Abstract
Understanding the factors that influence the accumulation of molecules beyond the mycomembrane of Mycobacterium tuberculosis (Mtb) – the main barrier to accumulation – is essential for developing effective antimycobacterial agents. In this study, we investigated two design principles commonly observed in natural products and mammalian cell-permeable peptides: backbone N-alkylation and macrocyclization. To assess how these structural edits impact molecule accumulation beyond the mycomembrane, we utilized our recently developed Peptidoglycan Accessibility Click-Mediated Assessment (PAC-MAN) assay for live-cell analysis. Our findings provide the first empirical evidence that peptide macrocyclization generally enhances accumulation in mycobacteria, while N-alkylation influences accumulation in a context-dependent manner. We examined these design principles in the context of two peptide antibiotics, tridecaptin A1 and griselimycin, which revealed the roles of N-alkylation and macrocyclization in improving both accumulation and antimicrobial activity against mycobacteria in specific contexts. Together, we present a working model for strategic structural modifications aimed at enhancing the accumulation of molecules past the mycomembrane. More broadly, our results also challenge the prevailing belief in the field that large and hydrophilic molecules, such as peptides, cannot readily traverse the mycomembrane.
Tuberculosis (TB) is a major global public health concern, with over 10 million cases reported worldwide in 2022.1 Often recognized as the deadliest infectious disease, TB has recently seen a surge in incidence, reversing a decade-long trend of decline.2 The causative agent of TB is Mycobacterium tuberculosis (Mtb). Mtb and other mycobacteria feature a complex cellular envelope that includes an outer mycomembrane, an arabinogalactan layer, a peptidoglycan (PG) layer, and an inner membrane.3 The mycomembrane is uniquely thick and hydrophobic, primarily composed of long-chain lipids (mycolic acids) and trehalose-based glycolipids.3 This mycomembrane is widely regarded as the primary permeation barrier to the entry of antibiotics, providing mycobacteria with a high level of intrinsic resistance.4–7
Most antibiotics currently used in the treatment of TB, such as isoniazid, ethambutol, and pyrazinamide, are small, hydrophobic molecules developed several decades ago.8 These restrictive molecular features of TB-active antibiotics are thought to result directly from the challenge of crossing the mycomembrane barrier. The overuse of these therapeutics, compounded by suboptimal patient adherence due to prolonged treatment regimens, has led to the emergence of multi-drug resistant (MDR) and total drug-resistant (XDR) strains of Mtb.9 In recent years, only two new antibiotics, bedaquiline and pretomanid (both small and hydrophobic), have been introduced to treat drug-resistant tuberculosis, underscoring the urgent need to expand the antimycobacterial pipeline.10
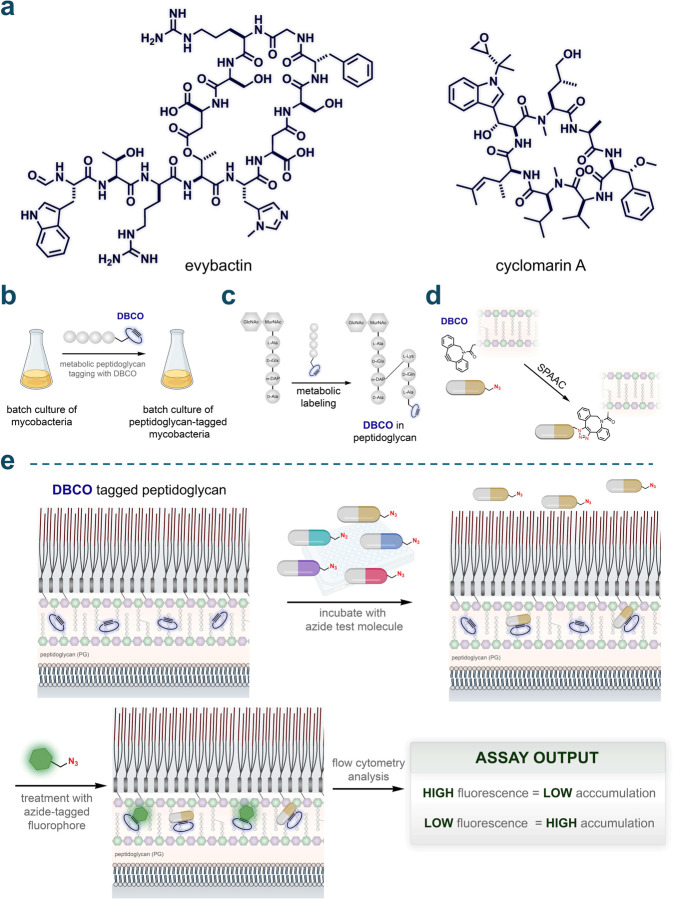
There are notable exceptions to the size constraints typically associated with anti-TB antibiotics (such as rifampicin), suggesting that larger hydrophilic molecules, including peptides, could be viable candidates for antimycobacterial therapy. Peptidic molecules that exceed Lipinski's Rule of Five (Ro5)11 have garnered considerable interest in drug design12–14 across various disease areas (e.g., oncology15 and metabolic16,17 disorders) due to their ability to bind targets with greater specificity and affinity. A significant example is the development of a new class of macrocyclic peptide antibiotics, Zosurabalpin (developed by Roche), which specifically targets the lipopolysaccharide transporter in carbapenem-resistant Acinetobacter baumannii.18 Additionally, peptide candidates are actively being developed for mycobacterial infections.19,20 This growing interest is exemplified by the recent discoveries of evybactin21 and cyclomarin A22 (Fig. 1a), which have served as the basis for a promising series of BacPROTACs.23–26
Fig. 1.
(a) Chemical structures of the peptides evybactin and cyclomarin A. (b) General schematic illustrating the labeling of mycobacteria with DBCO. Mycobacteria were grown in batches and metabolically labeled with DBCO using a modified exogenous stem peptide probe. (c) Schematic depicting site-specific tagging of the peptidoglycan layer with DBCO. An exogenous stem peptide modified with a DBCO moiety on its N-terminus is crosslinked onto the existing peptidoglycan framework. This occurs via the installation of a covalent link between the meso-α,ε-diaminopimelic acid (m-DAP) residue on a stem peptide of the existing peptidoglycan framework and the Lys residue on the exogenously added stem peptide, by transpeptidases. (d) Schematic illustration of strain-promoted azide-alkyne cycloaddition (SPAAC) occurring between the azide-tagged test molecules and the installed DBCO landmarks within the PG. (e) Schematic illustration of PAC-MAN. Incubation of azide-tagged molecules with the DBCO-labeled mycobacteria allows the registration of their arrival past the mycomembrane onto the PG through SPAAC. A subsequent incubation step with an azide-tagged fluorophore in a secondary SPAAC reveals the unoccupied DBCO landmarks. Cellular fluorescence is then measured by flow cytometry where high fluorescence values correspond to low accumulation and vice versa.
Backbone N-alkylation and macrocyclization are common modifications observed in natural peptide products.27–32 These structural changes have been extensively leveraged to enhance the passive membrane permeability of peptides in mammalian systems.33–37 However, these strategies have not yet been empirically validated in mycobacteria. A significant challenge in this field is the general lack of tools to readily measure the accumulation of molecules past the mycomembrane. In our previous work, we developed the Peptidoglycan Accessibility Click-Mediated Assessment (PAC-MAN) assay for live-cell analysis, demonstrating its capability to assess the accumulation of small molecules38 and antibiotics7 across the mycomembrane. Here, we expand upon this work to systematically evaluate the potential of backbone N-alkylation and macrocyclization in peptides to enhance their molecular accumulation levels in live mycobacteria. Through a comprehensive series of peptides, we demonstrate that these structural features can significantly influence accumulation levels. This discovery lays the groundwork for a set of prescriptive modifications that can be employed in developing more effective antibiotics targeting mycobacteria.
RESULTS
Establishing PAC-MAN Assay Parameters
Measuring molecular accumulation in bacteria presents notable technical challenges and remains an area of ongoing research in our laboratory.38–42 Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is considered the gold standard for quantifying whole-cell associated molecule concentrations in bacteria43–46 without the need for chemical tags. However, there are two principal limitations in its current application: (a) limited throughput capacity for conventional setups,45 and (b) a lack of precision in identifying the exact subcellular location of molecules, as it typically measures 'whole-cell' colocalization or association.44,47,48 Despite these challenges, recent efforts in the Hergenrother laboratory have led to the development of the eNTRy rules in Escherichia coli49 and the PASsagE rules in Pseudomonas aeruginosa50 using LC-MS/MS. In contrast, to the best of our knowledge, large-scale LC-MS/MS analyses in Mtb have not been previously described; the largest screen of molecule accumulation conducted prior to the development of PAC-MAN involved a series of ten sulfonyladenosines.51 Critically, PAC-MAN registers the arrival of compounds in the periplasmic space once they have passed the mycomembrane and reached the peptidoglycan (PG) layer.
We previously demonstrated that the PG layer of live mycobacteria can be metabolically labeled with fluorophore-tagged PG analogs.52–54 In a variation of this labeling strategy for PAC-MAN analysis, the fluorophore is replaced with a strained alkyne dibenzocyclooctyne (DBCO) unit (Fig. 1b). When mycobacterial cells are treated with DBCO-tagged PG analogs, transpeptidases incorporate these analogs into the existing PG matrix through crosslinking, creating a distinct, site-specific biorthogonal chemical landmark (Fig. 1c).38,52 The incubation of azide test molecules with the DBCO-tagged mycobacterial cells enables the detection of their arrival past the mycomembrane via a strain-promoted alkyne-azide cycloaddition (SPAAC) reaction (Fig. 1d). Subsequent treatment with an azide-tagged fluorophore reveals the unoccupied DBCO landmarks, making cellular fluorescence inversely proportional to the level of molecule accumulation (Fig. 1e). With this setup, accumulation profiles of hundreds of molecules can be obtained in a 96-well format in parallel within a single experiment. More importantly, the arrival of the molecules past the most critical barrier to entry is confirmed by covalent SPAAC reactions.
Initially, we aimed to build on our previous assay parameters using Mycobacterium smegmatis (Msm), a model organism that recapitulates key features of the pathogenic Mtb.55 Live cell PG labeling was initiated by incubating Msm cells with TetD, a PG analog featuring DBCO on the N-terminus of the stem peptide (Fig. 2a). Two azide-tagged peptides were assembled to establish parameters for the PAC-MAN assay in the context of peptides, which served as the baseline members of a backbone N-alkylated sub-library (Nmet0) (Fig. 2b) and a linear sub-library (Lin0) (Fig. S1). Phenylalanine residues were included to obtain definitive concentrations based on the UV absorbance of the chromophore, and lysine residues were incorporated to enhance water solubility.
Fig. 2.
(a) Schematic illustration and chemical structure of TetD. (b) Chemical structure of the peptide Nmet0. (c) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm. (d) Mass spectrometry analysis of sacculi fragments isolated from TetD treated cells that were subsequently treated with the dye Fl-az. (e) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm labeled with varying concentrations of TetD. (f) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm treated with different azide-tagged fluorophores, and their chemical structures. (g) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm treated with varying concentrations of Fl-az. (h) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm with varying Fl-az treatment durations. (i) Dose-response analysis showing the apparent accumulation of Nmet0 across the mycomembrane in Msm with varying Nmet0 treatment durations. (j) Schematic illustration of optimized assay conditions. For the rest of experiments, TetD labeled mycobacterial cells were incubated with 50 μM azide-peptides for 1 h or 2 h and washed with PBST (phosphate buffered saline with 0.05% tween80) twice. Cells were then treated with 50 μM azide-tagged fluorophore for 1 h and washed with PBST twice and fixed in 4% formaldehyde (in PBS) before high throughput analysis on flow cytometry. All dose-response analyses involving Nmet0 were performed with a 1 h incubation period with the peptide. Data are represented as mean ± SD (n= 3). For dose-response curves, Boltzmann sigmoidal curves were fitted to the data using GraphPad Prism.
In each assay, fluorescence intensities from cells without DBCO landmarks were considered as 0%, while fluorescence intensities from DBCO-tagged cells treated only with the azide-tagged fluorophore were regarded as 100% for the normalization of raw data. To facilitate visual interpretation, the data were processed as “1 − (normalized fluorescence intensity)”, a term referred to as ‘apparent accumulation’, which positively correlates with the accumulation of molecules. We began by testing varying concentrations of Nmet0 via the PAC-MAN assay. Our results showed a clear concentration-dependent increase in apparent accumulation, indicating that the peptide Nmet0 was able to arrive within the periplasmic space of Msm cells (Fig. 2c). The shape of the fitted curve is indeed similar to the concentration-dependent curves generated by the Chloroalkane Penetration Assay (CAPA), developed in the Kritzer lab for measuring penetration into mammalian cells.56 Given the size and hydrophilicity of this peptide, and considering the physicochemical properties of most antimycobacterial agents, the extent of its apparent accumulation is notable. The prevailing understanding in the field suggests that this type of peptide would likely show minimal accumulation past the mycomembrane.
