Abstract
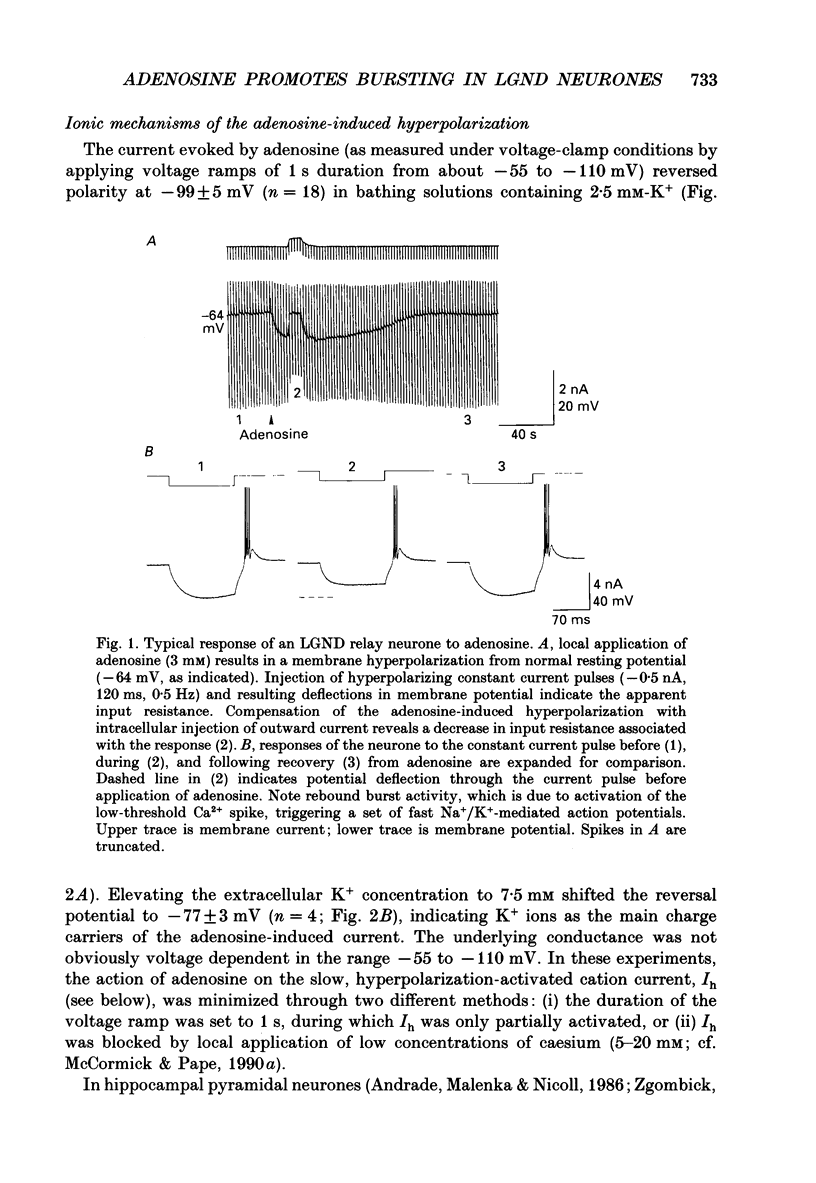
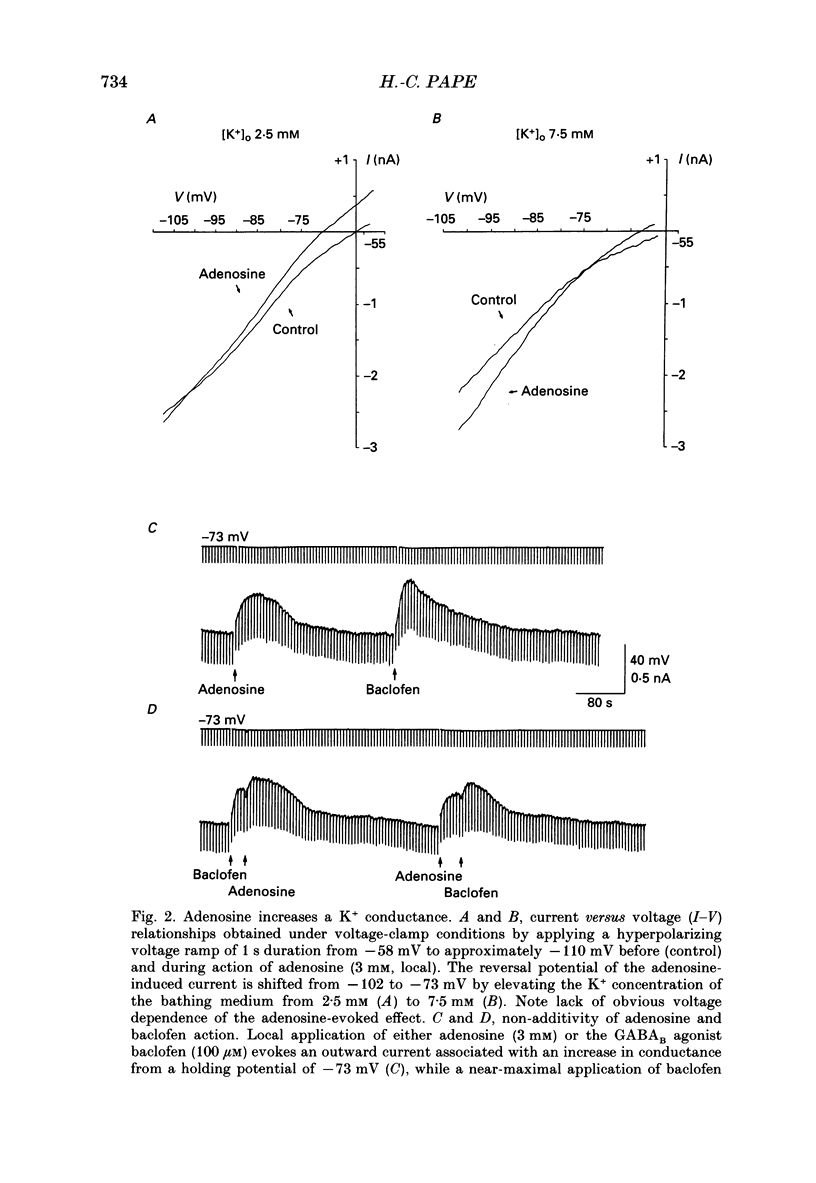
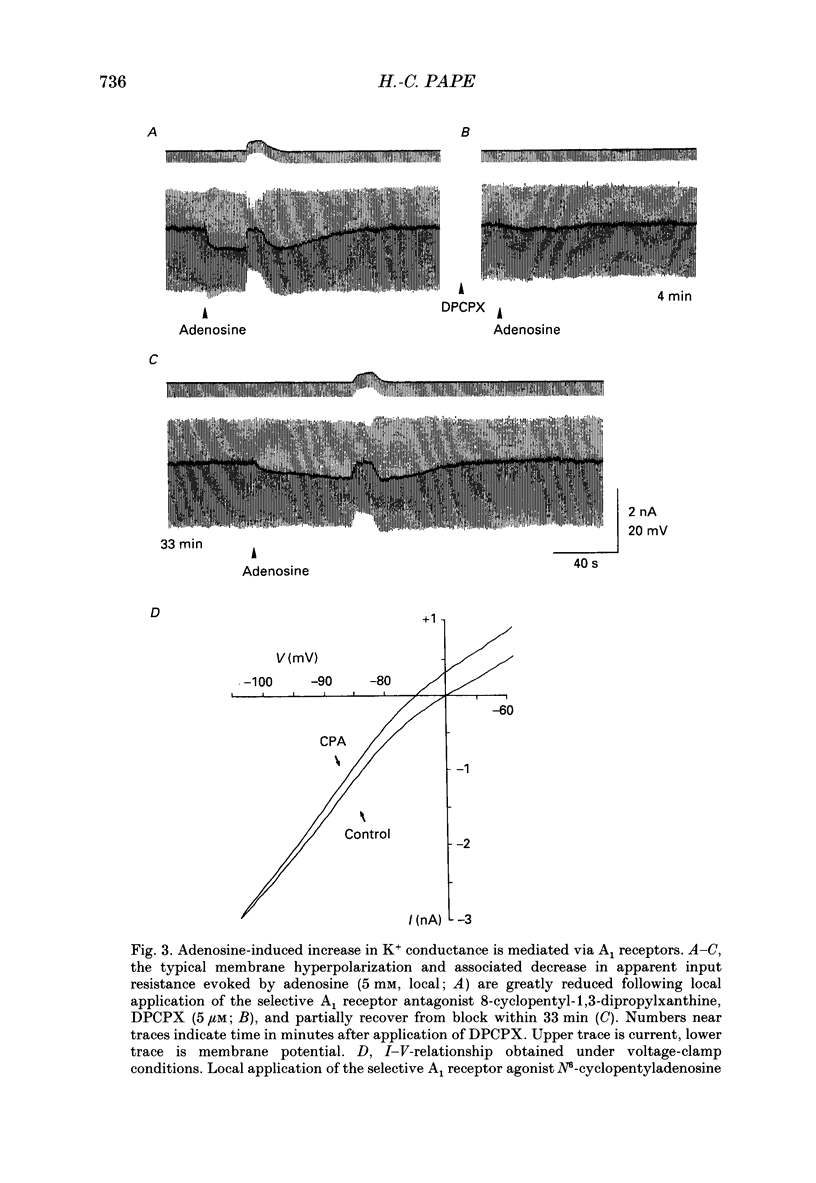
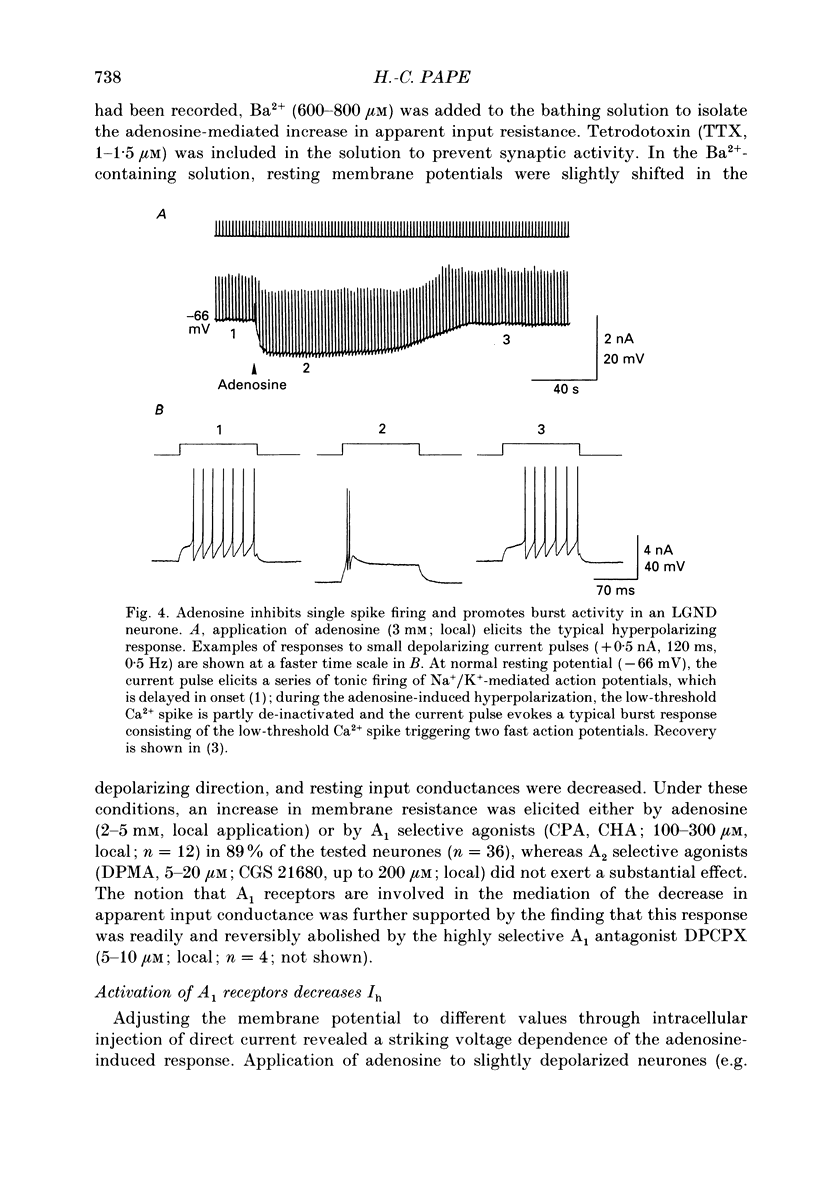
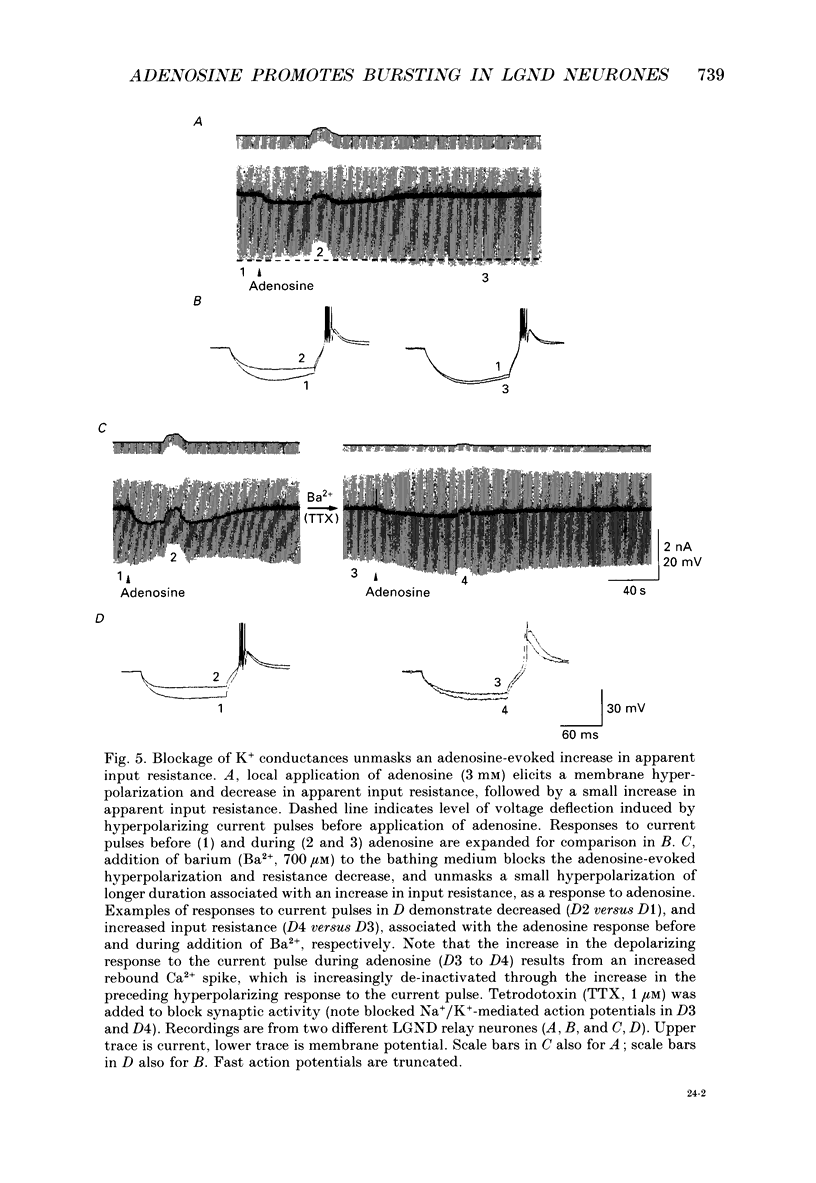
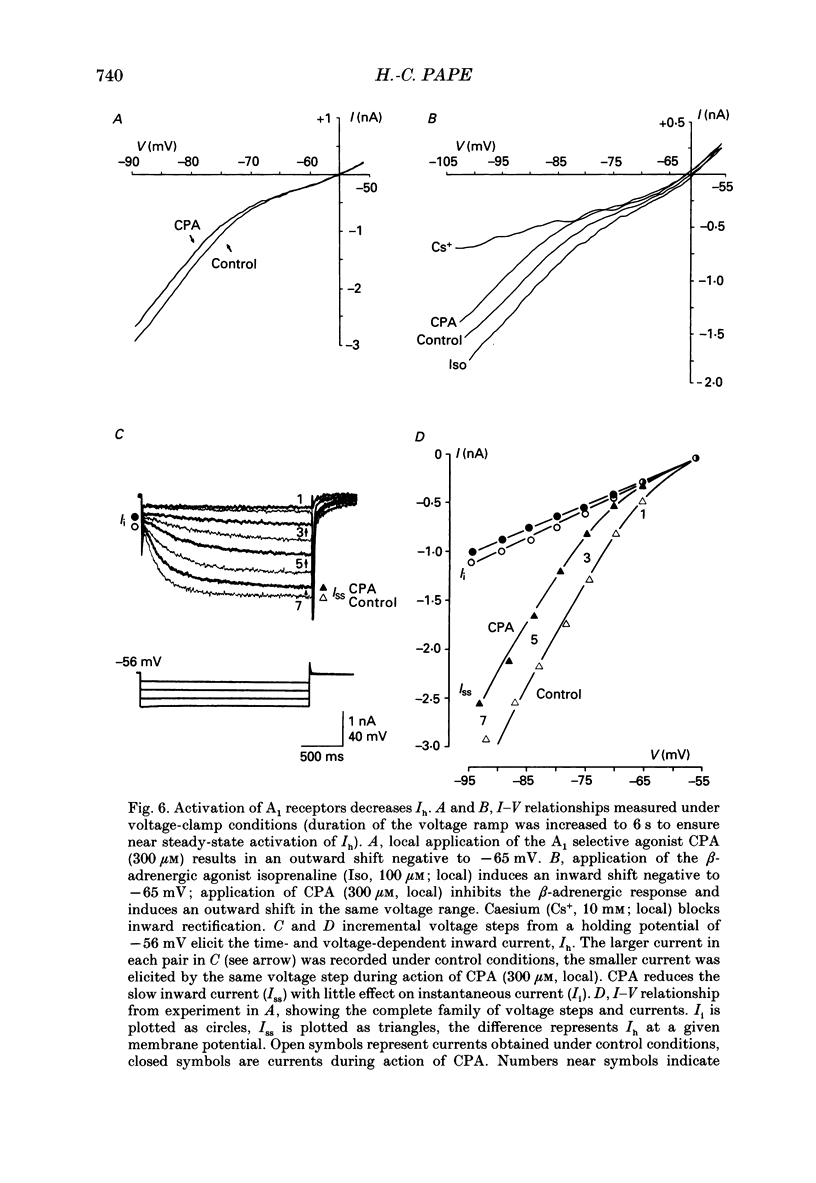
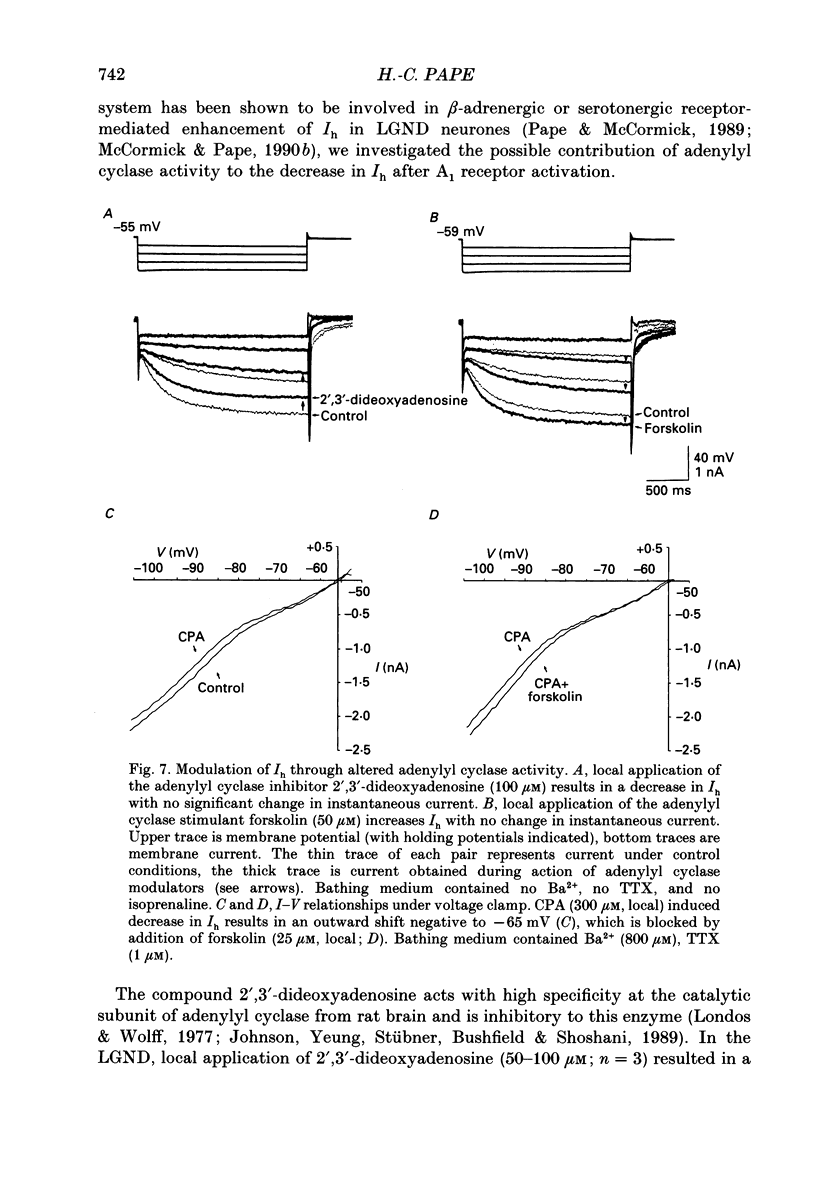
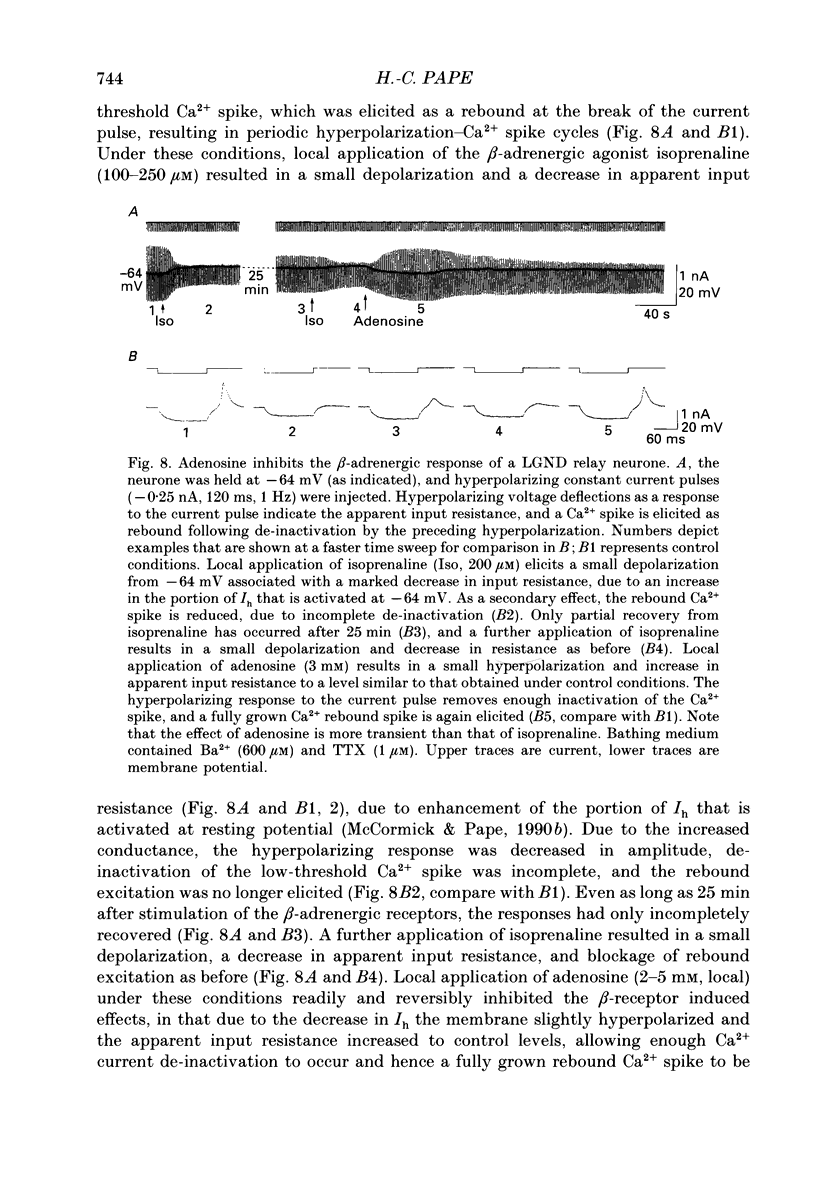
1. The mechanisms of action of adenosine were examined in relay neurones of the dorsal lateral geniculate nucleus (LGND) using in vitro intracellular recording techniques in guinea-pig thalamic slices. 2. Adenosine hyperpolarized LGND relay neurones due to an increase in membrane potassium conductance. The K+ currents generated by near maximal stimulation of adenosine and GABAB receptors were non-additive. 3. Blockage of membrane K+ conductances by barium unmasked a second response to adenosine; an outward shift of the current versus voltage relationship negative to -65 mV associated with an increase in membrane input resistance. The beta-adrenoceptor agonist isoprenaline elicited an inward current