Abstract
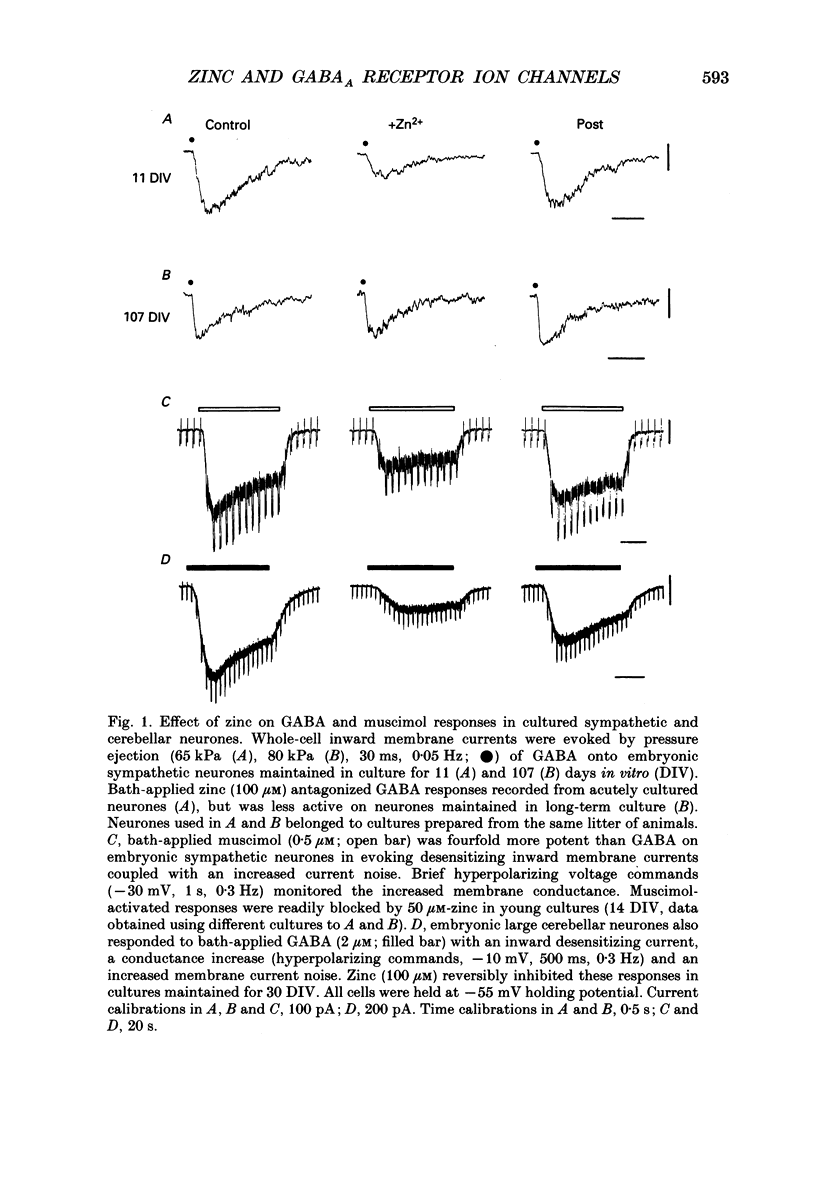
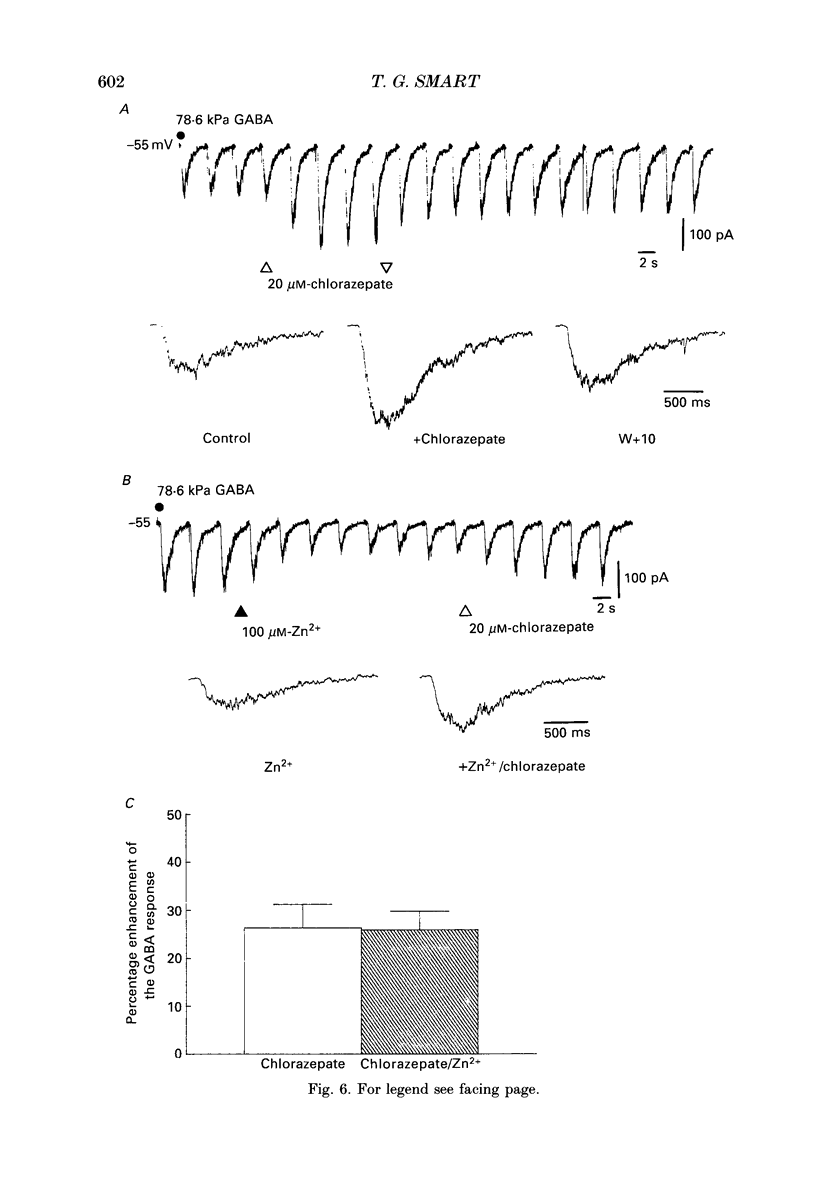
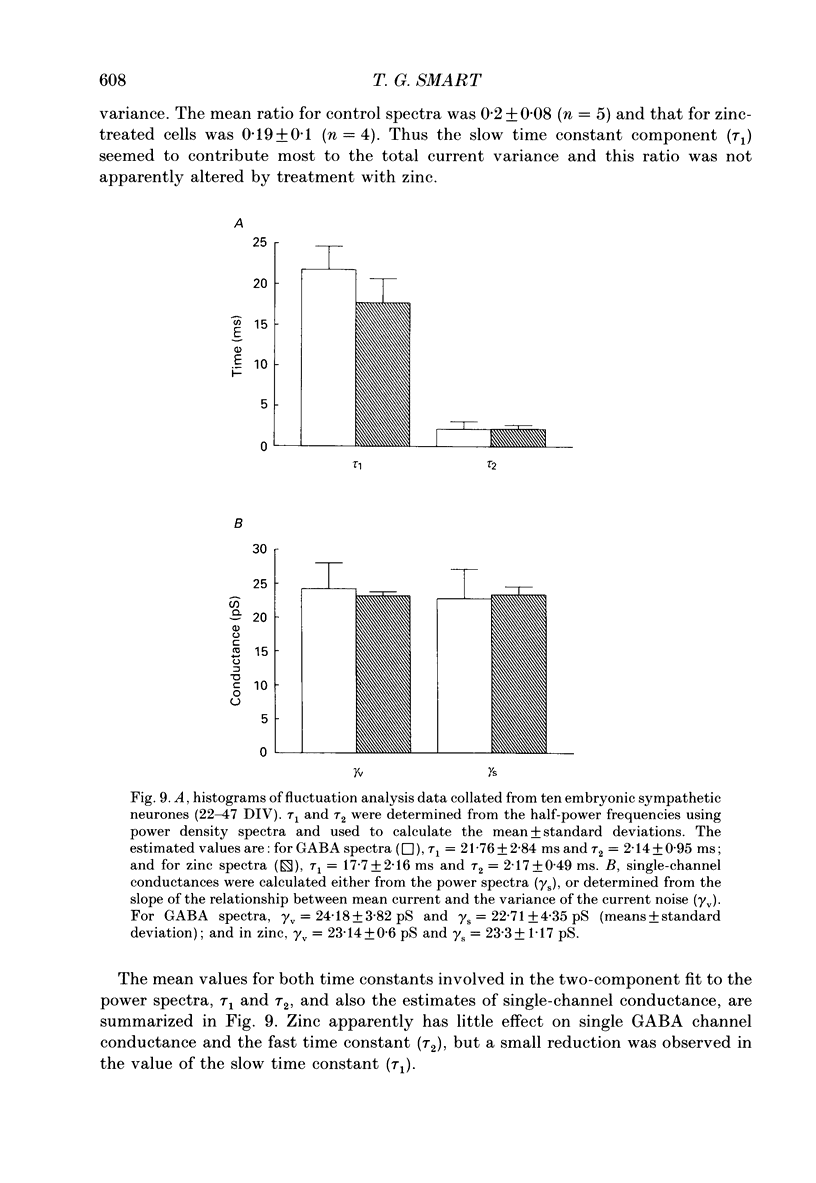
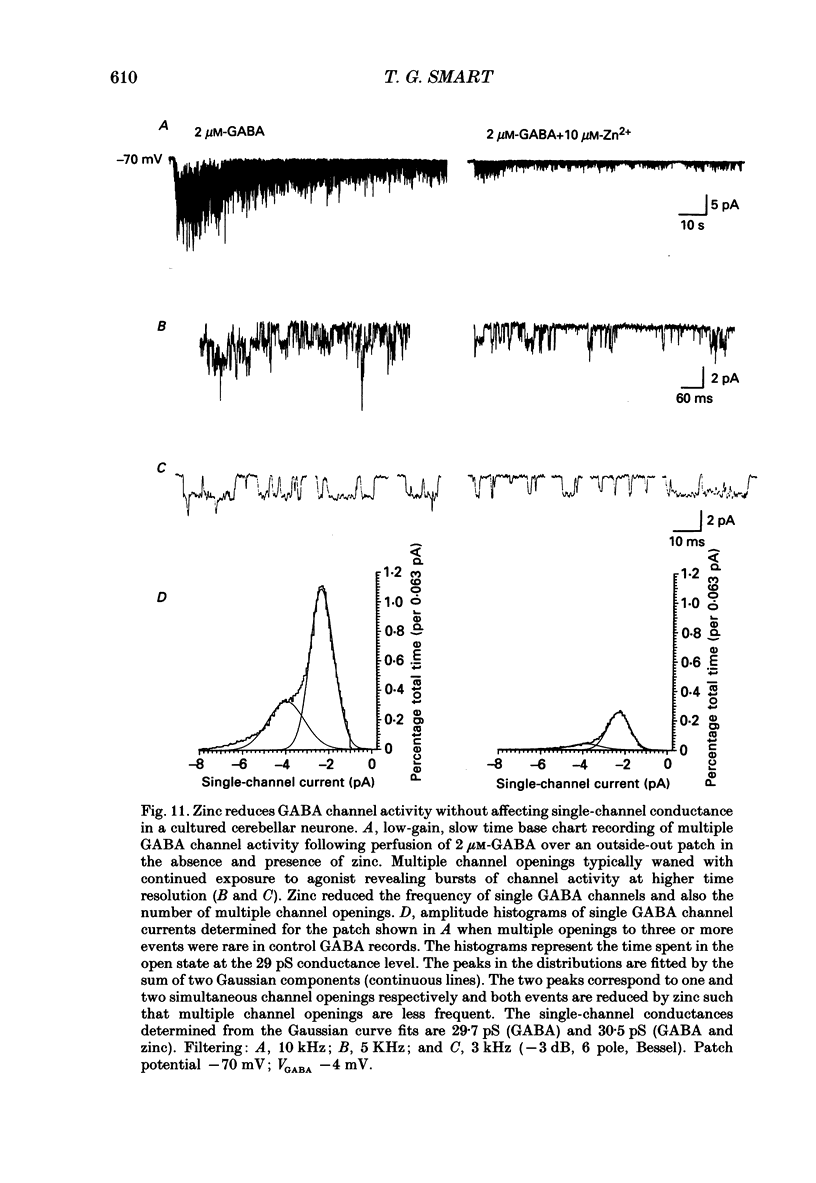
1. The properties of gamma-aminobutyric acidA (GABAA) receptor-ion channel complexes and the interaction with the transition metal zinc, were studied on rat sympathetic and cerebellar neurones in dissociated culture using patch clamp recording techniques. 2. The antagonism of GABA-induced membrane currents by zinc on sympathetic neurones was subject to developmental influence. Using embryonic sympathetic neurones acutely cultured for 24-72 h, GABA responses were more depressed by zinc when compared to responses evoked on adult neurones cultured for the same period. For neurones developing in vivo, the percentage inhibition of GABA responses produced by zinc in embryonic neurones was estimated to decline by 50% after 48.2 days following birth. 3. Embryonic sympathetic neurones maintained in culture for prolonged periods (40-50 days in vitro, DIV) became less sensitive to zinc when compared to neurones cultured for shorter periods (10-20 DIV). The decrease in the zinc inhibition for neurones maintained in vitro proceeded at an apparent rate of 0.55% per day. 4. Activation of the GABA receptor by muscimol (0.2-2 microM) was also antagonized by zinc (50-100 microM). 5. Lowering the pH of the perfusing Krebs solution did not affect the inhibition of GABA responses by zinc on sympathetic neurones. 6. Modulation of the GABAA receptor by some benzodiazepines, a barbiturate, a steroid based on pregnanolone, or antagonists bicuculline and picrotoxinin, did not interfere with the antagonism exerted by zinc on sympathetic neurones. A novel binding site for zinc on the GABAA receptor is proposed. 