A series of additional experiments was conducted to establish key aspects of the PAC-MAN assay. To confirm the installation of DBCO within the PG scaffold, sacculi were isolated from TetD-treated cells after treatment with the dye Fl-az. This isolation was performed using our previously reported procedures, which involved enzymatic depolymerization of the sacculi with mutanolysin and lysozyme.38,57 Fragments were analyzed by high-resolution quadrupole time-of-flight (Q-TOF) mass spectrometry, which revealed muropeptide fragments containing the click chemistry product (Fig. 2d). Despite the higher absolute fluorescence intensities resulting from treatment with elevated concentrations of TetD (Fig. S2), the apparent accumulation profiles for Nmet0 remained nearly identical across the tested concentration range (Fig. 2e). This indicates that upon normalization, the accumulation profiles for peptides are broadly unaffected by the absolute amount of DBCO landmarks within the PG scaffold, consistent with our previous findings.7 Based on these results, we designated 25 μM of TetD as the labeling concentration for PG labeling in all subsequent assays. Importantly, no significant changes in cell morphology or cell envelope integrity were observed upon treatment with TetD, as evidenced by microscopy (Fig. S3) and ethidium bromide (EtBr) accumulation/efflux experiments (Fig. S4). EtBr is used as an indicator of mycomembrane integrity, which when disrupted, results in increased intracellular accumulation of EtBr, leading to elevated cellular fluorescence.58–62
Next, we tested the tolerance of the PAC-MAN assay toward a range of azide-tagged fluorophores. In addition to Fl-az, we evaluated 3-azido-7-hydroxy coumarin (Co-az), azido-tetramethyl rhodamine (TMR-az), azido-sulfo Cy3 (sCy3-az), and azido-sulfo Cy5 (sCy5-az) with Msm cells (Fig. 2f). As expected, the resulting apparent accumulation curves overlapped across the different azide-tagged fluorophores used. This indicates that upon normalization, the accumulation profiles are largely unaffected by the choice of azide-tagged fluorophores used to detect the unoccupied DBCO landmarks within the PG scaffold. Considering that cellular fluorescence signals for cells treated with Fl-az, sCy3-az, or sCy5-az, all exhibited large signal-to-noise ratios (Fig. S5), we chose Fl-az, which is more widely available, for further experiments.
Similarly, we observed nearly identical normalized cellular fluorescence responses in the PAC-MAN assay when using various concentrations of Fl-az (Fig. 2g) or during varying incubation periods with Fl-az (Fig. 2h) in the chase step. However, longer incubation periods with the test molecule during the pulse step resulted in higher apparent accumulation (Fig. 2i), which is expected given the nature of detection upon periplasmic arrival, which is analogous to the mammalian CAPA.56 We note that the second baseline peptide (Lin0) underwent similar assay development steps as Nmet0, demonstrating comparable results (Fig. S6). Similar assay parameters and features for the PAC-MAN assay were also identified when optimized with azide-tagged small molecules.7 Together, these conditions established the workflow for analyzing test peptides in PAC-MAN (Fig. 2j), in which the concentration and incubation time of the test peptides were the most critical parameters.
Effect of Backbone N-alkylation on Peptide Accumulation
Methylation of the amide backbone in peptides and peptidic natural products has been previously evaluated for its ability to modulate membrane permeability in mammalian systems.31,63–66 This structural edit can alter the hydrogen bonding networks between the peptides and surrounding water molecules, potentially reducing the overall hydrophilicity of the peptide and promoting its desolvation prior to passive diffusion across a hydrophobic environment (Fig. S7). To investigate this feature using PAC-MAN, we constructed libraries of N-methylated peptides derived from the unmodified peptide, Nmet0. In the first subseries, peptides Nmet1 to Nmet5 contain a varying total number of methylation marks within their backbone (Fig. 3a).
Fig. 3.
(a) Chemical structures of the N-methylation sub-library with varying degrees of backbone N-methylation (Nmet0-5). (b) Apparent accumulation of the N-methylation sub-library with varying degrees of backbone N-methylation across the mycomembrane in Msm and Mtb following 1 h of treatment with 50 μM compound. (c) Chemical structures of the N-methylation sub-library with varying positions of backbone N-methylation (Nmet6–9 and Nmet1). (d) Apparent accumulation of the N-methylation sub-library with varying positions of backbone N-methylation across the mycomembrane in Msm and Mtb following 1 h of treatment with 50 μM compound. Data are represented as mean ± SD (n= 3). P-values were determined by a two-tailed t-test (ns = not significant, *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Our data revealed a significant increase in apparent accumulation with a single methylation added to the backbone of the C-terminal residue in Nmet1. This increase was even more pronounced with the addition of a second methylation for Nmet2. However, adding more than three methylation groups to the peptide did not further enhance its accumulation in Msm. Interestingly, the trimethylated molecule, Nmet3, deviated from this trend, exhibiting a reduction in apparent accumulation (Fig. 3b). Previous studies in mammalian systems found that a higher number of N-methyl groups may not necessarily correlate with improved permeability, potentially due to conformational preferences that are difficult to predict.67,68 In Mtb, no statistical differences were observed among the first three methylation marks, while an improvement was noted beyond three (Fig. 3b).
In the second subseries (Nmet6 to Nmet9), a single methylation was systematically installed across the peptide backbone of Nmet0 (Fig. 3c). Similar apparent accumulation was observed when methylation was applied to the central backbone amides (Nmet7–9); however, a slight decrease in accumulation was noted when the methylation mark was positioned at the N-terminus for Nmet6 (Fig. 3d). Interestingly, no significant differences in accumulation were found within the mono-methylation series in Mtb (Fig. 3d). Extensive work by the Kessler and Lokey laboratories has highlighted the structural elements related to the degree and positional effects of backbone N-methylation on mammalian permeability in the context of oral bioavailability.67–73 These data suggest an overall trend that backbone N-methylation can modulate peptide accumulation across the mycomembrane, although the optimal degree and position may be species-specific.
To explore the contribution of the backbone amides in a different but biologically relevant context, we constructed a peptoid library that retained the same side chain sequence as the N-methylation library. Similar to N-methylated peptides, peptoids lack the hydrogen bond donor capacity of the backbone amide74–79, which could potentially enhance their permeability compared to their peptide counterparts.74,80 Like the N-methylation peptide library, peptoids Nalk1 to Nalk5 were assembled with an increasing number of N-substituted glycine units from the N- to the C-terminus (Fig. S8a) to systematically evaluate different degrees of backbone N-alkylation. Peptoids Nalk5 to Nalk9 were synthesized to vary the positions of a single N-substituted glycine unit across the peptidic backbone (Fig. S8b). In contrast to our results for backbone N-methylated peptides (Nmet1-9), we observed a much greater impact and variability in apparent accumulation with N-alkylated peptoids in Msm. The apparent accumulation of the peptoids significantly increased, reaching a maximum level with three N-substituted glycine units; however, an unexpected drop was noted for the fully substituted peptoid, Nalk5. No significant difference in apparent accumulation was observed for the N-terminal alkylated peptoid Nalk6 compared to the base peptide Nmet0. Additionally, there was no preference for substitutions on the central residues for Nalk7–9 (Fig. S8c). Overall, our results suggest that backbone alkylation in peptoids can lead to higher accumulation across the mycomembrane compared to their corresponding canonical versions.
Next, we aimed to test potential differences in SPAAC reactivity within our series of azide-tagged molecules as they engage with the installed DBCO landmarks. Previous work from our group investigated the reactivity of azide-tagged small molecules using a bead-based assay.38 Flow cytometry-compatible DBCO-coated beads, chemically modified from commercially available amino-functionalized beads, served as surrogates for mycobacterial cells without the critical mycomembrane barrier. Similar to our live cell PAC-MAN assay, beads were incubated with the same concentration of azide-tagged test molecules, followed by a chase step with an azide-tagged fluorophore. Background fluorescence levels from unmodified beads treated with Fl-az were normalized to 0%, while fluorescence levels from DBCO-modified beads treated with the vehicle were normalized to 100%. All molecules in both the N-methylation library and the peptoid library fully reacted with the DBCO landmarks on the beads (Fig. S9, S10). These results strongly support the notion that our apparent accumulation findings across the mycomembrane reflect the level of molecule arrival within the periplasmic space. We also investigated the integrity of the mycobacterial cell envelope after metabolic labeling by TetD and subsequent treatment with our library by using EtBr accumulation measurements. No differences were detected in EtBr accumulation upon treatment with different azide molecules (Fig. S11, S12). These findings suggest that the mycomembrane is not artificially disrupted during incubation with the azide peptides, supporting our assay as a reliable method for testing the accumulation of modified peptides past this critical barrier.
Effect of Macrocyclization on Permeability
Cyclization has been extensively explored to improve the pharmacokinetic properties of large molecules such as peptides.81,82 Cyclic molecules present a more compact and favorable shape compared to their extended acyclic counterparts, which can facilitate their penetration through membrane barriers.29,34,83 The cyclic conformation may promote intramolecular hydrogen bonding and reduce the exposure of polar functional groups to the surrounding environment, thereby decreasing their solubility in water and promoting membrane integration (Fig. 4a). For the assembly of our macrocyclic peptide library, we chose a thioether-based cyclization strategy because of its metabolic stability and ease of formation under mild conditions.84 Additionally, thioether macrocyclic peptides are being extensively explored as potential drug scaffolds.85–88 We hypothesized that a combination of two modifications on peptides would enable in-solution cyclization: (1) an N-chloroacetyl group coupled onto the N-terminus of the peptide, and (2) a cysteine residue incorporated into the peptide sequence (Fig. S13). Importantly, we avoided placing cysteine residues at the C-terminal position due to concerns regarding potential epimerization89 and the formation of β-piperidinylalanine90 during peptide chain extension. For each macrocyclic peptide, we designed a linear counterpart with a largely preserved sequence, while replacing cysteine residues with serine and acetylating the N-terminus to closely mimic the composition of the cyclic peptide. We did not retain the cysteine residues in the linear peptides due to their susceptibility to rapid oxidation in the live cell assay, which could lead to various unique oxidized structures that are difficult to measure. Based on this macrocyclic library design, we synthesized three distinct sub-libraries combining standard solid-phase peptide synthesis and in-solution cyclization.
Fig. 4.
(a) Schematic illustration of a macrocyclic and a linear peptide interacting with the surrounding water molecules in an aqueous solution. Hydrogen bonds are indicated by dashed lines. Macrocyclization can promote intramolecular hydrogen bonding resulting in the reduction of hydrogen bonds between the backbone amides and the solvent, thereby potentially reducing membrane permeation associated desolvation penalty. (b) Chemical structures of the linear vs macrocyclic sub-library members containing macrocyclic peptides of increasing sizes (Cyc0-3) and their linear counterparts (Lin0–3). (c) Apparent accumulation of the linear vs macrocyclic sub-library across the mycomembrane in Msm and Mtb following 2 h of treatment with 50 μM compound. (d) Chemical structures of the ring size (lariat) sub-library members containing macrocyclic peptides of increasing ring sizes (Lar1-5) and their linear counterpart (Lar0). (e) Apparent accumulation of the ring size sub-library across the mycomembrane in Msm and Mtb following 2 h of treatment with 50 μM compound. (f) Chemical structures of the cyclization chemistry sub-library members containing a disulfide bonded macrocyclic peptide (Dit1), a bis-electrophilic linker based macrocyclic peptide (Dit2) and their linear counterpart (Dit0). (g) Apparent accumulation of the cyclization chemistry sub-library across the mycomembrane in Msm and Mtb following 2 h of treatment with 50 μM compound. Data are represented as mean ± SD (n= 3). P-values were determined by a two-tailed t-test (ns = not significant, *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Recognizing that the molecular size of macrocyclic peptides can influence membrane permeability,91,92 we designed a set of macrocyclic peptides with varying numbers of leucine and serine residues to systematically increase the cycle size (Cyc0-3) (Fig. 4b). This sub-library was then tested using our PAC-MAN assay in live mycobacteria. Generally, across all sizes tested, the macrocyclic peptides demonstrated better apparent accumulation across the mycomembrane compared to their linear counterparts, as observed in both Msm and Mtb (Fig. 4c). Additionally, in both organisms, the apparent accumulation of both linear and macrocyclic peptides diminished with increasing molecular size (Fig. 4c). Cyc0, the smallest macrocyclic peptide in our panel, exhibited better accumulation than any other member of this macrocyclic sub-library, which was comparable to the most permeable molecule identified in our previous work.38
Next, inspired by natural products, we focused our attention on lariat-shaped molecules. These molecules are characterized by a looped or "lasso" structure and have recently gained recognition for their utility as drug scaffolds93 and membrane permeators.68 A subset of these molecules has shown promising antimycobacterial activity, such as lassomycin.94 Their flexible linear tails provide additional functional versatility that can be modulated to specifically target proteins of interest. We prepared a series of lariat peptides by cyclizing between an N-terminal chloroacetyl group, and a cysteine side chain positioned at various distances from the N-terminus, thereby decreasing the size of their cyclic portion (Lar1-5) (Fig. 4d). The linear control, Lar0, was designed to be N-acetylated without any cysteine residues. The macrocyclic lariat-shaped peptides Lar1-4 demonstrated better accumulation profiles than the linear Lar0 in Msm (Fig. 4e). In contrast, Lar5 exhibited poor accumulation in Msm, which we attributed to the small size of its ring, leaving a substantial portion of the peptide flexible and exposed to the solvent (Fig. 4e). Within the lariat-shaped macrocyclic series (Lar1-5), no discernible trend was observed in either Mtb or Msm. However, Lar4 appeared to have a privileged scaffold over the other lariats and the linear control Lar0 in terms of accumulation into Mtb (Fig. 4e).
