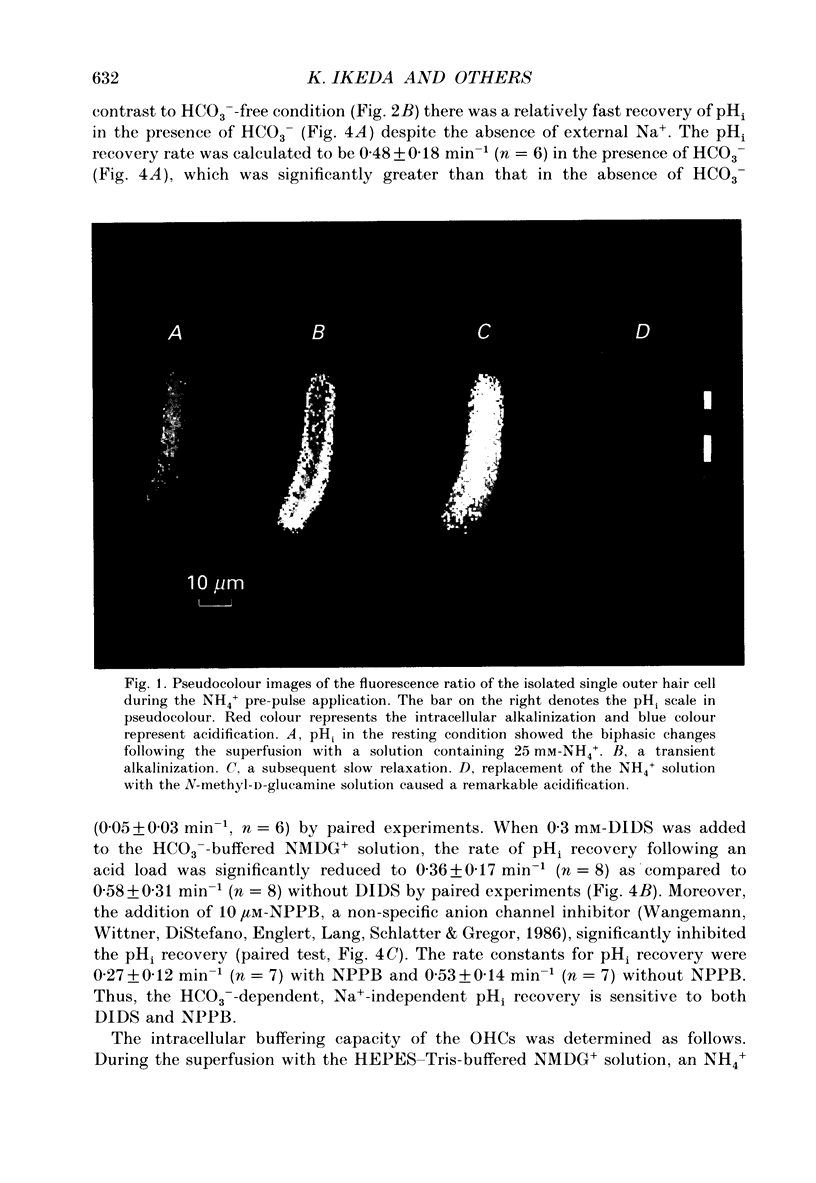
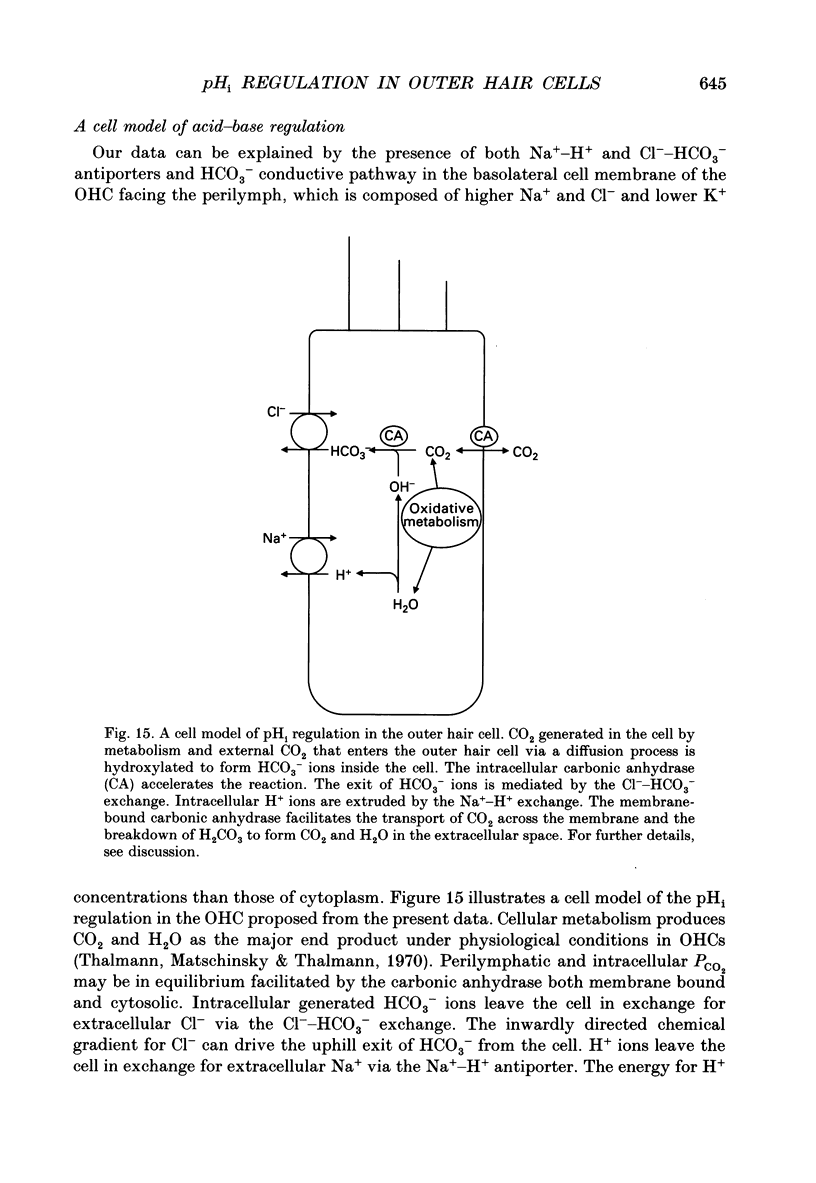
Abstract
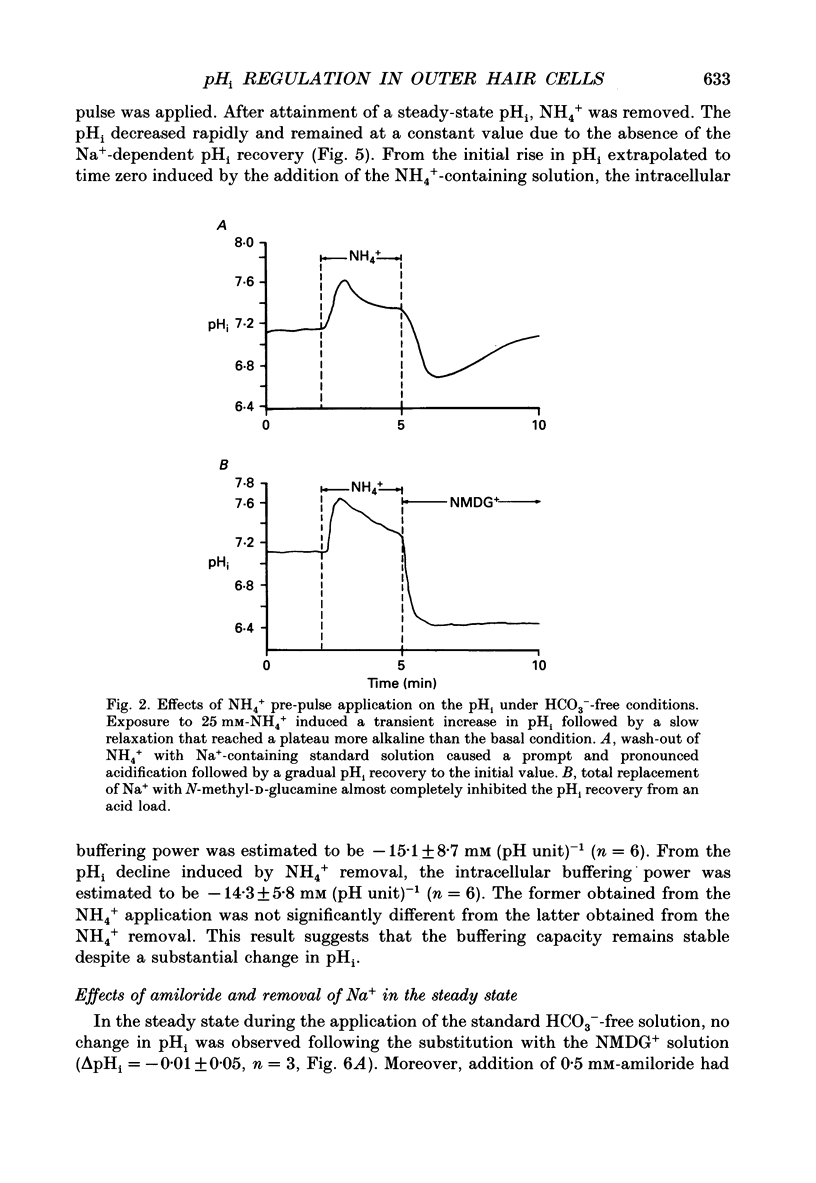
1. The intracellular pH (pHi) regulation mechanisms of the outer hair cell (OHC) isolated from the guinea-pig were studied using fluorescence ratio imaging microscopy. 2. The OHC pHi in the resting condition was 7.26 +/- 0.08 (mean +/- S.D., n = 49) when the standard solution buffered with HEPES-Tris was superfused. 3. Exposure to 25 mM-NH4+ in the absence of HCO3- caused biphasic changes in pHi; a transient increase (7.89 +/- 0.14, n = 22) followed by a slow decrease (7.57 +/- 0.12; mean +/- S.D.). Removal of external NH4+ by introducing the N-methyl-D-glucamine (NMDG+) solution in the absence of HCO3- markedly acidified the pHi to 6.38 +/- 0.12 with little pHi recovery. Subsequent application of the standard Na+ solution restored