7. Analysis of the GABA-activated current noise on sympathetic neurones revealed two kinetic components to the power spectra requiring a double Lorentzian fit. The time constant describing the fast component (tau 2, 2.1 ms) was unaffected by zinc, whereas the slow component time constant (tau 1, 21.7 ms) was slightly reduced to 17.1 ms. 8. The apparent single-channel conductance for GABA-activated ion channels was determined from the power spectra (gamma s = 22.7 pS) and also from the relationship between the mean GABA-induced inward current and the variance of the current (gamma v = 24 pS). Zinc (25-100 microM) did not affect the single-channel conductance. 9. Single GABA-activated ion channels were recorded from outside-out patches taken from the soma of large cerebellar neurones. Single GABA channels were capable of activation to multiple current amplitudes which were assessed into the following conductance levels: 8, 18, 23, 29 and 34 pS.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Borden L. A., Farb D. H. Mechanism of gamma-aminobutyric acid/benzodiazepine receptor turnover in neuronal cells: evidence for nonlysosomal degradation. Mol Pharmacol. 1988 Sep;34(3):354–362. [PubMed] [Google Scholar]
- Brehm P., Henderson L. Regulation of acetylcholine receptor channel function during development of skeletal muscle. Dev Biol. 1988 Sep;129(1):1–11. doi: 10.1016/0012-1606(88)90156-x. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A., Verdorn T. A., Ewert M., Seeburg P. H., Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990 Dec;5(6):781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Dvergsten C. L., Fosmire G. J., Ollerich D. A., Sandstead H. H. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. I. Impaired acquisition of granule cells. Brain Res. 1983 Jul 25;271(2):217–226. doi: 10.1016/0006-8993(83)90284-6. [DOI] [PubMed] [Google Scholar]
- Ebadi M., Babin D. The amino acid composition of the zinc-induced metallothionein isoforms in rat brain. Neurochem Res. 1989 Jan;14(1):69–73. doi: 10.1007/BF00969760. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Friedberg F. Effects of metal binding on protein structure. Q Rev Biophys. 1974 Feb;7(1):1–33. doi: 10.1017/s0033583500001335. [DOI] [PubMed] [Google Scholar]