We recognized that the method of cyclization could influence the conformation and structural rigidity of peptides, which might affect their interaction with the mycomembrane. It has been shown in mammalian systems that the structural features of the linker can alter peptide accumulation.95 In mycobacteria, we expanded our cyclic peptide library to include peptides cyclized using two cysteine side chains. First, we synthesized an N-acetylated dithiol peptide scaffold that possesses the same sequence as Cyc0, but with an additional cysteine residue at the N-terminus. This dithiol peptide was then directly oxidized to generate a disulfide bond between the two cysteine side chains, resulting in Dit1 (Fig. 4f). This analysis is significant due to the growing interest in disulfide-cyclized peptides as drug candidates.96–100 Additionally, we cyclized the linear dithiol peptide scaffold with a bis-electrophilic linker, yielding Dit2, which contains two thioether bonds (Fig. 4f). These types of linkers are frequently used in constructing macrocyclic peptide libraries due to their broad sequence compatibility and suitability for late-stage conformational diversification,101–104 prompting us to investigate a test case. Lastly, we synthesized a linear control, Dit0, by replacing the cysteine residues with serine (Fig. 4f).
Among this sub-library, we observed that the linear control, Dit0, exhibited the highest level of accumulation in Msm compared to its macrocyclic counterparts Dit1 and Dit2 (Fig. 4g). For Dit1, this may be partly due to interception by thiol/disulfide-displaying proteins in the cell envelope before it reaches the PG.105 Thiol-disulfide exchange reactions between disulfide-containing molecules and exofacial protein thiols/disulfides have previously been shown to modulate permeability.106,107 In the case of Dit2, we pose that the hydrophobicity of the linker itself may significantly contribute to peptide accumulation, similar to observations made in mammalian systems.95 In Mtb, we observed no statistical differences in apparent accumulation within this series (Fig. 4g). The difference in the apparent accumulation profiles of Cyc0 and Dit1 can be attributed to the difference of just one amino acid residue and the mode of cyclization. As previously established, we confirmed that the macrocyclic peptide library readily reacted with our DBCO-modified beads (Fig. S14) and that cell integrity remained unaffected upon treatment with the peptides, as indicated by EtBr analysis (Fig. S15). Taken together, our data suggest a general trend that macrocyclization can improve peptide accumulation across the mycomembrane, with additional considerations regarding ring size and the mode of cyclization.
We then sought to decipher the mechanistic basis for the enhanced accumulation of macrocyclic peptides in comparison to their linear counterparts. By evaluating the physicochemical properties of our libraries, we found that most library members, including the peptide with the highest accumulation levels, significantly exceeded the rule-of-five (Ro5) parameters established by Lipinski11 and Veber102 (Fig. S16). This finding highlights a gap in our understanding of the molecular characteristics that enable traversal of the mycomembrane. It is well established that intramolecular hydrogen bonding and the solvent-accessible surface area of a molecule are critical factors influencing permeability across membrane bilayers.109–112 This feature often informs the rationale for both the macrocyclization82,113 and N-methylation114 of peptides to improve membrane permeability by reducing solvent accessibility. Therefore, we aimed to determine whether variable solvent accessibility of the amide N−H between the linear and macrocyclic peptides is responsible for the differences observed in their accumulation across the mycomembrane.
To investigate this, we conducted hydrogen/deuterium exchange (HDX) experiments with the molecules Cyc0 and Lin0 using 1H-NMR (Fig. 5a). We characterized these molecules using 1D and 2D-NMR, assigning all N-H resonances. Generally, the backbone amide hydrogens of Lin0 exchanged more rapidly with the deuterated solvent molecules than those of Cyc0, showing complete exchange within an hour (Fig. 5b). As expected, not all amide hydrogens exchanged equally; for example, the amide hydrogen on the azide-lysine (Kaz) residue of Lin0 exhibited complete exchange within the dead time of the assay (~5 mins) (Fig. 5c). In contrast, the exchange of the amide hydrogen on Kaz of Cyc0 was not complete even at the 25-minute mark (Fig. 5c).
Fig. 5.
(a) Schematic illustration of the hydrogen-deuterium exchange (HDX) analysis of peptides. Peptides are incubated in a D2O buffer, allowing the exchange of amide protons (N-H) with deuterium, with the exchange rate reflecting the solvent accessibility of the N-H bonds. The HDX process is tracked using 1H-NMR spectroscopy. (b) 1H-NMR spectra illustrating the time-dependent HDX of the backbone amide protons in Lin0 and Cyc0 over 1 h. (c) Fraction of Kaz1 N-H protons of Lin0 and Cyc0 exchanged to N-D over time during HDX. (d) Fraction of L2 N-H protons of Lin0 and Cyc0 exchanged to N-D over time during HDX. (e) 3-D representation and chemical structure of Cyc0 showing hydrogen bonding observed through molecular dynamics simulations. (f) Comparison of serum stability of Lin0 and Cyc0 incubated in mouse serum at 37 °C over 1 h. For serum stability analyses, one-phase decay curves were fitted to the data using GraphPad Prism.
To further compare these two peptides, we focused on the backbone amide L2, which was present in both molecules and was well-resolved in the NMR spectra. The L2 amide on Cyc1 exhibited a half-life of 18 minutes, compared to 16 minutes for Lin0 (Fig. 5d). This observation highlights how macrocyclization can potentially shield the L2 backbone amide from solvent hydrogen bonding. The L2 backbone amide of Cyc0 may also participate in intramolecular hydrogen bonding, which could hinder exchange with solvent molecules. To support this analysis, molecular dynamics (MD) simulations were performed with Cyc0 and Lin0. Our results indicated that the Cyc0 backbone amides extensively participate in intramolecular hydrogen bonding (Fig. 5e), while no observable intramolecular hydrogen bonding was detected for the linear counterpart, Lin0.
Another well-recognized biochemical consequence of peptide cyclization is greater resistance to proteases.111,115–117 This protection may be partly attributed to the inability of the macrocyclic peptide to be recognized by the active site of serine proteases in the ideal conformation, given its limited flexibility.116,118 Upon testing the metabolic stability of both Cyc0 and Lin0, we observed that Lin0 fully degraded within 1 hour of serum incubation, while Cyc0 remained stable with no significant degradation (Fig. 5f). A similar observation was made regarding backbone N-methylation, where peptide Nmet5 exhibited considerably greater serum stability than peptide Nmet0, likely due to the steric hindrance introduced by N-methylation toward serine proteases (Fig. S17).119,120
Effect of Structural Edits on Peptide Antibiotics
Building on our findings, we set out to modulate the activity and accumulation profile of a peptidic antibiotic with potential activity against mycobacteria by applying these structural edits. Tridecaptin A1 is a non-ribosomal lipopeptide known for its antimicrobial activity against Gram-negative bacteria. Its mode of action is related to its binding to the bacterial cell wall precursor lipid II and subsequently disrupting the proton motive force.121,122 Binding to lipid II is a common mode of action for many potent antibiotics, including the glycopeptide vancomycin.123 To the best of our knowledge, tridecaptin A1 has not been shown to be active against mycobacteria. Given the theoretical necessity of crossing the mycomembrane to bind lipid II, the inherent lack of appreciable antimycobacterial activity may be attributed to insufficient accumulation of the peptide in the periplasmic space. Therefore, we propose that structural edits aimed at enhancing accumulation while maintaining target lipid II engagement could improve its antibacterial properties.
Considering the structural similarities between mycobacterial lipid II and Gram-negative lipid II, we hypothesized that backbone N-methylation or macrocyclization could potentially modulate the activity of tridecaptin A1 against mycobacteria by enhancing its accumulation past the mycomembrane. In redesigning tridecaptin, we started with the octanamide analog, Octyl-tridecaptin, as it retained biological activities and is more synthetically tractable.124 Based on previous studies, an alanine scan found that amino acid residues in positions 1, 4, 6, 10, and 11 can be substituted with alanine without significantly compromising their biological activity against Gram-negative pathogens.125 Furthermore, it was reported that the side chain carboxylate of Glu10 can be conjugated with antibiotic warheads to improve its activity in vitro.126 Relying on these prior efforts, we designed a series of analogs from the parental peptide, substituting the Glu10 residue with an azide-bearing lysine analog (tri) for compatibility with our assay (Fig. 6a).
Fig. 6.
(a) Chemical structure of the peptide tri, the tridecaptin parent analog in our series. (b) Chemical structures of the peptides triMe1, triMe2, triMe3, the N-methylated tridecaptin analogs in our series. (c) Dose-response analysis showing the apparent accumulation of the N-methylated tridecaptin analogs across the mycomembrane in Msm. (d) MIC values of the N-methylated tridecaptin analogs against Msm. (e) Chemical structure of triCyc, the macrocyclic tridecaptin analog in our series. (f) Dose-response analysis showing the apparent accumulation of the macrocyclic tridecaptin analog triCyc in comparison to the parent tridecaptin analog tri, across the mycomembrane in Msm. (g) MIC values of the macrocyclic and linear tridecaptin analogs triCyc and triLin, in comparison to the parent tridecaptin analog tri, against Msm. (h) Chemical structures of the peptides grisLin, grisCyc, grisMeLin, and grisMeCyc, the griselimycin analogs in our study. (i) Apparent accumulation of the griselimycin analogs across the mycomembrane in Msm following 6 h of treatment with 50 μM compound. (j) MIC values of the of the griselimycin analogs against Msm. All MIC values were obtained from three biological replicates which showed identical results. Other data are represented as mean ± SD (n= 3). For dose-response curves, Boltzmann sigmoidal curves were fitted to the data using GraphPad Prism. All dose-response analyses involving the tridecaptin series were performed with a 1 h incubation period with the peptides. P-values were determined by a two-tailed t-test (ns = not significant, * p < 0.1, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
N-methylation of the parent peptide should ideally not disrupt target binding. N-methylation sites were selected by examining the reported solution NMR structure of the tridecaptin A1-lipid II complex.121 The amide backbones of residues Ser6, Val11, or Ala13 were selected for methylation. Specifically, we generated monomethylated (triMe1 with meAla13), dimethylated (triMe2 with meVal11 and meAla13), and trimethylated (triMe3 with meSer6, meVal11, and meAla13) analogs of tri (Fig. 6b). The dose-response accumulation profiles of the N-methylated peptides were then obtained via live cell PAC-MAN in Msm (Fig. 6c, S18). triMe1 and triMe2 exhibited better accumulation compared to the unmethylated tri. However, triMe3 showed slightly worse accumulation than triMe2, suggesting that additional methylation on Ser6 negatively affects its apparent accumulation in mycobacteria. We noted that the unmethylated parent peptide tri demonstrated fluorescence intensities higher than those of the vehicle control at lower concentrations, resulting in negative apparent accumulation values (Fig. S18). We attributed this observation to potential cell envelope disruption by tri, which could theoretically modulate the accumulation level of the fluorophore and lead to higher fluorescence intensities. Indeed, incubation with tri significantly affected cell envelope integrity, allowing for higher EtBr accumulation (Fig. S19). No significant effects were observed in any of the N-methylated analogs. Importantly, we also ensured that the peptides readily reacted with our DBCO-modified beads (Fig. S20). Next, we studied the effect of N-methylation on the antimicrobial activity of tri against Msm via a Minimal Inhibitory Concentration (MIC) assay. A marked decrease in the MIC value of the peptide antibiotic was observed with an increasing degree of methylation, with trimethylated triMe3 exhibiting an 8-fold reduction in MIC value compared to unmethylated tri (Fig. 6d). Interestingly, the unmethylated analog tri exhibited fast amyloid-like aggregation compared to the trimethylated analog triMe3, indicated by a thioflavin T (ThT) fluorescence assay (Fig. S21). The ThT fluorescence assay is a semi-quantitative method used for the analysis of amyloid-like aggregates in the system.127,128 This feature has been reported as an critical mechanism of action for the antimicrobial activity of teixobactin.127–129 On the contrary, in our hands, diminished aggregation in the case of the trimethylated analog triMe3 was correlated with better antimycobacterial activity in comparison to tri. Overall, these data indicate that backbone N-methylation of tri enhances its activity against Msm by improving its apparent accumulation across the mycomembrane.
We then sought to analyze the effect of macrocyclization on the activity of tri against Msm. Octyl-tridecaptin A1 had previously been cyclized by replacing D-Trp5 and L-Phe9 with D- and L-cysteine residues, respectively, and the peptides were subsequently cyclized using bis-electrophilic linkers.130 Adopting a similar approach, we synthesized triCyc, wherein the peptide was cyclized with a bis-bromomethyl-benzene linker between the cysteine residues, and Glu10 was substituted with an azide-bearing lysine analog (Fig. 6e). The dose-response accumulation profile of triCyc was then analyzed via live cell PAC-MAN in Msm (Fig. 6f, S22). The cyclic form (triCyc) exhibited better accumulation across the mycomembrane compared to the parent peptide tri. To further elucidate the impact of macrocyclization, we also synthesized triLin, in which the D-Trp5 and L-Phe9 residues were replaced with D- and L-serine residues, respectively, to serve as a linear control (Fig. S23a). Interestingly, we observed no significant difference in accumulation between triCyc and triLin (Fig. S23b, c). Furthermore, EtBr accumulation experiments revealed that both triCyc and triLin had no significant effect on cell envelope integrity (Fig. S24). The peptides also readily reacted with our DBCO-labeled beads (Fig. S25). Next, we sought to examine the antimicrobial activity of triCyc and triLin against Msm. For triCyc, we also observed a 4-fold reduction in the MIC value when compared to the parent peptide tri (Fig. 6g). triCyc also exhibited over a 4-fold improvement in antimicrobial activity compared to triLin. Taken together, these data suggest that macrocyclization enhances the antimicrobial activity of tri against Msm.
