., Lambert B.L., Mattison J., McDade T., Pillet S., Murphy R. Cellular immunity is critical for assessing COVID-19 vaccine effectiveness in immunocompromised individuals. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.880784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahil S.K., Bechman K., Raharja A., Domingo-Vila C., Baudry D., Brown M.A., Cope A.P., Dasandi T., Graham C., Lechmere T., Malim M.H., Meynell F., Pollock E., Seow J., Sychowska K., Barker J.N., Norton S., Galloway J.B., Doores K.J., Tree T.I.M., Smith C.H. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. The Lancet Rheumatology. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassy Navarro S., Gil Villanueva N., Camino-López M., Alonso Fernández R., Lázaro-Martín B., Hernanz-Lobo A., Navarro Gómez M. Importance of optimizing routine immunization schedules in pediatric heart transplantation. Vacunas. 2024 doi: 10.1016/j.vacun.2024.07.002. S1576988724000712. [DOI] [Google Scholar]
- 29.Irene C., Elba M., Jiménez J.L., Mellado M.J., Muñoz-Fernández M.Á. HIV HGM biobank as a research platform for paediatric infectious diseases and COVID-19 pandemic. AIDS Res. Ther. 2022;19:22. doi: 10.1186/s12981-022-00448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranjbaran H., Ehteshaminia Y., Nadernezhad M., Jalali S.F., Jadidi-Niaragh F., Pagheh A.S., Enderami S.E., Kenari S.A., Hassannia H. Comparison of neutralization potency across passive immunotherapy approaches as potential treatments for emerging infectious diseases. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2023.e23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrando-Martínez S., Casazza J.P., Leal M., Machmach K., Muñoz-Fernández M.Á., Viciana P., Koup R.A., Ruiz-Mateos E. Differential gag-specific polyfunctional T cell maturation patterns in HIV-1 elite controllers. J. Virol. 2012;86:3667–3674. doi: 10.1128/JVI.07034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pernas M., Tarancón-Diez L., Rodríguez-Gallego E., Gómez J., Prado J.G., Casado C., Dominguez-Molina B., Olivares I., Coiras M., León A., Rodriguez C., Benito J.M., Rallón N., Plana M., Martinez-Madrid O., Dapena M., Iribarren J.A., Del Romero J., García F., Alcamí J., Muñoz-Fernández M., Vidal F., Leal M., Lopez-Galindez C., Ruiz-Mateos E. Factors leading to the loss of natural elite control of HIV-1 infection. J. Virol. 2018;92 doi: 10.1128/JVI.01805-17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., Demissie E.G., El-Qunni A.A., Haile A., Huang K., Kinnett B., Liebeskind M.J., Liu Z., McMorrow L.E., Paez D., Pawar N., Perantie D.C., Schriefer R.E., Sides S.E., Thapa M., Gergely M., Abushamma S., Akuse S., Klebert M., Mitchell L., Nix D., Graf J., Taylor K.E., Chahin S., Ciorba M.A., Katz P., Matloubian M., O'Halloran J.A., Presti R.M., Wu G.F., Whelan S.P.J., Buchser W.J., Gensler L.S., Nakamura M.C., Ellebedy A.H., Kim A.H.J. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann. Intern. Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgans H.A., Bradley T., Flebbe-Rehwaldt L., Selvarangan R., Bagherian A., Barnes A.P., Bass J., Cooper A.M., Fischer R., Kleiboeker S., Lee B.R., LeMaster C., Markus K., Morrison S., Myers A., Myers D., Payne E., Schuster J.E., Standley S., Wieser A., Warady B. Humoral and cellular response to the COVID-19 vaccine in immunocompromised children. Pediatr. Res. 2023;94:200–205. doi: 10.1038/s41390-022-02374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane C., Phebus E., Ingulli E. Immunologic response of mRNA SARS-CoV-2 vaccination in adolescent kidney transplant recipients. Pediatr. Nephrol. 2022;37:449–453. doi: 10.1007/s00467-021-05256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt T., Klemis V., Schub D., Schneitler S., Reichert M.C., Wilkens H., Sester U., Sester M., Mihm J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021;21:3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tychala A., Meletis G., Katsimpourlia E., Gkeka I., Dimitriadou R., Sidiropoulou E., Skoura L. Evaluation of the QuantiFERON SARS-CoV-2 assay to assess cellular immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in individuals with low and high humoral response. Hum. Vaccines Immunother. 