Abstract
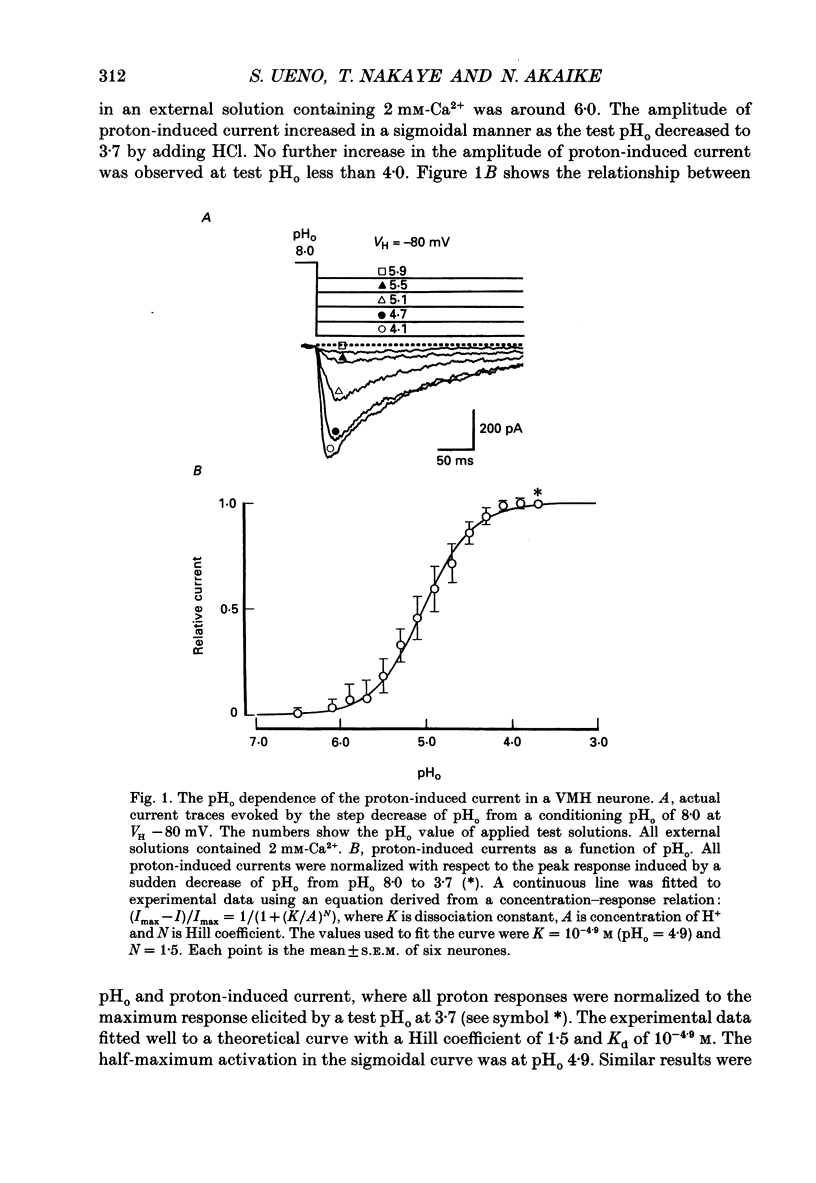
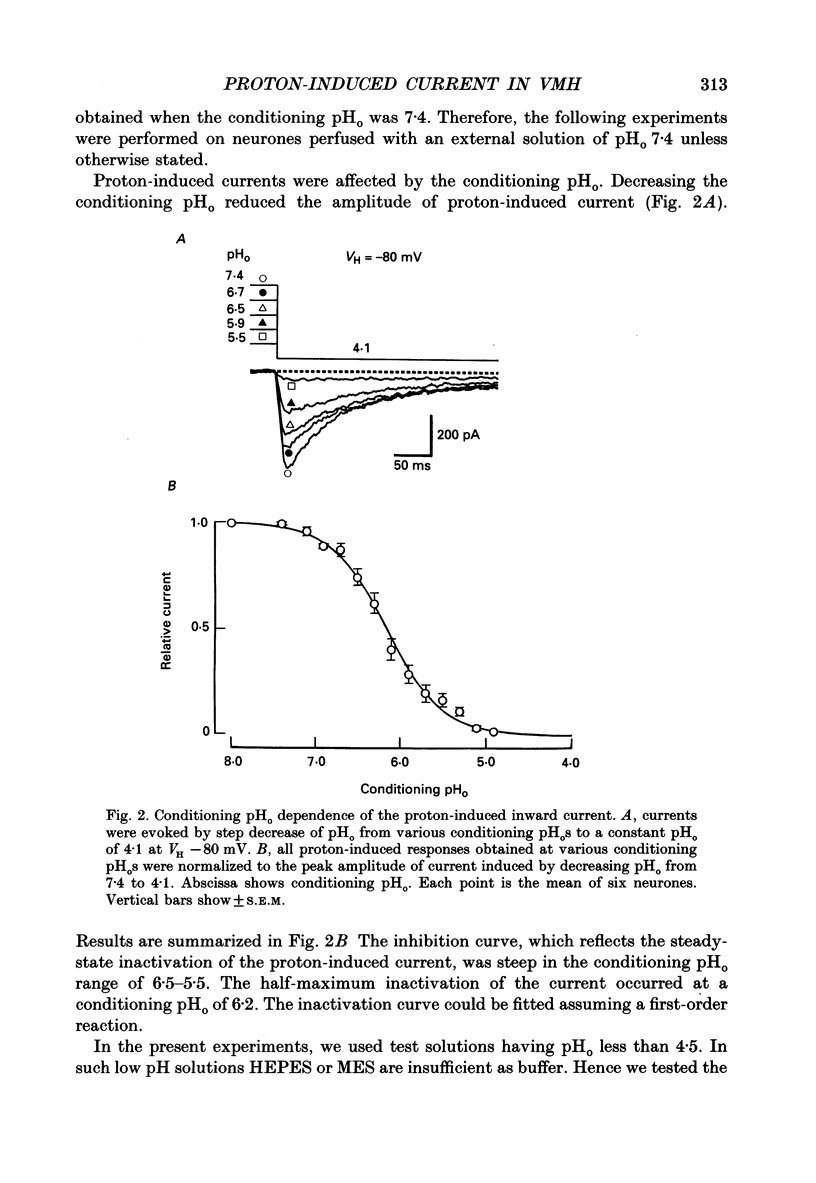
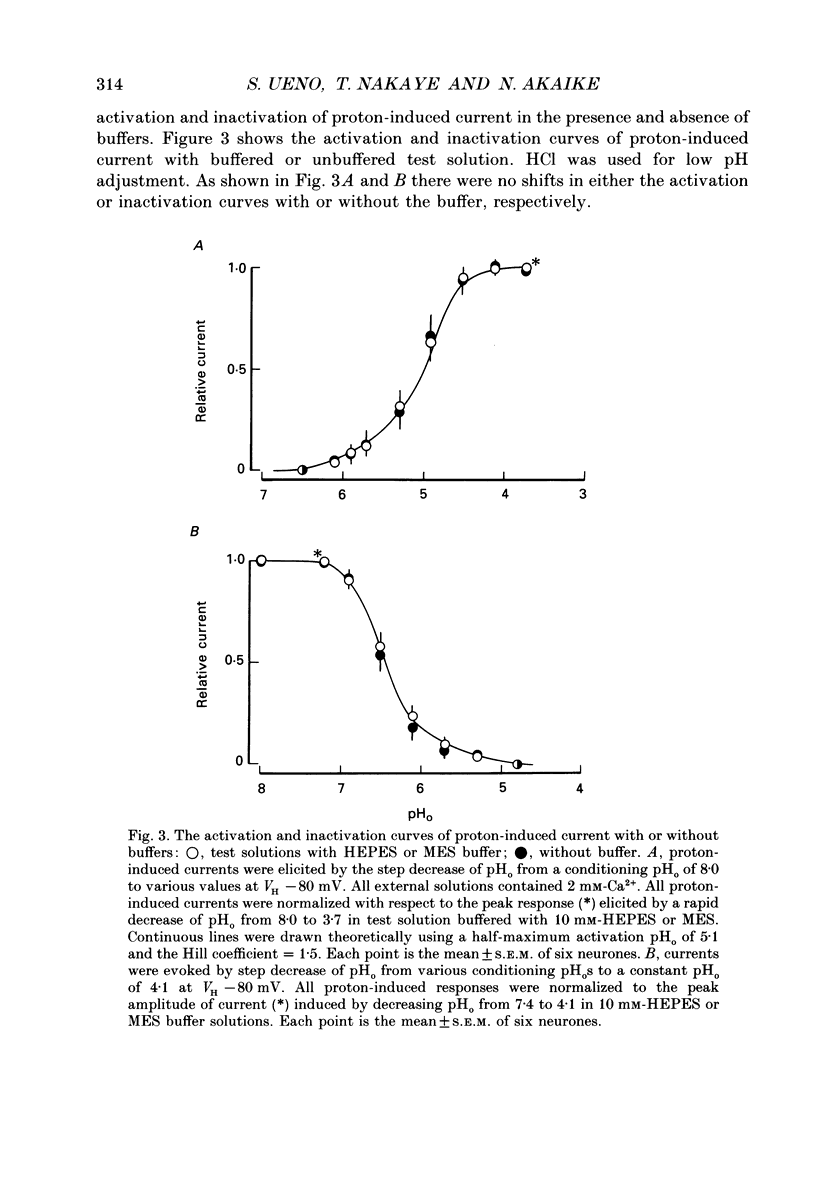
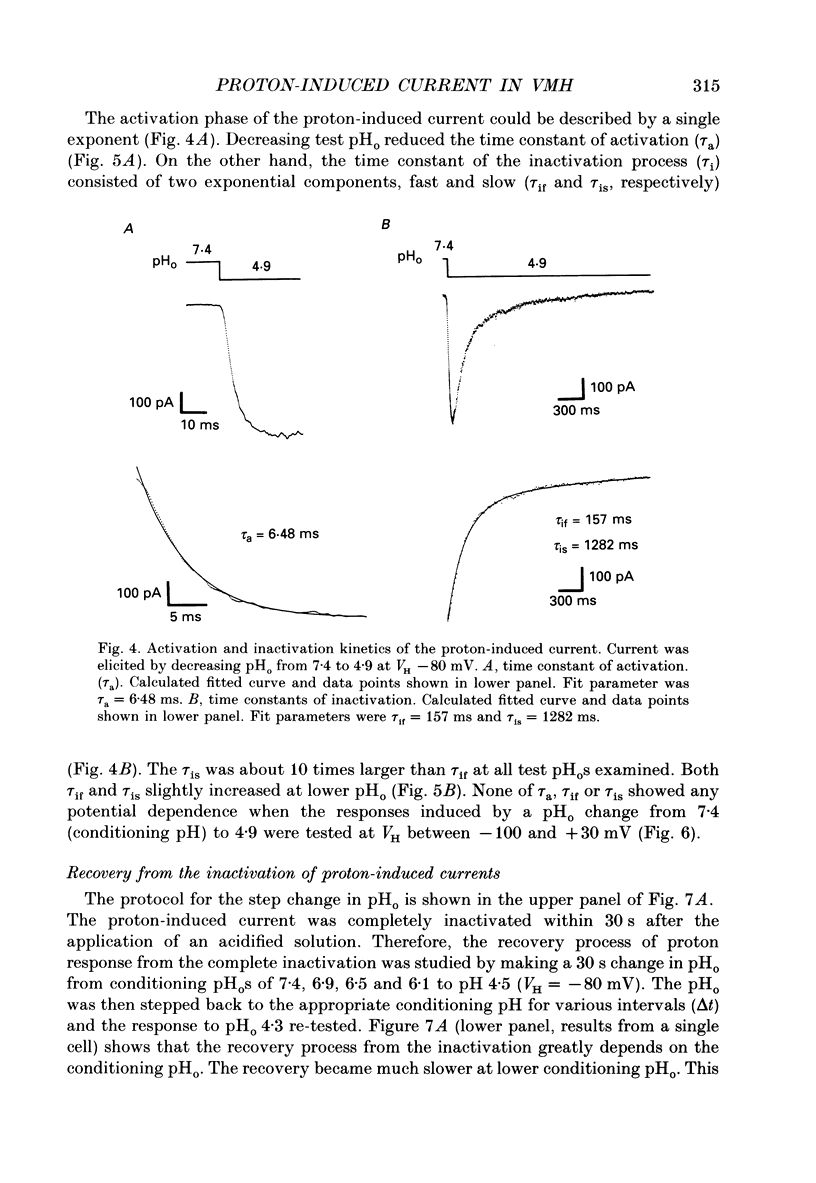
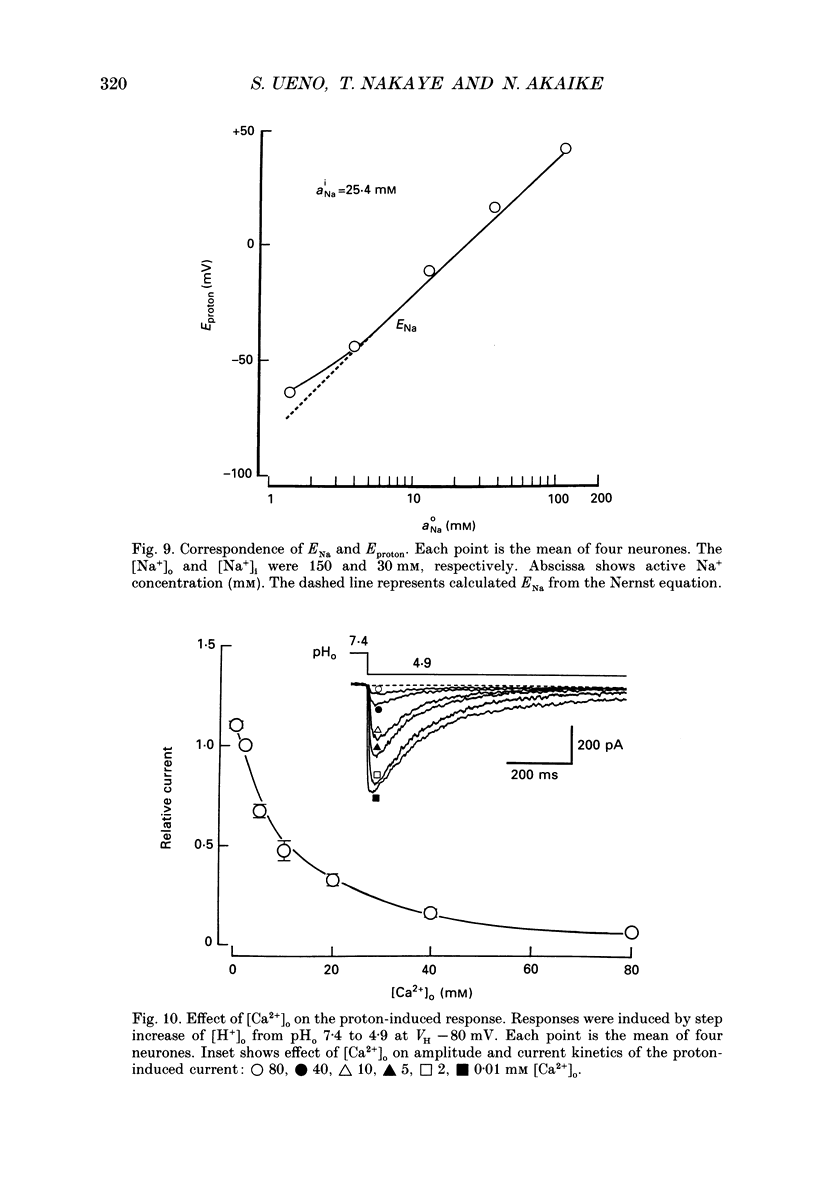
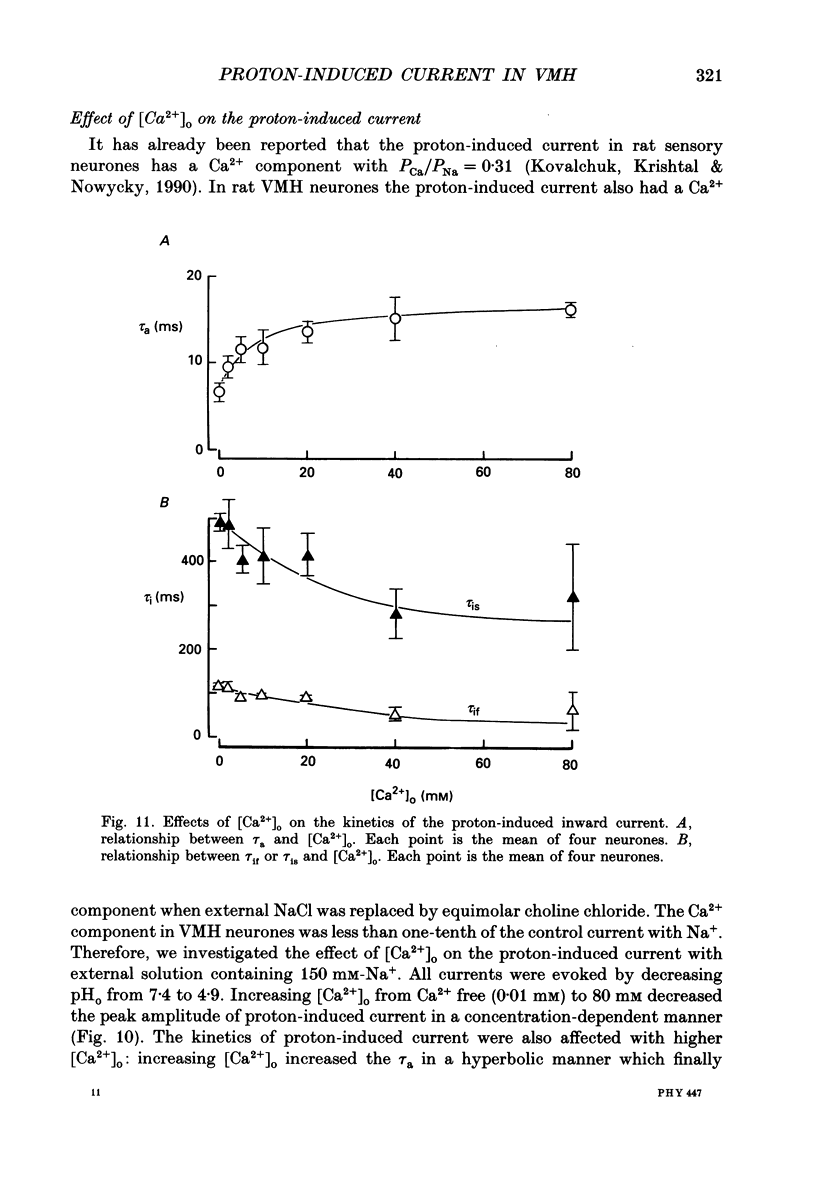
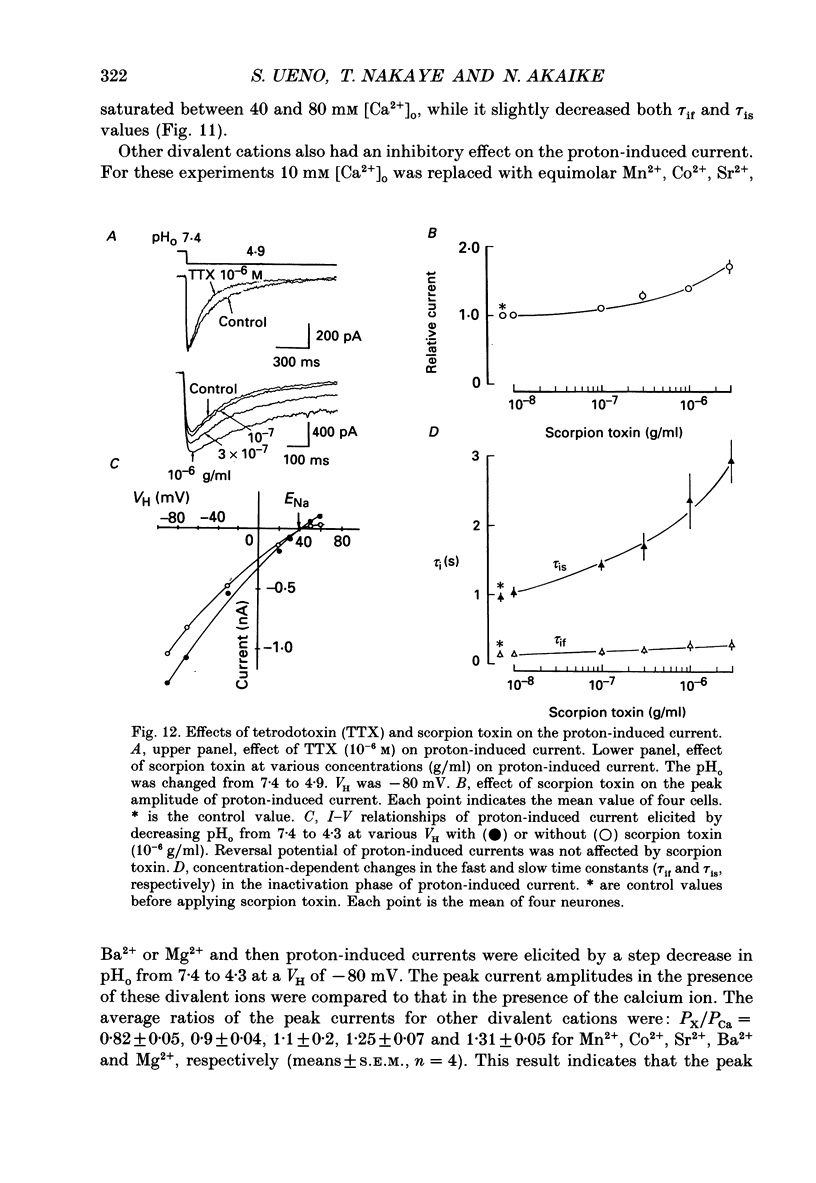
1. The proton-gated current was investigated in freshly dissociated ventromedial hypothalamic (VMH) neurones from 4-week-old Wistar rats, under whole-cell configuration by the use of the 'concentration-clamp' technique which combines intracellular perfusion with the rapid exchange of external solution within 1-2 ms under a single-electrode voltage-clamp condition. 2. The proton-gated current increased in a sigmoidal fashion as extracellular pH (pHo) decreased. In external solution containing 2 mM-Ca2+, the threshold of current activation was at pHo 6.5, and the maximum response appeared at pHo 4.1-3.9. The dissociation constant (Kd) and Hill coefficient were 10(-4.9) M (pHo = 4.9) and 1.5 respectively. 3. Decreasing extracellular Na+ concentration reduced the proton-gated current. The current reversed direction at the Na+ equilibrium potential (ENa), indicating that it was carried by Na+. 