We then aimed to investigate the influence of N-alkylation and macrocyclization on the antimicrobial activity and accumulation of a peptidic antibiotic that inherently possesses these structural features. Griselimycin, a peptide originally derived from Streptomyces,131 has been reported to exhibit antimicrobial activity against both Msm and Mtb.132–134 The antimicrobial activity of this peptide is mediated through its binding to the mycobacterial DNA polymerase III sliding clamp (DnaN), resulting in the disruption of DNA replication.132 Notably, the sliding clamps of other bacterial species have also been investigated as potential targets for griselimycin135,136 and other antimicrobial agents137–142. Structurally, griselimycin is a lariat-shaped peptide, cyclized through an ester bond between the C-terminus and the side chain hydroxyl group of its internal Thr3 residue. The peptide also contains four backbone N-methylation marks at residues - Val1, Thr3, Val7, and D-Leu9. To facilitate compatibility with the PAC-MAN assay, we designed analogs in which the N-terminal acetyl group present in the parental peptide was replaced with a 2-azido-acetyl group. Additionally, the 4-methyl-proline residues in the parental peptide were substituted with proline residues due to greater commercial availability, while acknowledging that this substitution may affect target engagement. The analogs were synthesized in both linear and cyclic forms, with or without backbone N-methylation, resulting in four distinct variants: grisMeCyc, grisCyc, grisMeLin, and grisLin (Fig. 6h).
The accumulation profiles of the griselimycin series were then obtained via live cell PAC-MAN in Msm. We observed that macrocyclization was important for the accumulation of grisLin, which had considerably lower accumulation than grisCyc. This effect was however absent in the case of grisMeCyc which did not significantly differ in its accumulation with respect to grisMeLin (Fig. 6i). The N-methylation data were interesting in that grisCyc in fact exhibited slightly improved accumulation over grisMeCyc, whereas grisLin accumulated to a much lower level than grisMeLin (Fig. 6i). As before, we ensure that the peptides readily reacted with our DBCO-labeled beads (Fig. S26)
Upon evaluating the antimicrobial activity of the peptides, the loss of macrocyclization in grisMeCyc resulted in over a 32-fold increase in the MIC value of grisMeLin (Fig. 6j). Similarly, the removal of methylation also led to a reduction in activity, with grisCyc performing weaker than grisMeCyc (Fig. 6j). The simultaneous loss of both macrocyclization and methylation was also detrimental to the peptide's activity. Collectively, these results highlight the critical role of macrocyclization and N-methylation in maintaining the antimicrobial activity of griselimycin against Msm.
DISCUSSION
Nature possesses a variety of biosynthetic machineries capable of constructing both small and large molecules. Over time, the evolution of small molecules with high molecular complexity and membrane permeability might lead us to anticipate that nature favors antibiotics with low molecular weights. After all, since most antibiotics target intracellular sites, they must overcome one or more membrane bilayers to reach these targets. However, this is not exclusively the case in nature. A substantial number of biologically active natural products, including many antibiotics, are large, polar, and often exceed the Rule of Five (Ro5) criteria. These molecules typically have a peptidic nature, a trait that can result from biosynthetic pathways, such as in ribosomally synthesized and post-translationally modified peptides (RiPPs). Larger peptide-like molecules offer significant advantages, primarily by interacting with their molecular targets more extensively, resulting in higher affinity and specificity. This size trade-off must be balanced with considerations of accumulation inside target cells. Consequently, it is commonly observed that peptidic antibiotics are extensively modified to maintain sufficient membrane permeability.
The assembly of most antibiotic peptides offers an avenue for unusual modifications, including the incorporation of structural elements that enhance permeability. While the range of structural alterations is vast, the alkylation of the amide backbone and macrocyclization are particularly notable. N-alkylation reduces the number of hydrogen bonds formed with the solvent, thereby decreasing the desolvation penalty associated with passive membrane diffusion. Some peptides, or molecules in general, can also exhibit chameleonic behavior during passive membrane diffusion, a trait enhanced by backbone alkylation.30,31,143 Indeed, N-methylation is used as an effective approach to improve peptide permeability through mammalian cell membranes.31,64–66,144 A similar approach to reducing the number of backbone hydrogen bond donors is to shift peptide side chains from the α-carbon to the backbone nitrogen atom to create peptidomimetics, i.e. peptoids. Peptoids are easy to synthesize from primary amines and allow the possibility of versatile side chain libraries – albeit at the cost of chiral centers on the backbone.145 Various peptoids have demonstrated effectiveness against drug-resistant bacterial pathogens.75–79 Proline residues, like peptoids, naturally lack backbone hydrogen bond donors due to alkylation. Notably, some peptide antibiotics against mycobacteria, such as griselimycin132 and callyaerin146 have three or more prolines in their structure. Similarly, macrocyclization enhances the suitability of peptides as drug candidates by improving passive permeability through intramolecular hydrogen bonding,35 thereby reducing the desolvation penalty associated with membrane permeation.109–111 Furthermore, the structural preorganization provided by macrocyclization mitigates entropic loss during target binding.147 Additionally, it can enhance other pharmacological properties, such as metabolic stability,17,148 which may be especially relevant in therapeutic contexts.
The field of de novo and natural product-based peptide antibiotics continues to expand, making the prospect of orally available peptide drugs increasingly realistic. However, it remains largely undefined how N-alkylation and macrocyclization affect accumulation in mycobacteria. Given the unique composition of the mycomembrane and its central role in determining which molecules can effectively impact mycobacterial cells, understanding the impact of these peptidic alterations is crucial for the design and redesign of natural product drug leads. In turn, a comprehensive understanding of how structural features drive accumulation will facilitate the identification of high-potential drug candidates and enable the strategic redesign of existing chemical scaffolds to enhance their accumulation within mycobacterial cells.
By assessing the accumulation of N-alkyl and peptoid-likes compounds in mycobacteria, our work demonstrated a general trend: higher degrees of N-alkylation usually led to higher accumulation levels past the mycomembrane.149 Moreover, in our panels of macrocyclic peptides, we observed that generally cyclic peptides had higher apparent accumulation across the mycomembrane than their linear counterparts, underscoring the significant impact of ring size and cyclization chemistry in some cases. It is important to note that the field differs on whether cyclization helps improve accumulation. A report from the Kodadek laboratory challenges the notion that macrocyclization necessarily enhances the cell permeability of linear peptides in every case,150 presenting findings that contradict other studies supporting such an improvement.35,151 Our findings reveal that the improvement in accumulation past the mycomembrane may not be absolute, but rather context-dependent. We propose that critical factors such as lipophilicity, ring size, and cyclization chemistry should be carefully considered when attempting to enhance permeability through macrocyclization. Such empirical evaluations are enabled by methodologies like our PAC-MAN assay.
Based on our findings regarding the impact of N-alkylation and macrocyclization, we applied these principles to a known antibiotic. Unexpectedly, we observed that the parent molecule, tri, inherently disrupted the mycobacterial cell envelop, whereas the N-methylation of the tridecaptin peptide mitigated the membrane disruption effect and improved its antimycobacterial activity. In contrast, previous studies reported that backbone N-methylation of teixobactin152 and other reported antimicrobial peptides153 negatively affected their antimicrobial activity. These results emphasize the value of empirical analyses of accumulation, separate from MIC assessments, in understanding how structure influences accumulation. Overall, both N-alkylation and macrocyclization enhanced the efficacy of tridecaptin A1 against mycobacteria, underscoring the importance of our new insights into the role of structural factors in improving accumulation beyond the mycomembrane. This case illustrates how redesigning a known antimicrobial molecule, previously not shown to be active against mycobacteria, can enhance its accumulation and activity. Notably, since nature frequently combines these structural features in molecular design, further analysis of tridecaptin A1 peptides and other molecules modified with both N-alkylation and macrocyclization presents an intriguing research opportunity. We are currently exploring additional modifications to tridecaptin to optimize its activity and potentially guide an analog toward clinical evaluation.
To investigate the impact of systematically removing these inherent structural features, we examined griselimycin, a macrocyclic, N-methylated peptide that is active against Msm. Our results revealed a significant loss of activity upon the deletion of one or both of these structural features, highlighting the critical role they play in preserving efficacy against Msm. Notably, the indispensability of these features in this case underscores how even subtle structural modifications can profoundly impact antimicrobial activity, highlighting the importance of optimizing these key elements in the design of effective antibiotics.
PAC-MAN offers several key advantages over other technologies, including ease of deployment, which makes it potentially adoptable by most laboratories; suitability for high-throughput analysis; and the capability to achieve subcellular resolution, as opposed to whole-cell analysis. However, a limitation of PAC-MAN is its inability to provide information regarding the arrival of the test molecule in the cytosol. Further work is needed to develop methodologies that identify structural modifications capable of enhancing accumulation into the cytoplasm. Since the mycomembrane is often regarded as the primary barrier to accumulation, we propose that establishing guidelines to facilitate passage through this membrane represents a critical first step in creating an antimycobacterial pipeline to combat deadly diseases, such as TB.
In this work, we present the first systematic governing rules for the rational redesign of potential antimycobacterial agents. Our comprehensive exploration of structural modifications – specifically N-alkylation and macrocyclization – provides key insights into designing more effective antimycobacterial agents. Our study elucidates how these modifications enhance peptide accumulation across the intricate mycomembrane, challenging the conventional belief that large hydrophilic molecules cannot efficiently penetrate this barrier. The novel application of these strategies to the peptide antibiotic tridecaptin A1 highlights their potential to overcome intrinsic resistance mechanisms and optimize antibacterial efficacy. Similarly, the marked loss of activity upon deletion of these structural features in griselimycin underscores their fundamental role in preserving its antimicrobial activity and thus the importance of structural optimization in antibiotic design. Moreover, our PAC-MAN assay not only serves as a high-throughput platform for evaluating accumulation dynamics but also lays the groundwork for future empirical investigations into improving cytoplasmic delivery. These findings significantly contribute to the growing arsenal against TB by guiding the rational design of next-generation therapeutics capable of breaching the formidable defenses of mycobacteria. Further research integrating these structural features with conventional drug frameworks may yield potent treatments tailored to combat the escalating threat of drug-resistant mycobacterial strains.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by the NIH grants GM124893-01 (M.M.P.), R01AI178975-01 (M.M.P. and W.I) and R01AI179080-01 (M.M.P., W.I., and M.S.S.). We thank Jeff Ellena, Department of Chemistry, University of Virginia, for NMR spectroscopy assistance.
Footnotes
SUPPORTING INFORMATION
Additional figures, tables, and materials/methods are included in the supporting information file.
REFERENCES
- (1).Global Tuberculosis Report 2023 - TB Incidence. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/tb-disease-burden/1-1-tb-incidence (accessed 2024-06-03).