- Friedman B., Price J. L. Fiber systems in the olfactory bulb and cortex: a study in adult and developing rats, using the timm method with the light and electron microscope. J Comp Neurol. 1984 Feb 10;223(1):88–109. doi: 10.1002/cne.902230108. [DOI] [PubMed] [Google Scholar]
- Gruol D. L., Franklin C. L. Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. J Neurosci. 1987 May;7(5):1271–1293. doi: 10.1523/JNEUROSCI.07-05-01271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoch W., Betz H., Becker C. M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989 Sep;3(3):339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Holm I. E., Andreasen A., Danscher G., Pérez-Clausell J., Nielsen H. Quantification of vesicular zinc in the rat brain. Histochemistry. 1988;89(3):289–293. doi: 10.1007/BF00493154. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Huck S., Lux H. D. Patch-clamp study of ion channels activated by GABA and glycine in cultured cerebellar neurons of the mouse. Neurosci Lett. 1987 Aug 18;79(1-2):103–107. doi: 10.1016/0304-3940(87)90679-3. [DOI] [PubMed] [Google Scholar]
- Ibata Y., Otsuka N. Electron microscopic demonstration of zinc in the hippocampal formation using Timm's sulfide silver technique. J Histochem Cytochem. 1969 Mar;17(3):171–175. doi: 10.1177/17.3.171. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Lambert J. D. Modulation of the responses to the GABA-mimetics, THIP and piperidine-4-sulphonic acid, by agents which interact with benzodiazepine receptors. An electrophysiological study on cultured mouse neurones. Neuropharmacology. 1984 Dec;23(12A):1441–1450. doi: 10.1016/0028-3908(84)90087-x. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Sturgess N. C., Hales T. G. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–82. doi: 10.1002/9780470513989.ch4. [DOI] [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. Noncompetitive inhibition of gamma-aminobutyric acidA channels by Zn. Mol Pharmacol. 