the pHi to the initial value. The recovery was inhibited by 0.5 mM-amiloride but not by 0.3 mM-DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid). 4. In the presence of HCO3-, removal of both external NH4+ and Na+ promptly caused an intracellular acidification followed by a pHi recovery. The pHi recovery from an acid load was inhibited by 0.3 mM-DIDS or 10 microM-NPPB (5-nitro-2-(3-phenylpropyl-amino)-benzoate). However, the pHi in the steady state in the presence or absence of HCO3- was not altered by addition of 0.5 mM-amiloride or NMDG+ solution. 5. The intracellular buffering power obtained from the NH4+ exposure and withdrawal was -15.1 +/- 8.7 mM (pH unit)-1 (n = 6) and -14.3 +/- 5.8 mM (pH unit)-1, respectively. 6. Replacement of external Cl- with gluconate in the HCO3- solution increased the pHi from 7.22 +/- 0.12 to 7.51 +/- 0.20 (n = 6), which was inhibited by 0.3 mM-DIDS. Moreover, addition of DIDS to the HCO3- solution increased the pHi by 0.13 +/- 0.08 (n = 8). 7. When the external standard solution buffered with HEPES-Tris was replaced with the HCO3- solution, the basal pHi (7.27 +/- 0.10) was promptly acidified to 6.87 +/- 0.10 then relaxed slowly to 7.00 +/- 0.15 (n = 16). 8. The pHi showed an initial alkalinization and a subsequent slow acidification after the HCO3(-)-free standard solution replaced the HCO3- solution. The slow acidification was inhibited by low external Cl- concentration or by addition of 0.3 mM-DIDS.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
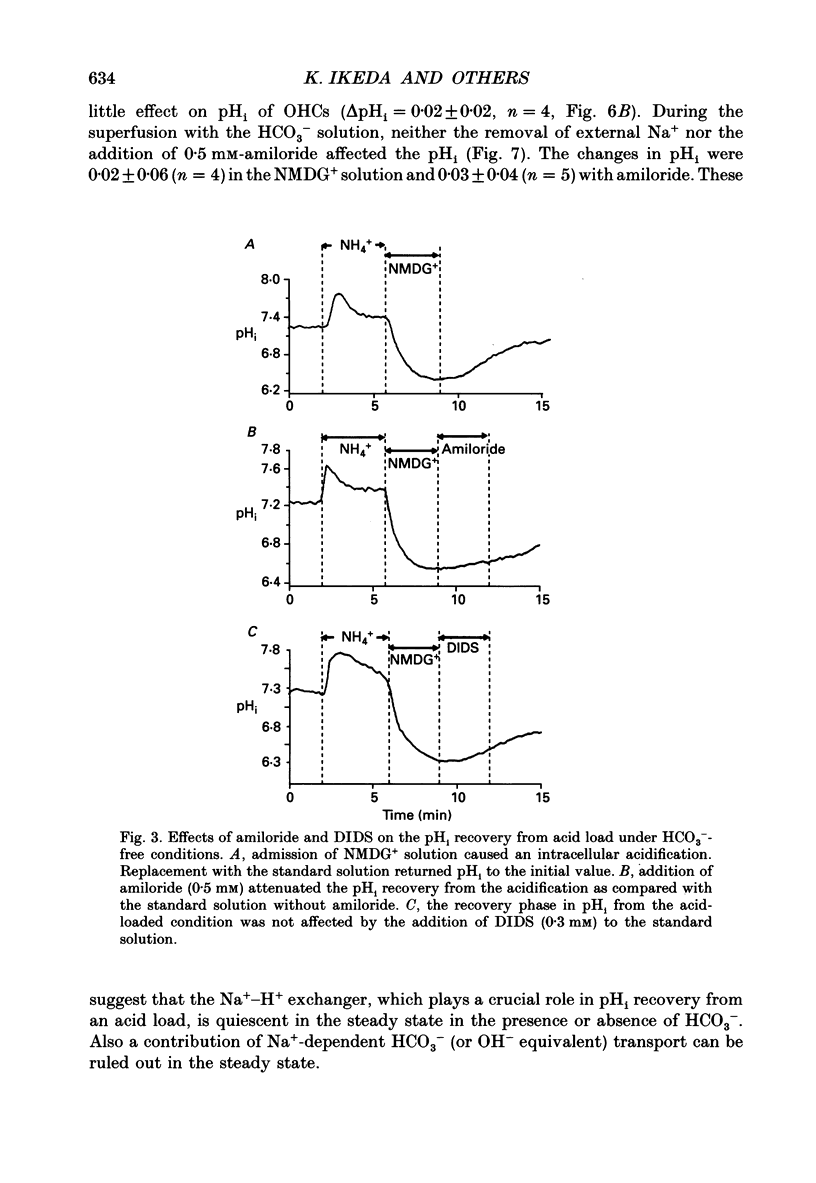
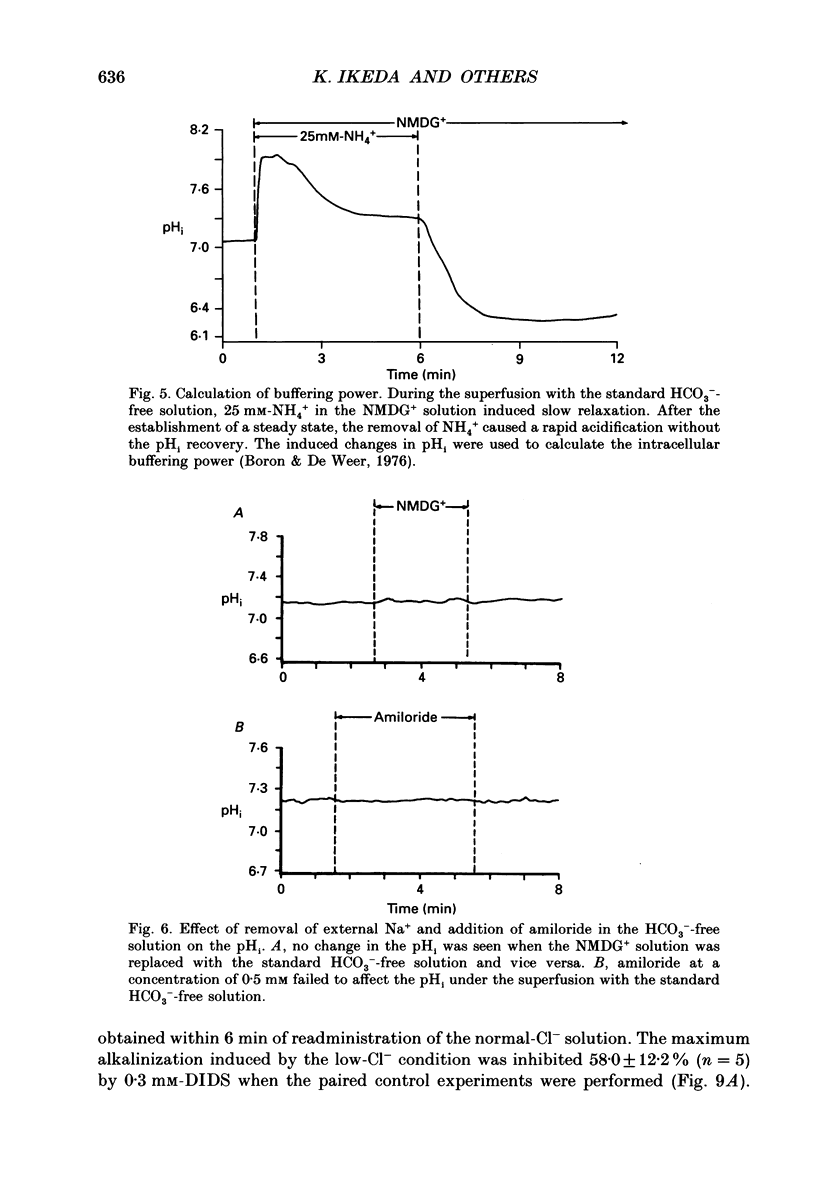
PDF



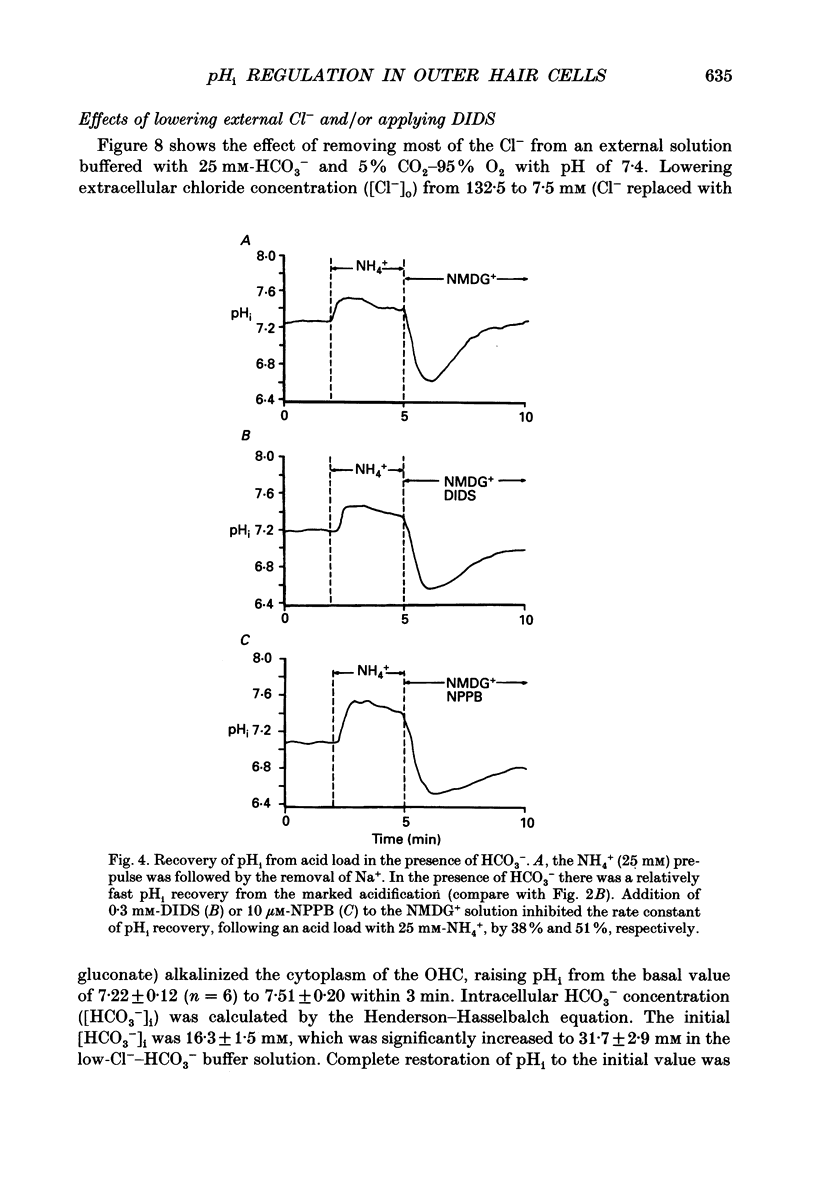
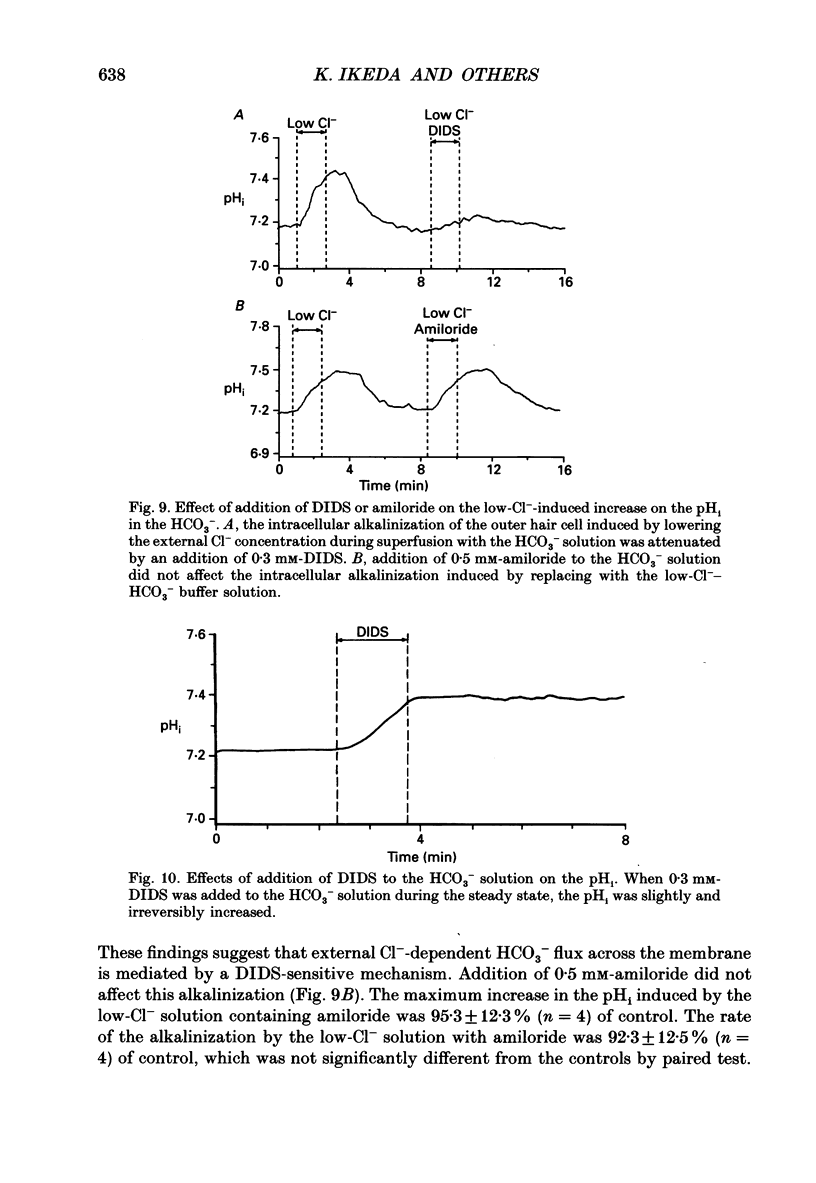
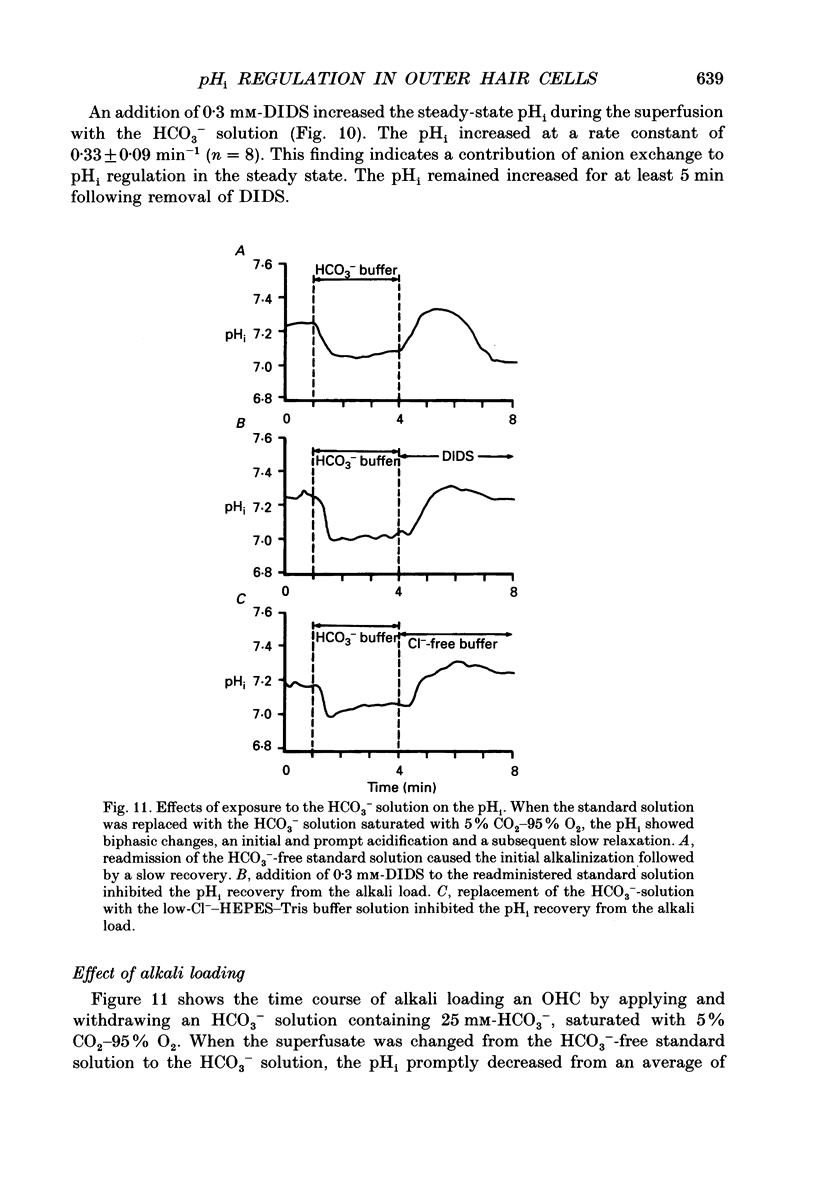