2021;17:5148–5149. doi: 10.1080/21645515.2021.1991710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Picchianti-Diamanti A., Aiello A., Laganà B., Agrati C., Castilletti C., Meschi S., Farroni C., Lapa D., Najafi Fard S., Cuzzi G., Cimini E., Grassi G., Vanini V., Di Rosa R., Salemi S., Nalli G., Salmi A., Repele F., Altera A.M.G., Maffongelli G., Palazzolo C., Vita S., Leone S., Puro V., Capobianchi M.R., Ippolito G., Nicastri E., Goletti D. ImmunosuppressiveTherapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers E.S., Cai W., Vivaldi G., Jolliffe D.A., Perdek N., Li W., Faustini S.E., Gibbons J.M., Pade C., Richter A.G., Coussens A.K., Martineau A.R. Influence of individuals' determinants including vaccine type on cellular and humoral responses to SARS-CoV-2 vaccination. Npj Vaccines. 2024;9:87. doi: 10.1038/s41541-024-00878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amodio D., Ruggiero A., Sgrulletti M., Pighi C., Cotugno N., Medri C., Morrocchi E., Colagrossi L., Russo C., Zaffina S., Di Matteo G., Cifaldi C., Di Cesare S., Rivalta B., Pacillo L., Santilli V., Giancotta C., Manno E.C., Ciofi Degli Atti M., Raponi M., Rossi P., Finocchi A., Cancrini C., Perno C.F., Moschese V., Palma P. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemieux J.E., Li A., Gentili M., Perugino C.A., Weiss Z.F., Bowman K., Ankomah P., Liu H., Lewis G.D., Bitar N., Lipiner T., Hacohen N., Pillai S.S., Goldberg M.B. Vaccine serologic responses among transplant patients associate with COVID-19 infection and T peripheral helper cells. Infectious Diseases (except HIV/AIDS) 2021 doi: 10.1101/2021.07.11.21260338. [DOI] [Google Scholar]
- 42.Genebat M., Tarancón-Díez L., de Pablo-Bernal R., Calderón A., Muñoz-Fernández M.Á., Leal M. Coronavirus disease (COVID-19): a perspective from immunosenescence. Aging and Disease. 2021;12:3. doi: 10.14336/AD.2020.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrando-Martinez S., De Pablo-Bernal R.S., De Luna-Romero M., De Ory S.J., Genebat M., Pacheco Y.M., Parras F.J., Montero M., Blanco J.R., Gutierrez F., Santos J., Vidal F., Koup R.A., Muñoz-Fernández M.Á., Leal M., Ruiz-Mateos E. Thymic function failure is associated with human immunodeficiency virus disease progression. Clin. Infect. Dis. 2017;64:1191–1197. doi: 10.1093/cid/cix095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klenerman P. The (gradual) rise of memory inflation. Immunol. Rev. 2018;283:99–112. doi: 10.1111/imr.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vafadari R., Kraaijeveld R., Weimar W., Baan C.C. Tacrolimus inhibits NF-κB activation in peripheral human T cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitallé J., Pérez-Gómez A., Ostos F.J., Gasca-Capote C., Jiménez-León M.R., Bachiller S., Rivas-Jeremías I., Silva-Sánchez M. del M., Ruiz-Mateos A.M., Martín-Sánchez M.Á., López-Cortes L.F., Rafii-El-Idrissi Benhnia M., Ruiz-Mateos E. Immune defects associated with lower SARS-CoV-2 BNT162b2 mRNA vaccine response in aged people. JCI Insight. 2022;7 doi: 10.1172/jci.insight.161045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sereti I., Herpin B., Metcalf J.A., Stevens R., Baseler M.W., Hallahan C.W., Kovacs J.A., Davey R.T., Lane H.C. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. AIDS. 2001;15:1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 48.Alonso R., Gil-Manso S., Catalán P., Sánchez-Arcilla I., Marzola M., Correa-Rocha R., Muñoz P., Pion M. The Gregorio Marañón Microbiology-ID COVID-19 Study Group, Neutralizing antibody levels detected early after mRNA-based vaccination do not predict by themselves subsequent breakthrough infections of SARS-CoV-2. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1341313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogolari M., Leoni B.D., De Cesaris M., Italiano R., Davini F., Miccoli G.A., Donati D., Clerico L., Stanziale A., Savini G., Petrosillo N., Ciccozzi M., Sommella L., Riva E., Fazii P., Angeletti S. Neutralizing antibodies against SARS-CoV-2 beta and omicron variants inhibition comparison after BNT162b2 mRNA booster doses with a new PETIA sVNT assay. Diagnostics. 2023;13:889. doi: 10.3390/diagnostics13050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Elslande J., Oyaert M., Ailliet S., Van Ranst M., Lorent N., Vande Weygaerde Y., André E., Lagrou K., Vandendriessche S., Vermeersch P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021;136 doi: 10.1016/j.jcv.2021.104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.