1991 Mar;39(3):267–274. [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., Kusano K., Muller J. M., Brownstein M. J., Mahan L. C. Cloning and expression of a novel rat GABAA receptor. FEBS Lett. 1989 Mar 27;246(1-2):145–148. doi: 10.1016/0014-5793(89)80271-6. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P., Sigel E., Baur R., Persohn E., Richards J. G., Möhler H. Functional expression and sites of gene transcription of a novel alpha subunit of the GABAA receptor in rat brain. FEBS Lett. 1990 Jan 29;260(2):261–265. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989 Aug;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Moonen G., Neale E. A., Macdonald R. L., Gibbs W., Nelson P. G. Cerebellar macroneurons in microexplant cell culture. Methodology, basic electrophysiology, and morphology after horseradish peroxidase injection. Brain Res. 1982 Sep;281(1):59–73. doi: 10.1016/0165-3806(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Neale E. A., Moonen G., Macdonald R. L., Nelson P. G. Cerebellar macroneurons in microexplant cell culture: ultrastructural morphology. Neuroscience. 1982;7(8):1879–1890. doi: 10.1016/0306-4522(82)90004-5. [DOI] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland C. F., Colquhoun D., Cull-Candy S. G. Single channels activated by high concentrations of GABA in superior cervical ganglion neurones of the rat. J Physiol. 1991 Jan;432:203–233. doi: 10.1113/jphysiol.1991.sp018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Pérez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985 Jun 24;337(1):91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- Ravindran A., Schild L., Moczydlowski E. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin. Zn2+ induces discrete substates in cardiac Na+ channels. J Gen Physiol. 1991 Jan;97(1):89–115. doi: 10.1085/jgp.97.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986 Aug 1;46(3):437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Classification of some GABA antagonists with regard to site of action and potency in slices of rat cuneate nucleus. Eur J Pharmacol. 