in the same voltage range, which was inhibited and replaced by an outward current during activation of adenosine receptors. The effects of adenosine were due to a decrease in amplitude and rate of rise of the hyperpolarization-activated cation current, Ih. Maximal reduction by 66% of Ih amplitude occurred near the range of half-activation. 4. Both responses to adenosine were mimicked by the selective A1 receptor agonists N6-cyclopentyladenosine or N6-cyclohexyladenosine, and reversibly blocked by the selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). 5. The decrease in Ih by adenosine may be mediated by an inhibition of adenylyl cyclase activity and hence a decrease in the intracellular level of cyclic AMP, since local application of the adenylyl cyclase inhibitor 2',3'-dideoxyadenosine imitated the decrease in Ih. Local application of the adenylyl cyclase stimulant forskolin or 8-bromo-cyclic AMP resulted in an enhancement in Ih, and forskolin inhibited the action on Ih evoked by N6-cyclopentyladenosine. 6. The adenosine-induced effects interacted with the intrinsic electrophysiological properties of LGND neurones in that (i) the hyperpolarization due to an increase in K+ conductance inhibited single spike firing and promoted calcium-mediated burst discharges, and (ii) the decrease in Ih inhibited the dampening effect on Ca(2+)-mediated rebound activity of beta-adrenergic receptor stimulation. 7. It is suggested that during increased levels of extracellular adenosine the response of LGND relay neurones to activating brainstem influences will be depressed, and a pattern of Ca(2+)-mediated burst firing will be favoured.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Bridges A. J., Bruns R. F., Ortwine D. F., Priebe S. R., Szotek D. L., Trivedi B. K. N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine and its uronamide derivatives. Novel adenosine agonists with both high affinity and high selectivity for the adenosine A2 receptor. J Med Chem. 1988 Jul;31(7):1282–1285. doi: 10.1021/jm00402a004. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Crunelli V., Leresche N., Parnavelas J. G. Membrane properties of morphologically identified X and Y cells in the lateral geniculate nucleus of the cat in vitro. J Physiol. 1987 Sep;390:243–256. doi: 10.1113/jphysiol.1987.sp016697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Gerber U., Greene R. W., Haas H. L., Stevens D. R. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J Physiol. 1989 Oct;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. R., Kuhar M. J., Hester L., Snyder S. H. Adenosine receptors: autoradiographic evidence for their location on axon terminals of excitatory neurons. Science. 1983 May 27;220(4600):967–969. doi: 10.1126/science.6302841. [DOI] [PubMed] [Google Scholar]
- Haulică I., Ababei L., Brănişteanu D., Topoliceanu F. Letter: Preliminary data on the possible hypnogenic role of adenosine. J Neurochem. 1973 Oct;21(4):1019–1020. doi: 10.1111/j.1471-4159.1973.tb07549.x. [DOI] [PubMed] [Google Scholar]
- Hirsch J. C., Fourment A., Marc M. E. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983 Jan 24;259(2):308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Yeung S. M., Stübner D., Bushfield M., Shoshani I. Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Mol Pharmacol. 1989 May;35(5):681–688. [PubMed] [Google Scholar]
- Kostopoulos G. K., Phillis J. W. Purinergic depression of neurons in different areas of the rat brain. Exp Neurol. 1977 Jun;55(3 Pt 1):719–724. doi: 10.1016/0014-4886(77)90296-5. [DOI] [PubMed] [Google Scholar]
- Lamarre Y., Filion M., Cordeau J. P. Neuronal discharges of the ventrolateral nucleus of the thalamus during sleep and wakefulness in the cat. I. Spontaneous activity. Exp Brain Res. 1971 Jun 29;12(5):480–498. doi: 10.1007/BF00234244. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Reddington M. Autoradiographic evidence for multiple CNS binding sites for adenosine derivatives. Neuroscience. 1986 Oct;19(2):535–549. doi: 10.1016/0306-4522(86)90279-4. [DOI] [PubMed] [Google Scholar]