4. The activation phase kinetics of proton-induced current was single exponential. The time constant of activation (tau a) did not have a potential dependence but decreased slightly by decreasing pHo. The inactivation phase kinetics was two-exponential. The time constant of inactivation (tau i) consisted of fast and slow components (tau if and tau is, respectively). Like tau a, both tau if and tau is did not have any potential dependence, but they slightly increased with decreasing pHo. 5. The steady-state inactivation curve, constructed by decreasing pHo from various conditioning pHos to 4.1, revealed that the proton-induced current had a half-maximum inactivation at pHo 6.2. 6. The proton-induced current was suppressed as the extracellular Ca2+ concentration ([Ca2+]o) increased from almost free (0.01 mM) to 80 mM. Increasing [Ca2+]o increased tau a, but slightly decreased both tau if and tau is. 7. Recovery of proton-induced current from complete inactivation of proton-induced current depended on the degree of pHo change. A bigger change in pHo induced faster recovery than a smaller change. 8. External divalent cations inhibited the proton-induced current, and the inhibitory potency was in the order of Mn2+ greater than Co2+ greater than Ca2+ greater than Sr2+ greater than Ba2+ greater than Mg2+. 9. Tetrodotoxin (TTX) at relatively low concentration (less than 10(-7) M) did not inhibit the peak amplitude of the proton-induced current, but at a higher concentration (10(-6) M) it slightly inhibited the peak amplitude of the current and accelerated the inactivation process. Scorpion toxin markedly increased the peak amplitude of the proton-induced current and prolonged the inactivation phase. The tau is was also increased by scorpion toxin in a concentration-dependent manner. Veratridine had no effect on the proton-induced current. 10. The membrane properties of the proton-operated channel were similar to those of the voltage-gated Na+ channel rather than the Ca2+ channel.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Kiyohara T. The K+-sensitive hyperpolarization of rat skeletal muscle and metabolic inhibitors. Jpn J Physiol. 1981;31(2):169–179. doi: 10.2170/jjphysiol.31.169. [DOI] [PubMed] [Google Scholar]