- (2).Williams P. M. Tuberculosis — United States, 2023. MMWR Morb Mortal Wkly Rep 2024, 73. 10.15585/mmwr.mm7312a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Chiaradia L.; Lefebvre C.; Parra J.; Marcoux J.; Burlet-Schiltz O.; Etienne G.; Tropis M.; Daffé M. Dissecting the Mycobacterial Cell Envelope and Defining the Composition of the Native Mycomembrane. Sci Rep 2017, 7 (1), 12807. 10.1038/s41598-017-12718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dulberger C. L.; Rubin E. J.; Boutte C. C. The Mycobacterial Cell Envelope — a Moving Target. Nat Rev Microbiol 2020, 18 (1), 47–59. 10.1038/s41579-019-0273-7. [DOI] [PubMed] [Google Scholar]
- (5).Bansal-Mutalik R.; Nikaido H. Mycobacterial Outer Membrane Is a Lipid Bilayer and the Inner Membrane Is Unusually Rich in Diacyl Phosphatidylinositol Dimannosides. Proc Natl Acad Sci U S A 2014, 111 (13), 4958–4963. 10.1073/pnas.1403078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kamariza M.; Shieh P.; Bertozzi C. R. Chapter Thirteen - Imaging Mycobacterial Trehalose Glycolipids. In Methods in Enzymology; Imperiali B., Ed.; Chemical Glycobiology Part B. Monitoring Glycans and their Interactions; Academic Press, 2018; Vol. 598, pp 355–369. 10.1016/bs.mie.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Lepori I.; Jackson K.; Liu Z.; Chordia M. D.; Wong M.; Rivera S. L.; Roncetti M.; Poliseno L.; Pires M. M.; Siegrist M. S. The Mycomembrane Differentially and Heterogeneously Restricts Antibiotic Permeation. bioRxiv 2025, 2024.12.31.630956. 10.1101/2024.12.31.630956. [DOI] [PubMed] [Google Scholar]
- (8).Smith I. Mycobacterium Tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clinical Microbiology Reviews 2003, 16 (3), 463–496. 10.1128/cmr.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Smith T.; Wolff K. A.; Nguyen L. Molecular Biology of Drug Resistance in Mycobacterium Tuberculosis. In Pathogenesis of Mycobacterium tuberculosis and its Interaction with the Host Organism; Pieters J., McKinney J. D., Eds.; Springer: Berlin, Heidelberg, 2013; pp 53–80. 10.1007/82_2012_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Francesca Conradie; Bagdasaryan Tatevik R.; Sergey Borisov; Pauline Howell; Lali Mikiashvili; Nosipho Ngubane; Anastasia Samoilova; Sergey Skornykova; Elena Tudor; Ebrahim Variava; Petr Yablonskiy; Daniel Everitt; Wills Genevieve H.; Eugene Sun; Morounfolu Olugbosi; Erica Egizi; Mengchun Li; Alda Holsta; Juliano Timm; Anna Bateson; Crook Angela M.; Fabiane Stella M.; Robert Hunt; McHugh Timothy D.; Tweed Conor D.; Foraida Salah; Mendel Carl M.; Melvin Spigelman. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. New England Journal of Medicine 2022, 387 (9), 810–823. 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings1. Advanced Drug Delivery Reviews 2001, 46 (1), 3–26. 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- (12).Wang L.; Wang N.; Zhang W.; Cheng X.; Yan Z.; Shao G.; Wang X.; Wang R.; Fu C. Therapeutic Peptides: Current Applications and Future Directions. Sig Transduct Target Ther 2022, 7 (1), 1–27. 10.1038/s41392-022-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Muttenthaler M.; King G. F.; Adams D. J.; Alewood P. F. Trends in Peptide Drug Discovery. Nat Rev Drug Discov 2021, 20 (4), 309–325. 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- (14).Doak B. C.; Over B.; Giordanetto F.; Kihlberg J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chemistry & Biology 2014, 21 (9), 1115–1142. 10.1016/j.chembiol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- (15).Vadevoo S. M. P.; Gurung S.; Lee H.-S.; Gunassekaran G. R.; Lee S.-M.; Yoon J.-W.; Lee Y.-K.; Lee B. Peptides as Multifunctional Players in Cancer Therapy. Exp Mol Med 2023, 55 (6), 1099–1109. 10.1038/s12276-023-01016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bailey C. J.; Flatt P. R.; Conlon J. M. An Update on Peptide-Based Therapies for Type 2 Diabetes and Obesity. Peptides 2023, 161, 170939. 10.1016/j.peptides.2023.170939. [DOI] [PubMed] [Google Scholar]
- (17).Peterson S. C.; Barry A. R. Effect of Glucagon-like Peptide-1 Receptor Agonists on All-Cause Mortality and Cardiovascular Outcomes: A Meta-Analysis. Current Diabetes Reviews 2018, 14 (3), 273–279. 10.2174/1573399813666170414101450. [DOI] [PubMed] [Google Scholar]
- (18).Zampaloni C.; Mattei P.; Bleicher K.; Winther L.; Thäte C.; Bucher C.; Adam J. M.; Alanine A.; Amrein K. E.; Baidin V.; Bieniossek C.; Bissantz C.; Boess F.; Cantrill C.; Clairfeuille T.; Dey F.; Di Giorgio P.; du Castel P.; Dylus D.; Dzygiel P.; Felici A.; García-Alcalde F.; Haldimann A.; Leipner M.; Leyn S.; Louvel S.; Misson P.; Osterman A.; Pahil K.; Rigo S.; Schäublin A.; Scharf S.; Schmitz P.; Stoll T.; Trauner A.; Zoffmann S.; Kahne D.; Young J. A. T.; Lobritz M. A.; Bradley K. A. A Novel Antibiotic Class Targeting the Lipopolysaccharide Transporter. Nature 2024, 625 (7995), 566–571. 10.1038/s41586-023-06873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mookherjee N.; Anderson M. A.; Haagsman H. P.; Davidson D. J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat Rev Drug Discov 2020, 19 (5), 311–332. 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- (20).Torres M. D. T.; Sothiselvam S.; Lu T. K.; de la Fuente-Nunez C. Peptide Design Principles for Antimicrobial Applications. Journal of Molecular Biology 2019, 431 (18), 3547–3567. 10.1016/j.jmb.2018.12.015. [DOI] [PubMed] [Google Scholar]
- (21).Imai Y.; Hauk G.; Quigley J.; Liang L.; Son S.; Ghiglieri M.; Gates M. F.; Morrissette M.; Shahsavari N.; Niles S.; Baldisseri D.; Honrao C.; Ma X.; Guo J. J.; Berger J. M.; Lewis K. Evybactin Is a DNA Gyrase Inhibitor That Selectively Kills Mycobacterium Tuberculosis. Nat Chem Biol 2022, 18 (11), 1236–1244. 10.1038/s41589-022-01102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Schmitt E. K.; Riwanto M.; Sambandamurthy V.; Roggo S.; Miault C.; Zwingelstein C.; Krastel P.; Noble C.; Beer D.; Rao S. P. S.; Au M.; Niyomrattanakit P.; Lim V.; Zheng J.; Jeffery D.; Pethe K.; Camacho L. R. The Natural Product Cyclomarin Kills Mycobacterium Tuberculosis by Targeting the ClpC1 Subunit of the Caseinolytic Protease. Angewandte Chemie International Edition 2011, 50 (26), 5889–5891. 10.1002/anie.201101740. [DOI] [PubMed] [Google Scholar]
- (23).Junk L.; Schmiedel V. M.; Guha S.; Fischel K.; Greb P.; Vill K.; Krisilia V.; van Geelen L.; Rumpel K.; Kaur P.; Krishnamurthy R. V.; Narayanan S.; Shandil R. K.; Singh M.; Kofink C.; Mantoulidis A.; Biber P.; Gmaschitz G.; Kazmaier U.; Meinhart A.; Leodolter J.; Hoi D.; Junker S.; Morreale F. E.; Clausen T.; Kalscheuer R.; Weinstabl H.; Boehmelt G. Homo-BacPROTAC-Induced Degradation of ClpC1 as a Strategy against Drug-Resistant Mycobacteria. Nat Commun 2024, 15 (1), 2005. 10.1038/s41467-024-46218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hoi D. M.; Junker S.; Junk L.; Schwechel K.; Fischel K.; Podlesainski D.; Hawkins P. M. E.; van Geelen L.; Kaschani F.; Leodolter J.; Morreale F. E.; Kleine S.; Guha S.; Rumpel K.; Schmiedel V. M.; Weinstabl H.; Meinhart A.; Payne R. J.; Kaiser M.; Hartl M.; Boehmelt G.; Kazmaier U.; Kalscheuer R.; Clausen T. Clp-Targeting BacPROTACs Impair Mycobacterial Proteostasis and Survival. Cell 2023, 186 (10), 2176–2192.e22. 10.1016/j.cell.2023.04.009. [DOI] [PubMed] [Google Scholar]
- (25).Bonjorno A. F.; Pavan A. R.; Fernandes G. F. S.; Scarim C. B.; Castagnolo D.; Dos Santos J. L. BacPROTACs Targeting Clp Protease: A Promising Strategy for Anti-Mycobacterial Drug Discovery. Front. Chem. 2024, 12. 10.3389/fchem.2024.1358539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Morreale F. E.; Kleine S.; Leodolter J.; Junker S.; Hoi D. M.; Ovchinnikov S.; Okun A.; Kley J.; Kurzbauer R.; Junk L.; Guha S.; Podlesainski D.; Kazmaier U.; Boehmelt G.; Weinstabl H.; Rumpel K.; Schmiedel V. M.; Hartl M.; Haselbach D.; Meinhart A.; Kaiser M.; Clausen T. BacPROTACs Mediate Targeted Protein Degradation in Bacteria. Cell 2022, 185 (13), 2338–2353.e18. 10.1016/j.cell.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Vinogradov A. A.; Yin Y.; Suga H. Macrocyclic Peptides as Drug Candidates: Recent Progress and Remaining Challenges. J. Am. Chem. Soc. 2019, 141 (10), 4167–4181. 10.1021/jacs.8b13178. [DOI] [PubMed] [Google Scholar]
- (28).White C. J.; Yudin A. K. Contemporary Strategies for Peptide Macrocyclization. Nature Chem 2011, 3 (7), 509–524. 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- (29).Dougherty P. G.; Sahni A.; Pei D. Understanding Cell Penetration of Cyclic Peptides. Chem. Rev. 2019, 119 (17), 10241–10287. 10.1021/acs.chemrev.9b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ono S.; Naylor M. R.; Townsend C. E.; Okumura C.; Okada O.; Lee H.-W.; Lokey R. S. Cyclosporin A: Conformational Complexity and Chameleonicity. J. Chem. Inf. Model. 2021, 61 (11), 5601–5613. 10.1021/acs.jcim.1c00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li Y.; Li W.; Xu Z. Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Marine Drugs 2021, 19 (6), 311. 10.3390/md19060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Bockus A. T.; McEwen C. M.; Lokey R. S. Form and Function in Cyclic Peptide Natural Products: A Pharmacokinetic Perspective. http://www.eurekaselect.com. [DOI] [PubMed] [Google Scholar]
- (33).Nischan N.; Herce H. D.; Natale F.; Bohlke N.; Budisa N.; Cardoso M. C.; Hackenberger C. P. R. Covalent Attachment of Cyclic TAT Peptides to GFP Results in Protein Delivery into Live Cells with Immediate Bioavailability. Angewandte Chemie International Edition 2015, 54 (6), 1950–1953. 10.1002/anie.201410006. [DOI] [PubMed] [Google Scholar]
- (34).Okumu F. W.; Pauletti G. M.; Vander Velde D. G.; Siahaan T. J.; Borchardt R. T. Effect of Restricted Conformational Flexibility on the Permeation of Model Hexapeptides Across Caco-2 Cell Monolayers. Pharm Res 1997, 14 (2), 169–175. 10.1023/A:1012092409216. [DOI] [PubMed] [Google Scholar]
- (35).Price A., D.; Eng H.; Farley A., K.; Goetz H., G.; Huang Y.; Jiao Z.; Kalgutkar S., A.; Kablaoui M., N.; Khunte B.; Liras S.; Limberakis C.; Mathiowetz M., A.; Ruggeri B., R.; Quan J.-M.; Yang Z. Comparative Pharmacokinetic Profile of Cyclosporine (CsA) with a Decapeptide and a Linear Analogue. Organic & Biomolecular Chemistry 2017, 15 (12), 2501–2506. [DOI] [PubMed] [Google Scholar]
- (36).Yang Q.-Q.; Zhu L.-J.; Xi T.-K.; Zhu H.-Y.; Chen X.-X.; Wu M.; Sun C.; Xu C.; Fang G.-M.; Meng X. Delivery of Cell Membrane Impermeable Peptides into Living Cells by Using Head-to-Tail Cyclized Mitochondria-Penetrating Peptides. Org. Biomol. Chem. 2019, 17 (45), 9693–9697. 10.1039/C9OB02075F. [DOI] [PubMed] [Google Scholar]
- (37).Furukawa A.; Schwochert J.; Pye C. R.; Asano D.; Edmondson Q. D.; Turmon A. C.; Klein V. G.; Ono S.; Okada O.; Lokey R. S. Drug-like Properties in Macrocycles above MW 1000: Backbone Rigidity vs. Side-Chain Lipophilicity. Angewandte Chemie (International ed. in English) 2020, 59 (48), 21571. 10.1002/anie.202004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Liu Z.; Lepori I.; Chordia M. D.; Dalesandro B. E.; Guo T.; Dong J.; Siegrist M. S.; Pires M. M. A Metabolic-Tag-Based Method for Assessing the Permeation of Small Molecules Across the Mycomembrane in Live Mycobacteria. Angew Chem Int Ed Engl 2023, 62 (20), e202217777. 10.1002/anie.202217777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kelly J. J.; Dalesandro B. E.; Liu Z.; Chordia M. D.; Ongwae G. M.; Pires M. M. Measurement of Accumulation of Antibiotics to Staphylococcus Aureus in Phagosomes of Live Macrophages. Angewandte Chemie International Edition 2024, 63 (3), e202313870. 10.1002/anie.202313870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ongwae G. M.; Lepori I.; Chordia M. D.; Dalesandro B. E.; Apostolos A. J.; Siegrist M. S.; Pires M. M. Measurement of Small Molecule Accumulation into Diderm Bacteria. ACS Infect. Dis. 2023, 9 (1), 97–110. 10.1021/acsinfecdis.2c00435. [DOI] [PubMed] [Google Scholar]