1982 Jun 4;80(4):347–358. doi: 10.1016/0014-2999(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. A novel effect of zinc on the lobster muscle GABA receptor. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):327–341. doi: 10.1098/rspb.1982.0045. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Constanti A. Differential effect of zinc on the vertebrate GABAA-receptor complex. Br J Pharmacol. 1990 Apr;99(4):643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G., Moss S. J., Xie X., Huganir R. L. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991 Aug;103(4):1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart T. G. Uncultured lobster muscle, cultured neurons and brain slices: the neurophysiology of zinc. J Pharm Pharmacol. 1990 Jun;42(6):377–387. doi: 10.1111/j.2042-7158.1990.tb06576.x. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Zorec R., McBurney R. N. Conductance states activated by glycine and GABA in rat cultured spinal neurones. J Membr Biol. 1989 Apr;108(1):45–52. doi: 10.1007/BF01870424. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989 Mar;25(3):213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- Vicini S., Mienville J. M., Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J Pharmacol Exp Ther. 1987 Dec;243(3):1195–1201. [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Xie X. M., Smart T. G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991 Feb 7;349(6309):521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Wesson L., Eisenman G., Eisenberg D. Where metal ions bind in proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5648–5652. doi: 10.1073/pnas.87.15.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Wisden W., Werner P., Keinänen K., Schofield P. R., Sprengel R., Pritchett D. B., Seeburg P. H. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990 Oct;9(10):3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Schofield P. R., Draguhn A., Werner P., Köhler M., Seeburg P. H. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989 Jun;8(6):1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