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Krishtal O. A., Maruyama T. Proton-induced sodium current in frog isolated dorsal root ganglion cells. J Neurophysiol. 1990 Apr;63(4):805–813. doi: 10.1152/jn.1990.63.4.805. [DOI] [PubMed] [Google Scholar]
- Akaike N., Krishtal O. A., Maruyama T. Proton-induced sodium current in frog isolated dorsal root ganglion cells. J Neurophysiol. 1990 Apr;63(4):805–813. doi: 10.1152/jn.1990.63.4.805. [DOI] [PubMed] [Google Scholar]
- BRINK F. The role of calcium ions in neural processes. Pharmacol Rev. 1954 Sep;6(3):243–298. [PubMed] [Google Scholar]
- Balestrino M., Somjen G. G. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988 Feb;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Danko M. Hydrogen ion block of the sodium pore in squid giant axons. J Gen Physiol. 1983 Nov;82(5):599–618. doi: 10.1085/jgp.82.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. Do protons block Na+ channels by binding to a site outside the pore? Nature. 1982 Jul 8;298(5870):165–167. doi: 10.1038/298165a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg P., Patterson L., Purves M. J. The pH of brain extracellular fluid in the cat. J Physiol. 1977 Oct;272(1):137–166. doi: 10.1113/jphysiol.1977.sp012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R., Lux H. D. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neurosci Lett. 1988 Jun 29;89(2):198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Rectification of single GABA-gated chloride channels in adult hippocampal neurons. J Neurophysiol. 1985 Jul;54(1):134–142. doi: 10.1152/jn.1985.54.1.134. [DOI] [PubMed] [Google Scholar]
- Gruol D. L., Barker J. L., Huang L. Y., MacDonald J. F., Smith T. G., Jr Hydrogen ions have multiple effects on the excitability of cultured mammalian neurons. Brain Res. 1980 Feb 3;183(1):247–252. doi: 10.1016/0006-8993(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U., Lux H. D. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res. 1989 Dec 29;505(2):225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Oyama Y., Ikemoto Y., Akaike N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain Res. 1989 Apr 10;484(1-2):348–351. doi: 10.1016/0006-8993(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Oyama Y., Ikemoto Y., Akaike N. Scorpion toxin prolongs an inactivation phase of the voltage-dependent sodium current in rat isolated single hippocampal neurons. Brain Res. 1989 May 15;487(1):192–195. doi: 10.1016/0006-8993(89)90958-x. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk YuN, Krishtal O. A., Nowycky M. C. The proton-activated inward current of rat sensory neurons includes a calcium component. Neurosci Lett. 1990 Jul 31;115(2-3):237–242. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6(12):2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Shirasaki T., Tateishi N., Murase K., Akaike N. Effects of antagonists on N-methyl-D-aspartate response in acutely isolated nucleus tractus solitarii neurons of the rat. Neurosci Lett. 1990 May 31;113(2):169–174. doi: 10.1016/0304-3940(90)90298-n. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Akaike N. Nicergoline inhibits T-type Ca2+ channels in rat isolated hippocampal CA1 pyramidal neurones. Br J Pharmacol. 1990 Aug;100(4):705–710. doi: 10.1111/j.1476-5381.1990.tb14079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Ogawa T. Effects of lowered pH of the extracellular solution on non-transsynaptic component of evoked potentials in the rat's in vitro cerebral cortical slices. Tohoku J Exp Med. 1987 May;152(1):63–65. doi: 10.1620/tjem.152.63. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. The influence of pH on equilibrium effects of tetrodotoxin on myelinated nerve fibres of Rana esculenta. J Physiol. 1975 Oct;252(1):159–184. doi: 10.1113/jphysiol.1975.sp011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M., Kaneda M., Oyama Y., Akaike N. Effects of chlordiazepoxide, chlorpromazine, diazepam, diphenylhydantoin, flunitrazepam and haloperidol on the voltage-dependent sodium current of isolated mammalian brain neurons. Brain Res. 1989 Aug 14;494(2):374–378. doi: 10.1016/0006-8993(89)90607-0. [DOI] [PubMed] [Google Scholar]
- Yakushiji T., Tokutomi N., Akaike N., Carpenter D. O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987 Sep;22(3):1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