- (41).Ongwae G. M.; Liu Z.; Chordia M. D.; Dalesandro B. E.; Guo T.; Dong J.; Pires M. M. Determination of Accumulation of Molecules in Escherichia Coli. bioRxiv June 23, 2023, p 2023.06.20.545103. 10.1101/2023.06.20.545103. [DOI] [Google Scholar]
- (42).Dash R.; Holsinger K. A.; Chordia M. D.; Gh. M. S.; Pires M. M. Bioluminescence-Based Determination of Cytosolic Accumulation of Antibiotics in Escherichia Coli. ACS Infect. Dis. 2024, 10 (5), 1602–1611. 10.1021/acsinfecdis.3c00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Six D. A.; Krucker T.; Leeds J. A. Advances and Challenges in Bacterial Compound Accumulation Assays for Drug Discovery. Current Opinion in Chemical Biology 2018, 44, 9–15. 10.1016/j.cbpa.2018.05.005. [DOI] [PubMed] [Google Scholar]
- (44).Iyer R.; Ye Z.; Ferrari A.; Duncan L.; Tanudra M. A.; Tsao H.; Wang T.; Gao H.; Brummel C. L.; Erwin A. L. Evaluating LC–MS/MS To Measure Accumulation of Compounds within Bacteria. ACS Infect. Dis. 2018, 4 (9), 1336–1345. 10.1021/acsinfecdis.8b00083. [DOI] [PubMed] [Google Scholar]
- (45).Widya M.; Pasutti W. D.; Sachdeva M.; Simmons R. L.; Tamrakar P.; Krucker T.; Six D. A. Development and Optimization of a Higher-Throughput Bacterial Compound Accumulation Assay. ACS Infect. Dis. 2019, 5 (3), 394–405. 10.1021/acsinfecdis.8b00299. [DOI] [PubMed] [Google Scholar]
- (46).Bhat J.; Narayan A.; Venkatraman J.; Chatterji M. LC–MS Based Assay to Measure Intracellular Compound Levels in Mycobacterium Smegmatis: Linking Compound Levels to Cellular Potency. Journal of Microbiological Methods 2013, 94 (2), 152–158. 10.1016/j.mimet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- (47).Prochnow H.; Fetz V.; Hotop S.-K.; García-Rivera M. A.; Heumann A.; Brönstrup M. Subcellular Quantification of Uptake in Gram-Negative Bacteria. Anal. Chem. 2019, 91 (3), 1863–1872. 10.1021/acs.analchem.8b03586. [DOI] [PubMed] [Google Scholar]
- (48).Cama J.; Henney A. M.; Winterhalter M. Breaching the Barrier: Quantifying Antibiotic Permeability across Gram-Negative Bacterial Membranes. Journal of Molecular Biology 2019, 431 (18), 3531–3546. 10.1016/j.jmb.2019.03.031. [DOI] [PubMed] [Google Scholar]
- (49).Richter M. F.; Drown B. S.; Riley A. P.; Garcia A.; Shirai T.; Svec R. L.; Hergenrother P. J. Predictive Compound Accumulation Rules Yield a Broad-Spectrum Antibiotic. Nature 2017, 545 (7654), 299–304. 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Geddes E. J.; Gugger M. K.; Garcia A.; Chavez M. G.; Lee M. R.; Perlmutter S. J.; Bieniossek C.; Guasch L.; Hergenrother P. J. Porin-Independent Accumulation in Pseudomonas Enables Antibiotic Discovery. Nature 2023, 624 (7990), 145–153. 10.1038/s41586-023-06760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Davis T. D.; Gerry C. J.; Tan D. S. General Platform for Systematic Quantitative Evaluation of Small-Molecule Permeability in Bacteria. ACS Chem. Biol. 2014, 9 (11), 2535–2544. 10.1021/cb5003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Pidgeon S. E.; Apostolos A. J.; Nelson J. M.; Shaku M.; Rimal B.; Islam M. N.; Crick D. C.; Kim S. J.; Pavelka M. S.; Kana B. D.; Pires M. M. L,D-Transpeptidase Specific Probe Reveals Spatial Activity of Peptidoglycan Cross-Linking. ACS Chemical Biology 2019. 10.1021/acschembio.9b00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Apostolos A. J.; Nelson J. M.; Silva J. R. A.; Lameira J.; Achimovich A. M.; Gahlmann A.; Alves C. N.; Pires M. M. Facile Synthesis and Metabolic Incorporation of M-DAP Bioisosteres Into Cell Walls of Live Bacteria. ACS Chemical Biology 2020. 10.1021/acschembio.0c00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Apostolos A. J.; Pidgeon S. E.; Pires M. M. Remodeling of Cross-Bridges Controls Peptidoglycan Cross-Linking Levels in Bacterial Cell Walls. ACS Chemical Biology 2020. 10.1021/acschembio.0c00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sparks I. L.; Derbyshire K. M.; Jacobs W. R.; Morita Y. S. Mycobacterium Smegmatis: The Vanguard of Mycobacterial Research. Journal of Bacteriology 2023, 205 (1), e00337–22. 10.1128/jb.00337-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Peraro L.; Deprey K. L.; Moser M. K.; Zou Z.; Ball H. L.; Levine B.; Kritzer J. A. Cell Penetration Profiling Using the Chloroalkane Penetration Assay. J. Am. Chem. Soc. 2018, 140 (36), 11360–11369. 10.1021/jacs.8b06144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mahapatra S.; Crick D. C.; McNeil M. R.; Brennan P. J. Unique Structural Features of the Peptidoglycan of Mycobacterium Leprae. Journal of Bacteriology 2008, 190 (2), 655–661. 10.1128/jb.00982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Rodrigues L.; Ramos J.; Couto I.; Amaral L.; Viveiros M. Ethidium Bromide Transport across Mycobacterium Smegmatis Cell-Wall: Correlation with Antibiotic Resistance. BMC Microbiol 2011, 11 (1), 35. 10.1186/1471-2180-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Papadopoulos A. O.; Ealand C.; Gordhan B. G.; VanNieuwenhze M.; Kana B. D. Characterisation of a Putative M23-Domain Containing Protein in Mycobacterium Tuberculosis. PLOS ONE 2021, 16 (11), e0259181. 10.1371/journal.pone.0259181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Bisson G. P.; Mehaffy C.; Broeckling C.; Prenni J.; Rifat D.; Lun D. S.; Burgos M.; Weissman D.; Karakousis P. C.; Dobos K. Upregulation of the Phthiocerol Dimycocerosate Biosynthetic Pathway by Rifampin-Resistant, rpoB Mutant Mycobacterium Tuberculosis. Journal of Bacteriology 2012, 194 (23), 6441–6452. 10.1128/jb.01013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Chuang Y.-M.; Bandyopadhyay N.; Rifat D.; Rubin H.; Bader J. S.; Karakousis P. C. Deficiency of the Novel Exopolyphosphatase Rv1026/PPX2 Leads to Metabolic Downshift and Altered Cell Wall Permeability in Mycobacterium Tuberculosis. mBio 2015, 6 (2), 10.1128/mbio.02428-14. https://doi.org/10.1128/mbio.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Campodónico V. L.; Rifat D.; Chuang Y.-M.; Ioerger T. R.; Karakousis P. C. Altered Mycobacterium Tuberculosis Cell Wall Metabolism and Physiology Associated With RpoB Mutation H526D. Front. Microbiol. 2018, 9. 10.3389/fmicb.2018.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Bockus A. T.; Lexa K. W.; Pye C. R.; Kalgutkar A. S.; Gardner J. W.; Hund K. C. R.; Hewitt W. M.; Schwochert J. A.; Glassey E.; Price D. A.; Mathiowetz A. M.; Liras S.; Jacobson M. P.; Lokey R. S. Probing the Physicochemical Boundaries of Cell Permeability and Oral Bioavailability in Lipophilic Macrocycles Inspired by Natural Products. J. Med. Chem. 2015, 58 (11), 4581–4589. 10.1021/acs.jmedchem.5b00128. [DOI] [PubMed] [Google Scholar]
- (64).Hewitt W. M.; Leung S. S. F.; Pye C. R.; Ponkey A. R.; Bednarek M.; Jacobson M. P.; Lokey R. S. Cell-Permeable Cyclic Peptides from Synthetic Libraries Inspired by Natural Products. J. Am. Chem. Soc. 2015, 137 (2), 715–721. 10.1021/ja508766b. [DOI] [PubMed] [Google Scholar]
- (65).Lee D.; Choi J.; Yang M. J.; Park C.-J.; Seo J. Controlling the Chameleonic Behavior and Membrane Permeability of Cyclosporine Derivatives via Backbone and Side Chain Modifications. J. Med. Chem. 2023, 66 (18), 13189–13204. 10.1021/acs.jmedchem.3c01140. [DOI] [PubMed] [Google Scholar]
- (66).Barlow N.; Chalmers D. K.; Williams-Noonan B. J.; Thompson P. E.; Norton R. S. Improving Membrane Permeation in the Beyond Rule-of-Five Space by Using Prodrugs to Mask Hydrogen Bond Donors. ACS Chem. Biol. 2020, 15 (8), 2070–2078. 10.1021/acschembio.0c00218. [DOI] [PubMed] [Google Scholar]
- (67).Bockus A. T.; Schwochert J. A.; Pye C. R.; Townsend C. E.; Sok V.; Bednarek M. A.; Lokey R. S. Going Out on a Limb: Delineating The Effects of β-Branching, N-Methylation, and Side Chain Size on the Passive Permeability, Solubility, and Flexibility of Sanguinamide A Analogues. J. Med. Chem. 2015, 58 (18), 7409–7418. 10.1021/acs.jmedchem.5b00919. [DOI] [PubMed] [Google Scholar]
- (68).Kelly C. N.; Townsend C. E.; Jain A. N.; Naylor M. R.; Pye C. R.; Schwochert J.; Lokey R. S. Geometrically Diverse Lariat Peptide Scaffolds Reveal an Untapped Chemical Space of High Membrane Permeability. J. Am. Chem. Soc. 2021, 143 (2), 705–714. 10.1021/jacs.0c06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Ovadia O.; Greenberg S.; Chatterjee J.; Laufer B.; Opperer F.; Kessler H.; Gilon C.; Hoffman A. The Effect of Multiple N-Methylation on Intestinal Permeability of Cyclic Hexapeptides. Mol. Pharmaceutics 2011, 8 (2), 479–487. 10.1021/mp1003306. [DOI] [PubMed] [Google Scholar]
- (70).Biron E.; Chatterjee J.; Ovadia O.; Langenegger D.; Brueggen J.; Hoyer D.; Schmid H. A.; Jelinek R.; Gilon C.; Hoffman A.; Kessler H. Improving Oral Bioavailability of Peptides by Multiple N-Methylation: Somatostatin Analogues. Angewandte Chemie International Edition 2008, 47 (14), 2595–2599. 10.1002/anie.200705797. [DOI] [PubMed] [Google Scholar]
- (71).Räder A. F. B.; Reichart F.; Weinmüller M.; Kessler H. Improving Oral Bioavailability of Cyclic Peptides by N-Methylation. Bioorganic & Medicinal Chemistry 2018, 26 (10), 2766–2773. 10.1016/j.bmc.2017.08.031. [DOI] [PubMed] [Google Scholar]
- (72).Beck J. G.; Chatterjee J.; Laufer B.; Kiran M. U.; Frank A. O.; Neubauer S.; Ovadia O.; Greenberg S.; Gilon C.; Hoffman A.; Kessler H. Intestinal Permeability of Cyclic Peptides: Common Key Backbone Motifs Identified. J. Am. Chem. Soc. 2012, 134 (29), 12125–12133. 10.1021/ja303200d. [DOI] [PubMed] [Google Scholar]
- (73).Chatterjee J.; Gilon C.; Hoffman A.; Kessler H. N-Methylation of Peptides: A New Perspective in Medicinal Chemistry. Acc. Chem. Res. 2008, 41 (10), 1331–1342. 10.1021/ar8000603. [DOI] [PubMed] [Google Scholar]
- (74).Schwochert J.; Turner R.; Thang M.; Berkeley R. F.; Ponkey A. R.; Rodriguez K. M.; Leung S. S. F.; Khunte B.; Goetz G.; Limberakis C.; Kalgutkar A. S.; Eng H.; Shapiro M. J.; Mathiowetz A. M.; Price D. A.; Liras S.; Jacobson M. P.; Lokey R. S. Peptide to Peptoid Substitutions Increase Cell Permeability in Cyclic Hexapeptides. Org. Lett. 2015, 17 (12), 2928–2931. 10.1021/acs.orglett.5b01162. [DOI] [PubMed] [Google Scholar]
- (75).Kapoor R.; Wadman M. W.; Dohm M. T.; Czyzewski A. M.; Spormann A. M.; Barron A. E. Antimicrobial Peptoids Are Effective against Pseudomonas Aeruginosa Biofilms. Antimicrob Agents Chemother 2011, 55 (6), 3054–3057. 10.1128/AAC.01516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Kapoor R.; Eimerman P. R.; Hardy J. W.; Cirillo J. D.; Contag C. H.; Barron A. E. Efficacy of Antimicrobial Peptoids against Mycobacterium Tuberculosis. Antimicrobial Agents and Chemotherapy 2011, 55 (6), 3058–3062. 10.1128/aac.01667-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Zuckermann R. N.; Kodadek T. Peptoids as Potential Therapeutics. Curr Opin Mol Ther 2009, 11 (3), 299–307. [PubMed] [Google Scholar]