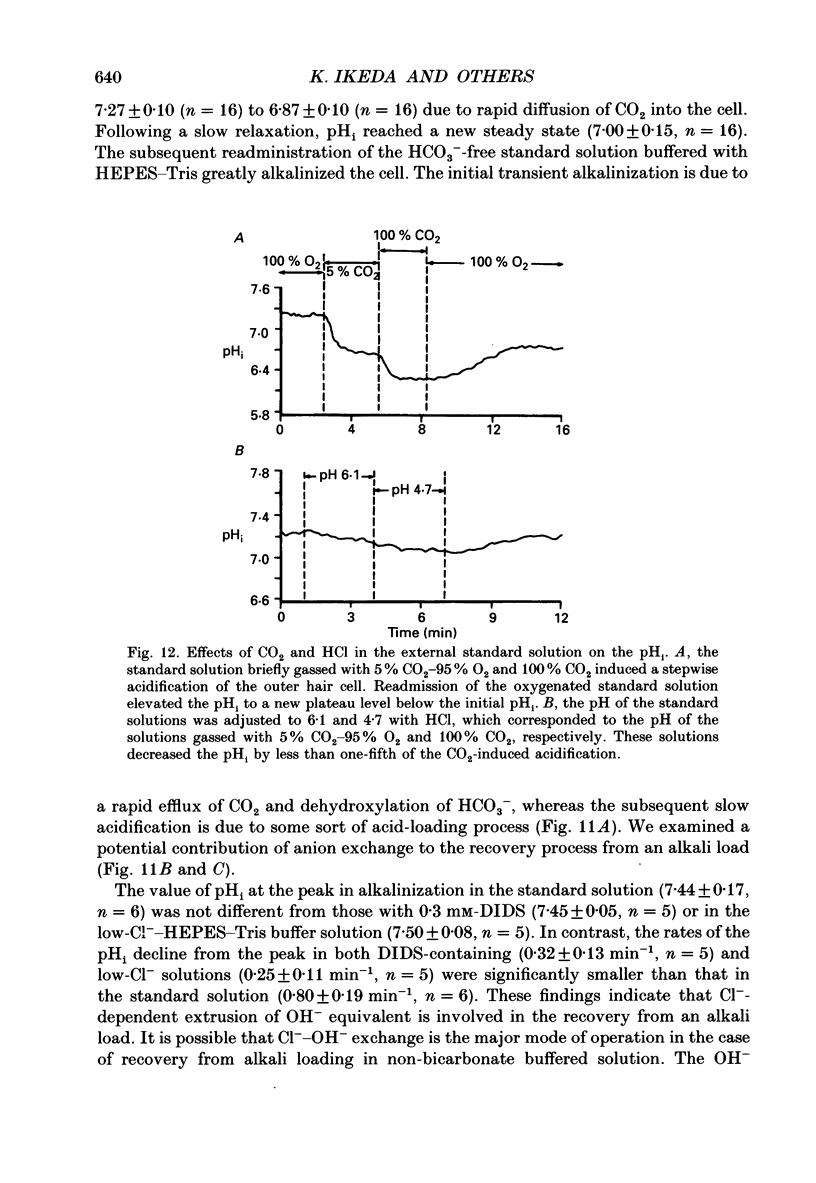

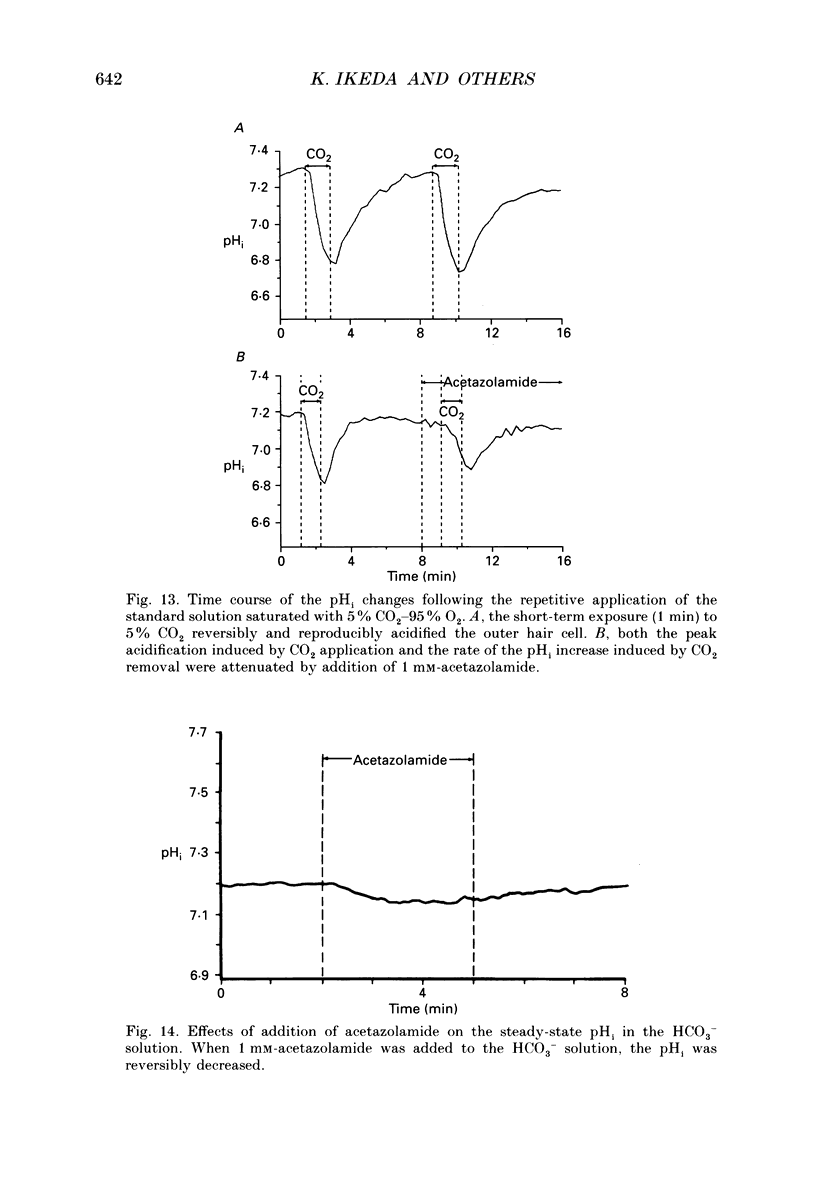





Images in this article
Selected References
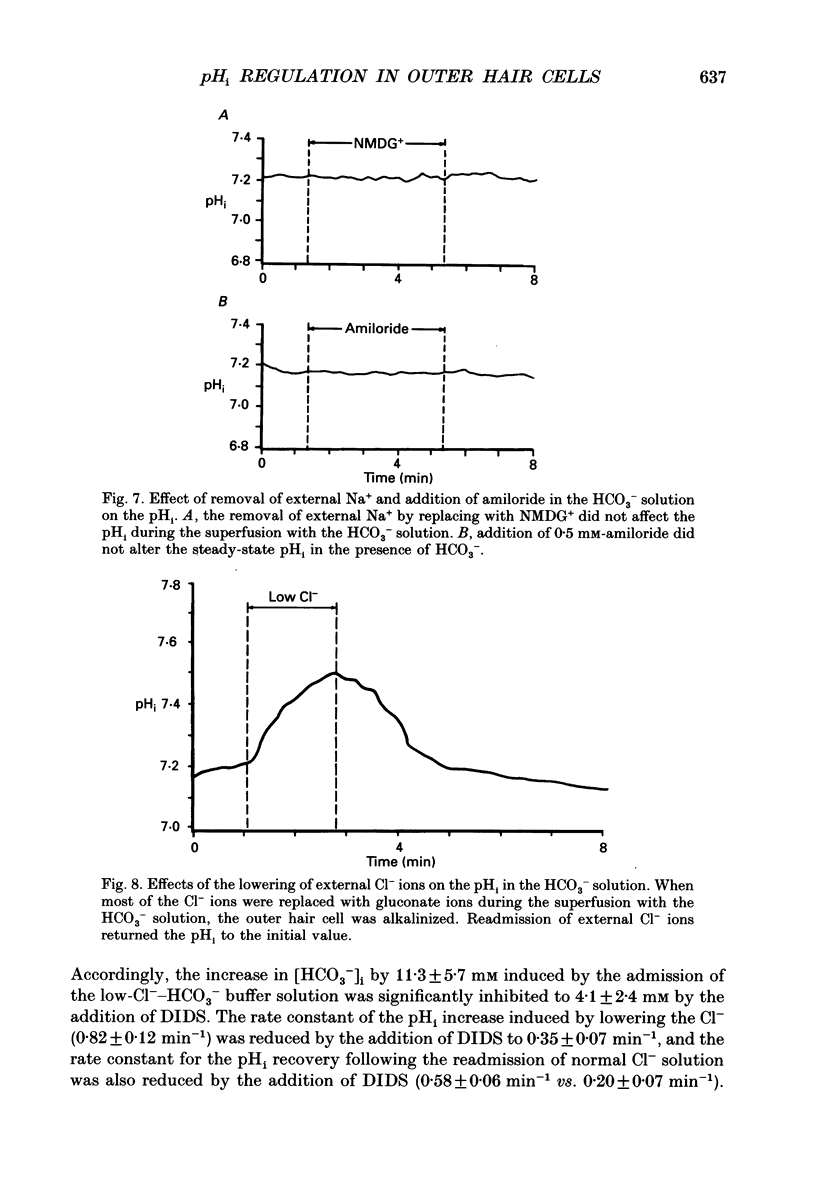
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg D. A., Rebhun L. I., Hyatt H. Structural organization of actin in the sea urchin egg cortex: microvillar elongation in the absence of actin filament bundle formation. J Cell Biol. 1982 Apr;93(1):24–32. doi: 10.1083/jcb.93.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K. The nature of the negative endocochlear potentials produced by anoxia and ethacrynic acid in the rat and guinea-pig. J Physiol. 1979 Aug;293:329–345. doi: 10.1113/jphysiol.1979.sp012892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B. Mechanisms and consequences of pH-mediated cell regulation. Annu Rev Physiol. 1986;48:389–402. doi: 10.1146/annurev.ph.48.030186.002133. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Drescher D. G. Purification of a carbonic anhydrase from the inner ear of the guinea pig. Proc Natl Acad Sci U S A. 1977 Mar;74(3):892–896. doi: 10.1073/pnas.74.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERULKAR S. D., MAREN T. H. Carbonic anhydrase and the inner ear. Nature. 1961 Feb 11;189:459–460. doi: 10.1038/189459a0. [DOI] [PubMed] [Google Scholar]
- Fidelman M. L., Seeholzer S. H., Walsh K. B., Moore R. D. Intracellular pH mediates action of insulin on glycolysis in frog skeletal muscle. Am J Physiol. 1982 Jan;242(1):C87–C93. doi: 10.1152/ajpcell.1982.242.1.C87. [DOI] [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Gitter A. H., Zenner H. P., Frömter E. Membrane potential and ion channels in isolated outer hair cells of guinea pig cochlea. ORL J Otorhinolaryngol Relat Spec. 1986;48(2):68–75. doi: 10.1159/000275848. [DOI] [PubMed] [Google Scholar]
- Gonnella P. A., Nachmias V. T. Platelet activation and microfilament bundling. J Cell Biol. 1981 Apr;89(1):146–151. doi: 10.1083/jcb.89.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. J., Nomura Y. Carbonic anhydrase activity in the inner ear. Acta Otolaryngol Suppl. 1985;418:1–42. [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Kusakari J., Takasaka T., Saito Y. Early effects of acetazolamide on anionic activities of the guinea pig endolymph: evidence for active function of carbonic anhydrase in the cochlea. Hear Res. 1987 Dec 31;31(3):211–216. doi: 10.1016/0378-5955(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Kusakari J., Takasaka T., Saito Y. The Ca2+ activity of cochlear endolymph of the guinea pig and the effect of inhibitors. Hear Res. 1987;26(1):117–125. doi: 10.1016/0378-5955(87)90040-2. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Morizono T. Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res. 1989 Jun 1;39(3):279–286. doi: 10.1016/0378-5955(89)90047-6. [DOI] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kuijpers W., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XXIV. Localization and properties of ATPase in the inner ear of the guinea pig. Biochim Biophys Acta. 1969 Apr;173(3):477–485. doi: 10.1016/0005-2736(69)90012-1. [DOI] [PubMed] [Google Scholar]
- Lim D. J., Karabinas C., Trune D. R. Histochemical localization of carbonic anhydrase in the inner ear. Am J Otolaryngol. 1983 Jan-Feb;4(1):33–42. doi: 10.1016/s0196-0709(83)80005-2. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Swenson E. R., Addink A. D. Rates of ion movement from plasma to endolymph in the dogfish. Ann Otol Rhinol Laryngol. 1975 Nov-Dec;84(6):847–858. doi: 10.1177/000348947508400618. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W. J., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol. 1981 Jul;316:293–308. doi: 10.1113/jphysiol.1981.sp013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Biochemical characterization of the amiloride-sensitive Na+/H+ antiport in Chinese hamster lung fibroblasts. J Biol Chem. 1983 Mar 25;258(6):3503–3508. [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Zenner H. P. Motile responses in outer hair cells. Hear Res. 1986;22:83–90. doi: 10.1016/0378-5955(86)90082-1. [DOI] [PubMed] [Google Scholar]
- Zenner H. P., Zimmermann U., Gitter A. H. Fast motility of isolated mammalian auditory sensory cells. Biochem Biophys Res Commun. 1987 Nov 30;149(1):304–308. doi: 10.1016/0006-291x(87)91639-1. [DOI] [PubMed] [Google Scholar]