- Leresche N., Jassik-Gerschenfeld D., Haby M., Soltesz I., Crunelli V. Pacemaker-like and other types of spontaneous membrane potential oscillations of thalamocortical cells. Neurosci Lett. 1990 May 18;113(1):72–77. doi: 10.1016/0304-3940(90)90497-w. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley R. W., Benoit O., Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983 Oct;50(4):798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989 Jun;12(6):215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Feeser H. R. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–113. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988 Jul 21;334(6179):246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson W. B., Kuruvilla A., Watlington T., Goehl K., Paul S. M., Skolnick P. Sedative and electroencephalographic actions of erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA): relationship to inhibition of brain adenosine deaminase. Psychopharmacology (Berl) 1983;79(2-3):126–129. doi: 10.1007/BF00427798. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Okada Y., Saito M. Inhibitory action adenosine, 5-HT (serotonin) and GABA (gamma-aminobutyric acid) on the postsynaptic potential (PSP) or slices from olfactory cortex and superior colliculus in correlation to the level of cyclic AMP. Brain Res. 1979 Jan 12;160(2):368–371. doi: 10.1016/0006-8993(79)90434-7. [DOI] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Radulovacki M., Miletich R. S., Green R. D. N6 (L-phenylisopropyl) adenosine (L-PHA) increases slow-wave sleep (S2) and decreases wakefulness in rats. Brain Res. 1982 Aug 19;246(1):178–180. doi: 10.1016/0006-8993(82)90161-5. [DOI] [PubMed] [Google Scholar]
- Radulovacki M., Virus R. M., Djuricic-Nedelson M., Green R. D. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984 Feb;228(2):268–274. [PubMed] [Google Scholar]
- Radulovacki M., Virus R. M., Djuricic-Nedelson M., Green R. D. Hypnotic effects of deoxycorformycin in rats. Brain Res. 1983 Jul 25;271(2):392–395. doi: 10.1016/0006-8993(83)90309-8. [DOI] [PubMed] [Google Scholar]
- Schubert P., Lee K., West M., Deadwyler S., Lynch G. Stimulation-dependent release of 3H-adenosine derivatives from central axon terminals to target neurones. Nature. 1976 Apr 8;260(5551):541–542. doi: 10.1038/260541a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Crunelli V. Optic tract stimulation evokes GABAA but not GABAB IPSPs in the rat ventral lateral geniculate nucleus. Brain Res. 1989 Feb 6;479(1):49–55. doi: 10.1016/0006-8993(89)91334-6. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Braunwalder A., Erickson T. J. Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue. Naunyn Schmiedebergs Arch Pharmacol. 1986 Feb;332(2):179–183. doi: 10.1007/BF00511410. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- Zgombick J. M., Beck S. G., Mahle C. D., Craddock-Royal B., Maayani S. Pertussis toxin-sensitive guanine nucleotide-binding protein(S) couple adenosine A1 and 5-hydroxytryptamine1A receptors to the same effector systems in rat hippocampus: biochemical and electrophysiological studies. Mol Pharmacol. 1989 Apr;35(4):484–494. [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