- (78).Chongsiriwatana N. P.; Patch J. A.; Czyzewski A. M.; Dohm M. T.; Ivankin A.; Gidalevitz D.; Zuckermann R. N.; Barron A. E. Peptoids That Mimic the Structure, Function, and Mechanism of Helical Antimicrobial Peptides. Proceedings of the National Academy of Sciences 2008, 105 (8), 2794–2799. 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Simon R. J.; Kania R. S.; Zuckermann R. N.; Huebner V. D.; Jewell D. A.; Banville S.; Ng S.; Wang L.; Rosenberg S.; Marlowe C. K. Peptoids: A Modular Approach to Drug Discovery. Proceedings of the National Academy of Sciences 1992, 89 (20), 9367–9371. 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Tan N. C.; Yu P.; Kwon Y.-U.; Kodadek T. High-Throughput Evaluation of Relative Cell Permeability between Peptoids and Peptides. Bioorganic & Medicinal Chemistry 2008, 16 (11), 5853–5861. 10.1016/j.bmc.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Zhang H.; Chen S. Cyclic Peptide Drugs Approved in the Last Two Decades (2001–2021). RSC Chemical Biology 2022, 3 (1), 18–31. 10.1039/D1CB00154J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Nielsen D. S.; Shepherd N. E.; Xu W.; Lucke A. J.; Stoermer M. J.; Fairlie D. P. Orally Absorbed Cyclic Peptides. Chem. Rev. 2017, 117 (12), 8094–8128. 10.1021/acs.chemrev.6b00838. [DOI] [PubMed] [Google Scholar]
- (83).Hess S.; Ovadia O.; Shalev D. E.; Senderovich H.; Qadri B.; Yehezkel T.; Salitra Y.; Sheynis T.; Jelinek R.; Gilon C.; Hoffman A. Effect of Structural and Conformation Modifications, Including Backbone Cyclization, of Hydrophilic Hexapeptides on Their Intestinal Permeability and Enzymatic Stability. J. Med. Chem. 2007, 50 (24), 6201–6211. 10.1021/jm070836d. [DOI] [PubMed] [Google Scholar]
- (84).Iwasaki K.; Goto Y.; Katoh T.; Suga H. Selective Thioether Macrocyclization of Peptides Having the N-Terminal 2-Chloroacetyl Group and Competing Two or Three Cysteine Residues in Translation. Org. Biomol. Chem. 2012, 10 (30), 5783–5786. 10.1039/C2OB25306B. [DOI] [PubMed] [Google Scholar]
- (85).Yu H.; Dranchak P.; Li Z.; MacArthur R.; Munson M. S.; Mehzabeen N.; Baird N. J.; Battalie K. P.; Ross D.; Lovell S.; Carlow C. K. S.; Suga H.; Inglese J. Macrocycle Peptides Delineate Locked-Open Inhibition Mechanism for Microorganism Phosphoglycerate Mutases. Nat Commun 2017, 8 (1), 14932. 10.1038/ncomms14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Vamisetti G. B.; Saha A.; Huang Y. J.; Vanjari R.; Mann G.; Gutbrod J.; Ayoub N.; Suga H.; Brik A. Selective Macrocyclic Peptide Modulators of Lys63-Linked Ubiquitin Chains Disrupt DNA Damage Repair. Nat Commun 2022, 13 (1), 6174. 10.1038/s41467-022-33808-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Nishio K.; Belle R.; Katoh T.; Kawamura A.; Sengoku T.; Hanada K.; Ohsawa N.; Shirouzu M.; Yokoyama S.; Suga H. Thioether Macrocyclic Peptides Selected against TET1 Compact Catalytic Domain Inhibit TET1 Catalytic Activity. ChemBioChem 2018, 19 (9), 979–985. 10.1002/cbic.201800047. [DOI] [PubMed] [Google Scholar]
- (88).Merz M. L.; Habeshian S.; Li B.; David J.-A. G. L.; Nielsen A. L.; Ji X.; Il Khwildy K.; Duany Benitez M. M.; Phothirath P.; Heinis C. De Novo Development of Small Cyclic Peptides That Are Orally Bioavailable. Nat Chem Biol 2024, 20 (5), 624–633. 10.1038/s41589-023-01496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Kaiser T.; Nicholson G. J.; Kohlbau H. J.; Voelter W. Racemization Studies of Fmoc-Cys(Trt)-OH during Stepwise Fmoc-Solid Phase Peptide Synthesis. Tetrahedron Letters 1996, 37 (8), 1187–1190. 10.1016/0040-4039(95)02406-9. [DOI] [Google Scholar]
- (90).Han Y.; Albericio F.; Barany G. Occurrence and Minimization of Cysteine Racemization during Stepwise Solid-Phase Peptide Synthesis1,2. J. Org. Chem. 1997, 62 (13), 4307–4312. 10.1021/jo9622744. [DOI] [PubMed] [Google Scholar]
- (91).Pye C. R.; Hewitt W. M.; Schwochert J.; Haddad T. D.; Townsend C. E.; Etienne L.; Lao Y.; Limberakis C.; Furukawa A.; Mathiowetz A. M.; Price D. A.; Liras S.; Lokey R. S. Nonclassical Size Dependence of Permeation Defines Bounds for Passive Adsorption of Large Drug Molecules. J. Med. Chem. 2017, 60 (5), 1665–1672. 10.1021/acs.jmedchem.6b01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Peraro L.; Kritzer J. A. Emerging Methods and Design Principles for Cell-Penetrant Peptides. Angewandte Chemie International Edition 2018, 57 (37), 11868–11881. 10.1002/anie.201801361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Muratspahić E.; Deibler K.; Han J.; Tomašević N.; Jadhav K. B.; Olivé-Marti A. L.; Hochrainer N.; Hellinger R.; Koehbach J.; Fay J. F.; Rahman M. H.; Hegazy L.; Craven T. W.; Varga B. R.; Bhardwaj G.; Appourchaux K.; Majumdar S.; Muttenthaler M.; Hosseinzadeh P.; Craik D. J.; Spetea M.; Che T.; Baker D.; Gruber C. W. Design and Structural Validation of Peptide–Drug Conjugate Ligands of the Kappa-Opioid Receptor. Nat Commun 2023, 14 (1), 8064. 10.1038/s41467-023-43718-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Gavrish E.; Sit C. S.; Cao S.; Kandror O.; Spoering A.; Peoples A.; Ling L.; Fetterman A.; Hughes D.; Bissell A.; Torrey H.; Akopian T.; Mueller A.; Epstein S.; Goldberg A.; Clardy J.; Lewis K. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium Tuberculosis by Targeting the ATP-Dependent Protease ClpC1P1P2. Chem Biol 2014, 21 (4), 509–518. 10.1016/j.chembiol.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Abrigo N. A.; Dods K. K.; Makovsky C. A.; Lohan S.; Mitra K.; Newcomb K. M.; Le A.; Hartman M. C. T. Development of a Cyclic, Cell Penetrating Peptide Compatible with In Vitro Selection Strategies. ACS Chem. Biol. 2023, 18 (4), 746–755. 10.1021/acschembio.2c00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Góngora-Benítez M.; Tulla-Puche J.; Albericio F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 2014, 114 (2), 901–926. 10.1021/cr400031z. [DOI] [PubMed] [Google Scholar]
- (97).Habeshian S.; Merz M. L.; Sangouard G.; Mothukuri G. K.; Schüttel M.; Bognár Z.; Díaz-Perlas C.; Vesin J.; Bortoli Chapalay J.; Turcatti G.; Cendron L.; Angelini A.; Heinis C. Synthesis and Direct Assay of Large Macrocycle Diversities by Combinatorial Late-Stage Modification at Picomole Scale. Nat Commun 2022, 13 (1), 3823. 10.1038/s41467-022-31428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Lu S.; Fan S.; Xiao S.; Li J.; Zhang S.; Wu Y.; Kong C.; Zhuang J.; Liu H.; Zhao Y.; Wu C. Disulfide-Directed Multicyclic Peptide Libraries for the Discovery of Peptide Ligands and Drugs. J. Am. Chem. Soc. 2023, 145 (3), 1964–1972. 10.1021/jacs.2c12462. [DOI] [PubMed] [Google Scholar]
- (99).Zhou L.; Cai F.; Li Y.; Gao X.; Wei Y.; Fedorova A.; Kirchhofer D.; Hannoush R. N.; Zhang Y. Disulfide-Constrained Peptide Scaffolds Enable a Robust Peptide-Therapeutic Discovery Platform. PLoS One 2024, 19 (3), e0300135. 10.1371/journal.pone.0300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Akondi K. B.; Muttenthaler M.; Dutertre S.; Kaas Q.; Craik D. J.; Lewis R. J.; Alewood P. F. Discovery, Synthesis, and Structure–Activity Relationships of Conotoxins. Chem. Rev. 2014, 114 (11), 5815–5847. 10.1021/cr400401e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Muppidi A.; Wang Z.; Li X.; Chen J.; Lin Q. Achieving Cell Penetration with Distance-Matching Cysteine Cross-Linkers: A Facile Route to Cell -Permeable Peptide Dual Inhibitors of Mdm2/Mdmx. Chemical Communications 2011, 47 (33), 9396–9398. 10.1039/C1CC13320A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Todorova-Balvay D.; Stoilova I.; Gargova S.; Vijayalakshmi M. A. An Efficient Two Step Purification and Molecular Characterization of β-Galactosidases from Aspergillus Oryzae. Journal of Molecular Recognition 2006, 19 (4), 299–304. 10.1002/jmr.788. [DOI] [PubMed] [Google Scholar]
- (103).Jo H.; Meinhardt N.; Wu Y.; Kulkarni S.; Hu X.; Low K. E.; Davies P. L.; DeGrado W. F.; Greenbaum D. C. Development of α-Helical Calpain Probes by Mimicking a Natural Protein–Protein Interaction. J. Am. Chem. Soc. 2012, 134 (42), 17704–17713. 10.1021/ja307599z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Smeenk L. E. J.; Dailly N.; Hiemstra H.; van Maarseveen J. H.; Timmerman P. Synthesis of Water-Soluble Scaffolds for Peptide Cyclization, Labeling, and Ligation. Org. Lett. 2012, 14 (5), 1194–1197. 10.1021/ol203259a. [DOI] [PubMed] [Google Scholar]
- (105).Mejia-Santana A.; Collins R.; Doud E. H.; Landeta C. Disulfide Bonds Are Required for Cell Division, Cell Envelope Biogenesis and Antibiotic Resistance Proteins in Mycobacteria. bioRxiv January 28, 2025, p 2025.01.27.635063. 10.1101/2025.01.27.635063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Klußmann M.; Stillger K.; Ruppel M.; Sticker C.-L.; Neundorf I. Investigating the Impact of Thiol Reactivity and Disulfide Formation on Cellular Uptake of Cell-Permeable Peptides. Journal of Peptide Science n/a (n/a), e3604. 10.1002/psc.3604. [DOI] [PubMed] [Google Scholar]
- (107).Li T.; Gao W.; Liang J.; Zha M.; Chen Y.; Zhao Y.; Wu C. Biscysteine-Bearing Peptide Probes To Reveal Extracellular Thiol–Disulfide Exchange Reactions Promoting Cellular Uptake. Anal. Chem. 2017, 89 (16), 8501–8508. 10.1021/acs.analchem.7b02084. [DOI] [PubMed] [Google Scholar]
- (108).Veber D. F.; Johnson S. R.; Cheng H.-Y.; Smith B. R.; Ward K. W.; Kopple K. D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45 (12), 2615–2623. 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- (109).Rossi Sebastiano M.; Doak B. C.; Backlund M.; Poongavanam V.; Over B.; Ermondi G.; Caron G.; Matsson P.; Kihlberg J. Impact of Dynamically Exposed Polarity on Permeability and Solubility of Chameleonic Drugs Beyond the Rule of 5. J. Med. Chem. 2018, 61 (9), 4189–4202. 10.1021/acs.jmedchem.8b00347. [DOI] [PubMed] [Google Scholar]
- (110).Wang C. K.; Northfield S. E.; Colless B.; Chaousis S.; Hamernig I.; Lohman R.-J.; Nielsen D. S.; Schroeder C. I.; Liras S.; Price D. A.; Fairlie D. P.; Craik D. J. Rational Design and Synthesis of an Orally Bioavailable Peptide Guided by NMR Amide Temperature Coefficients. Proceedings of the National Academy of Sciences 2014, 111 (49), 17504–17509. 10.1073/pnas.1417611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Rezai T.; Bock J. E.; Zhou M. V.; Kalyanaraman C.; Lokey R. S.; Jacobson M. P. Conformational Flexibility, Internal Hydrogen Bonding, and Passive Membrane Permeability: Successful in Silico Prediction of the Relative Permeabilities of Cyclic Peptides. J. Am. Chem. Soc. 2006, 128 (43), 14073–14080. 10.1021/ja063076p. [DOI] [PubMed] [Google Scholar]
- (112).Liras S.; Mcclure K. F. Permeability of Cyclic Peptide Macrocycles and Cyclotides and Their Potential as Therapeutics. ACS Med Chem Lett 2019, 10 (7), 1026–1032. 10.1021/acsmedchemlett.9b00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Bhardwaj G.; O’Connor J.; Rettie S.; Huang Y.-H.; Ramelot T. A.; Mulligan V. K.; Alpkilic G. G.; Palmer J.; Bera A. K.; Bick M. J.; Di Piazza M.; Li X.; Hosseinzadeh P.; Craven T. W.; Tejero R.; Lauko A.; Choi R.; Glynn C.; Dong L.; Griffin R.; van Voorhis W. C.; Rodriguez J.; Stewart L.; Montelione G. T.; Craik D.; Baker D. Accurate de Novo Design of Membrane-Traversing Macrocycles. Cell 2022, 185 (19), 3520–3532.e26. 10.1016/j.cell.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).White T. R.; Renzelman C. M.; Rand A. C.; Rezai T.; McEwen C. M.; Gelev V. M.; Turner R. A.; Linington R. G.; Leung S. S. F.; Kalgutkar A. S.; Bauman J. N.; Zhang Y.; Liras S.; Price D. A.; Mathiowetz A. M.; Jacobson M. P.; Lokey R. S. On-Resin N-Methylation of Cyclic Peptides for Discovery of Orally Bioavailable Scaffolds. Nat Chem Biol 2011, 7 (11), 810–817. 10.1038/nchembio.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).van Neer R. H. P.; Dranchak P. K.; Liu L.; Aitha M.; Queme B.; Kimura H.; Katoh T.; Battaile K. P.; Lovell S.; Inglese J.; Suga H. Serum-Stable and Selective Backbone-N-Methylated Cyclic Peptides That Inhibit Prokaryotic Glycolytic Mutases. ACS Chem. Biol. 2022, 17 (8), 2284–2295. 10.1021/acschembio.2c00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Ji X.; Nielsen A. L.; Heinis C. Cyclic Peptides for Drug Development. Angewandte Chemie International Edition 2024, 63 (3), e202308251. 10.1002/anie.202308251. [DOI] [PubMed] [Google Scholar]
- (117).Ngambenjawong C.; Gustafson H. H.; Sylvestre M.; Pun S. H. A Facile Cyclization Method Improves Peptide Serum Stability and Confers Intrinsic Fluorescence. ChemBioChem 2017, 18 (24), 2395–2398. 10.1002/cbic.201700446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Khatri B.; Nuthakki V. R.; Chatterjee J. Strategies to Enhance Metabolic Stabilities. In Cyclic Peptide Design; Goetz G., Ed.; Springer: New York, NY, 2019; pp 17–40. 10.1007/978-1-4939-9504-2_2. [DOI] [PubMed] [Google Scholar]
- (119).Janecka A.; Kruszynski R.; Fichna J.; Kosson P.; Janecki T. Enzymatic Degradation Studies of Endomorphin-2 and Its Analogs Containing N-Methylated Amino Acids. Peptides 2006, 27 (1), 131–135. 10.1016/j.peptides.2005.06.015. [DOI] [PubMed] [Google Scholar]
- (120).Gazdik M.; O’Neill M. T.; Lopaticki S.; Lowes K. N.; Smith B. J.; Cowman A. F.; Boddey J. A.; Sleebs B. E. The Effect of N-Methylation on Transition State Mimetic Inhibitors of the Plasmodium Protease, Plasmepsin V. Med. Chem. Commun. 2015, 6 (3), 437–443. 10.1039/C4MD00409D. [DOI] [Google Scholar]
- (121).Cochrane S. A.; Findlay B.; Bakhtiary A.; Acedo J. Z.; Rodriguez-Lopez E. M.; Mercier P.; Vederas J. C. Antimicrobial Lipopeptide Tridecaptin A1 Selectively Binds to Gram-Negative Lipid II. Proceedings of the National Academy of Sciences 2016, 113 (41), 11561–11566. 10.1073/pnas.1608623113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Bann S. J.; Ballantine R. D.; Cochrane S. A. The Tridecaptins: Non-Ribosomal Peptides That Selectively Target Gram-Negative Bacteria. RSC Med. Chem. 2021, 12 (4), 538–551. 10.1039/D0MD00413H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Breukink E.; de Kruijff B. Lipid II as a Target for Antibiotics. Nat Rev Drug Discov 2006, 5 (4), 321–323. 10.1038/nrd2004. [DOI] [PubMed] [Google Scholar]
- (124).Cochrane S. A.; Lohans C. T.; Brandelli J. R.; Mulvey G.; Armstrong G. D.; Vederas J. C. Synthesis and Structure–Activity Relationship Studies of N-Terminal Analogues of the Antimicrobial Peptide Tridecaptin A1. J. Med. Chem. 2014, 57 (3), 1127–1131. 10.1021/jm401779d. [DOI] [PubMed] [Google Scholar]
- (125).Cochrane S. A.; Findlay B.; Vederas J. C.; Ratemi E. S. Key Residues in Octyl-Tridecaptin A1 Analogues Linked to Stable Secondary Structures in the Membrane. ChemBioChem 2014, 15 (9), 1295–1299. 10.1002/cbic.201402024. [DOI] [PubMed] [Google Scholar]
- (126).Cochrane S. A.; Li X.; He S.; Yu M.; Wu M.; Vederas J. C. Synthesis of Tridecaptin–Antibiotic Conjugates with in Vivo Activity against Gram-Negative Bacteria. J. Med. Chem. 2015, 58 (24), 9779–9785. 10.1021/acs.jmedchem.5b01578. [DOI] [PubMed] [Google Scholar]
- (127).Yang H.; Wierzbicki M.; Du Bois D. R.; Nowick J. S. X-Ray Crystallographic Structure of a Teixobactin Derivative Reveals Amyloid-like Assembly. J. Am. Chem. Soc. 2018, 140 (43), 14028–14032. 10.1021/jacs.8b07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Shukla R.; Lavore F.; Maity S.; Derks M. G. N.; Jones C. R.; Vermeulen B. J. A.; Melcrová A.; Morris M. A.; Becker L. M.; Wang X.; Kumar R.; Medeiros-Silva J.; van Beekveld R. A. M.; Bonvin A. M. J. J.; Lorent J. H.; Lelli M.; Nowick J. S.; MacGillavry H. D.; Peoples A. J.; Spoering A. L.; Ling L. L.; Hughes D. E.; Roos W. H.; Breukink E.; Lewis K.; Weingarth M. Teixobactin Kills Bacteria by a Two-Pronged Attack on the Cell Envelope. Nature 2022, 608 (7922), 390–396. 10.1038/s41586-022-05019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Shukla R.; Medeiros-Silva J.; Parmar A.; Vermeulen B. J. A.; Das S.; Paioni A. L.; Jekhmane S.; Lorent J.; Bonvin A. M. J. J.; Baldus M.; Lelli M.; Veldhuizen E. J. A.; Breukink E.; Singh I.; Weingarth M. Mode of Action of Teixobactins in Cellular Membranes. Nat Commun 2020, 11 (1), 2848. 10.1038/s41467-020-16600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Ballantine R. D.; Li Y.-X.; Qian P.-Y.; Cochrane S. A. Rational Design of New Cyclic Analogues of the Antimicrobial Lipopeptide Tridecaptin A1. Chem. Commun. 2018, 54 (75), 10634–10637. 10.1039/C8CC05790G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Terlain B.; Thomas J. P. Structure of griselimycin, polypeptide antibiotic extracted Streptomyces cultures. I. Identification of the products liberated by hydrolysis. Bull Soc Chim Fr 1971, 6, 2349–2356. [PubMed] [Google Scholar]
- (132).Kling A.; Lukat P.; Almeida D. V.; Bauer A.; Fontaine E.; Sordello S.; Zaburannyi N.; Herrmann J.; Wenzel S. C.; König C.; Ammerman N. C.; Barrio M. B.; Borchers K.; Bordon-Pallier F.; Brönstrup M.; Courtemanche G.; Gerlitz M.; Geslin M.; Hammann P.; Heinz D. W.; Hoffmann H.; Klieber S.; Kohlmann M.; Kurz M.; Lair C.; Matter H.; Nuermberger E.; Tyagi S.; Fraisse L.; Grosset J. H.; Lagrange S.; Müller R. Targeting DnaN for Tuberculosis Therapy Using Novel Griselimycins. Science 2015, 348 (6239), 1106–1112. 10.1126/science.aaa4690. [DOI] [PubMed] [Google Scholar]
- (133).Toyohara M. Aspects of the Antituberculous Activity of 27753-RP, a New Semisynthetic Derivative of Griselimycine. Annales de l’Institut Pasteur / Microbiologie 1987, 138 (6), 737–744. 10.1016/0769-2609(87)90151-7. [DOI] [PubMed] [Google Scholar]
- (134).Fu C.; Liu Y.; Walt C.; Rasheed S.; Bader C. D.; Lukat P.; Neuber M.; Haeckl F. P. J.; Blankenfeldt W.; Kalinina O. V.; Müller R. Elucidation of Unusual Biosynthesis and DnaN-Targeting Mode of Action of Potent Anti-Tuberculosis Antibiotics Mycoplanecins. Nat Commun 2024, 15 (1), 791. 10.1038/s41467-024-44953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Fenwick M. K.; Pierce P. G.; Abendroth J.; Barrett K. F.; Barrett L. K.; Bowatte K.; Choi R.; Chun I.; Conrady D. G.; Craig J. K.; Dranow D. M.; Hammerson B.; Higgins T.; Lorimer D. D.; Lukat P.; Mayclin S. J.; Hewitt S. N.; Peng Y. P.; Shanbhogue A.; Smutney H.; Stigliano M. Z. Z.; Tillery L. M.; Udell H. S.; Wallace E. G.; DeRocher A. E.; Phan I. Q.; Staker B. L.; Subramanian S.; Van Voorhis W. C.; Blankenfeldt W.; Müller R.; Edwards T. E.; Myler P. J. Exquisite Selectivity of Griselimycin Extends to Beta Subunit of DNA Polymerases from Gram-Negative Bacterial Pathogens. Commun Biol 2024, 7 (1), 1–13. 10.1038/s42003-024-07175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Aragaw W. W.; Roubert C.; Fontaine E.; Lagrange S.; Zimmerman M. D.; Dartois V.; Gengenbacher M.; Dick T. Cyclohexyl-Griselimycin Is Active against Mycobacterium Abscessus in Mice. Antimicrob Agents Chemother 66 (1), e01400–21. 10.1128/AAC.01400-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Holzgrabe U. New Griselimycins for Treatment of Tuberculosis. Chemistry & Biology 2015, 22 (8), 981–982. 10.1016/j.chembiol.2015.08.002. [DOI] [PubMed] [Google Scholar]
- (138).Yin Z.; Whittell L. R.; Wang Y.; Jergic S.; Ma C.; Lewis P. J.; Dixon N. E.; Beck J. L.; Kelso M. J.; Oakley A. J. Bacterial Sliding Clamp Inhibitors That Mimic the Sequential Binding Mechanism of Endogenous Linear Motifs. J. Med. Chem. 2015, 58 (11), 4693–4702. 10.1021/acs.jmedchem.5b00232. [DOI] [PubMed] [Google Scholar]
- (139).Yin Z.; Whittell L. R.; Wang Y.; Jergic S.; Liu M.; Harry E. J.; Dixon N. E.; Beck J. L.; Kelso M. J.; Oakley A. J. Discovery of Lead Compounds Targeting the Bacterial Sliding Clamp Using a Fragment-Based Approach. J. Med. Chem. 2014, 57 (6), 2799–2806. 10.1021/jm500122r. [DOI] [PubMed] [Google Scholar]
- (140).Wolff P.; Amal I.; Oliéric V.; Chaloin O.; Gygli G.; Ennifar E.; Lorber B.; Guichard G.; Wagner J.; Dejaegere A.; Burnouf D. Y. Differential Modes of Peptide Binding onto Replicative Sliding Clamps from Various Bacterial Origins. J. Med. Chem. 2014, 57 (18), 7565–7576. 10.1021/jm500467a. [DOI] [PubMed] [Google Scholar]
- (141).Kjelstrup S.; Hansen P. M. P.; Thomsen L. E.; Hansen P. R.; Løbner-Olesen A. Cyclic Peptide Inhibitors of the β-Sliding Clamp in Staphylococcus Aureus. PLOS ONE 2013, 8 (9), e72273. 10.1371/journal.pone.0072273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Georgescu R. E.; Yurieva O.; Kim S.-S.; Kuriyan J.; Kong X.-P.; O’Donnell M. Structure of a Small-Molecule Inhibitor of a DNA Polymerase Sliding Clamp. Proceedings of the National Academy of Sciences 2008, 105 (32), 11116–11121. 10.1073/pnas.0804754105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Poongavanam V.; Wieske L. H. E.; Peintner S.; Erdélyi M.; Kihlberg J. Molecular Chameleons in Drug Discovery. Nat Rev Chem 2024, 8 (1), 45–60. 10.1038/s41570-023-00563-1. [DOI] [PubMed] [Google Scholar]
- (144).Linker S. M.; Schellhaas C.; Kamenik A. S.; Veldhuizen M. M.; Waibl F.; Roth H.-J.; Fouché M.; Rodde S.; Riniker S. Lessons for Oral Bioavailability: How Conformationally Flexible Cyclic Peptides Enter and Cross Lipid Membranes. J. Med. Chem. 2023, 66 (4), 2773–2788. 10.1021/acs.jmedchem.2c01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Zuckermann R. N. Peptoid Origins. Peptide Science 2011, 96 (5), 545–555. 10.1002/bip.21573. [DOI] [PubMed] [Google Scholar]
- (146).Podlesainski D.; Adeniyi E. T.; Gröner Y.; Schulz F.; Krisilia V.; Rehberg N.; Richter T.; Sehr D.; Xie H.; Simons V. E.; Kiffe-Delf A.-L.; Kaschani F.; Ioerger T. R.; Kaiser M.; Kalscheuer R. The Anti-Tubercular Callyaerins Target the Mycobacterium Tuberculosis-Specific Non-Essential Membrane Protein Rv2113. Cell Chemical Biology 2024, 31 (10), 1755–1771.e73. 10.1016/j.chembiol.2024.06.002. [DOI] [PubMed] [Google Scholar]
- (147).Joo S. H. Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol Ther (Seoul) 2012, 20 (1), 19–26. 10.4062/biomolther.2012.20.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Ricardo M. G.; Ali A. M.; Plewka J.; Surmiak E.; Labuzek B.; Neochoritis C. G.; Atmaj J.; Skalniak L.; Zhang R.; Holak T. A.; Groves M.; Rivera D. G.; Dömling A. Multicomponent Peptide Stapling as a Diversity-Driven Tool for the Development of Inhibitors of Protein–Protein Interactions. Angewandte Chemie International Edition 2020, 59 (13), 5235–5241. 10.1002/anie.201916257. [DOI] [PubMed] [Google Scholar]
- (149).Calabretta L. O.; Yang J.; Raines R. T. Nα-Methylation of Arginine: Implications for Cell-Penetrating Peptides. Journal of Peptide Science 2023, 29 (5), e3468. 10.1002/psc.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Kwon Y.-U.; Kodadek T. Quantitative Comparison of the Relative Cell Permeability of Cyclic and Linear Peptides. Chemistry & Biology 2007, 14 (6), 671–677. 10.1016/j.chembiol.2007.05.006. [DOI] [PubMed] [Google Scholar]
- (151).Rezai T.; Yu B.; Millhauser G. L.; Jacobson M. P.; Lokey R. S. Testing the Conformational Hypothesis of Passive Membrane Permeability Using Synthetic Cyclic Peptide Diastereomers. J. Am. Chem. Soc. 2006, 128 (8), 2510–2511. 10.1021/ja0563455. [DOI] [PubMed] [Google Scholar]
- (152).Velkov T.; Swarbrick J. D.; Hussein M. H.; Schneider-Futschik E. K.; Hoyer D.; Li J.; Karas J. A. The Impact of Backbone N-Methylation on the Structure-Activity Relationship of Leu10-Teixobactin. Journal of Peptide Science 2019, 25 (9), e3206. 10.1002/psc.3206. [DOI] [PubMed] [Google Scholar]
- (153).Humpola M. V.; Spinelli R.; Erben M.; Perdomo V.; Tonarelli G. G.; Albericio F.; Siano A. S. D- and N-Methyl Amino Acids for Modulating the Therapeutic Properties of Antimicrobial Peptides and Lipopeptides. Antibiotics 2023, 12 (5), 821. 10.3390/antibiotics12050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.