Abstract
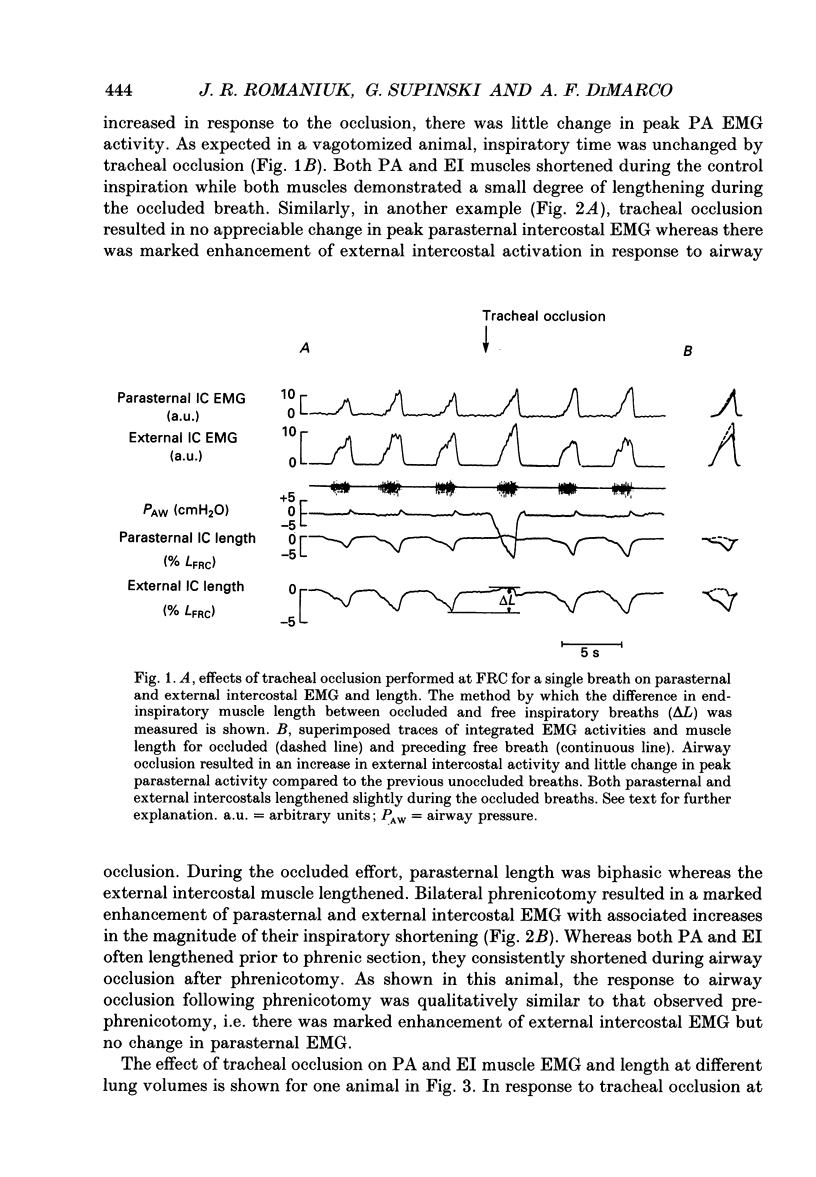
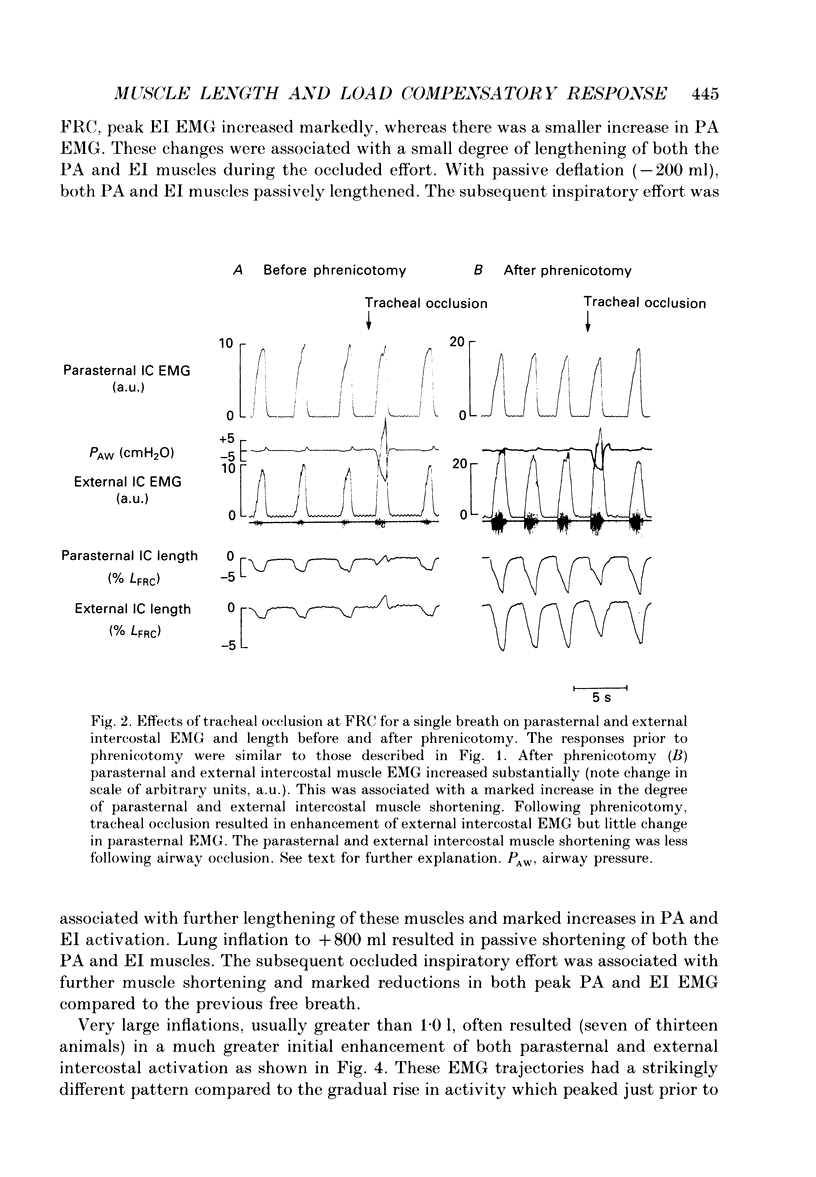
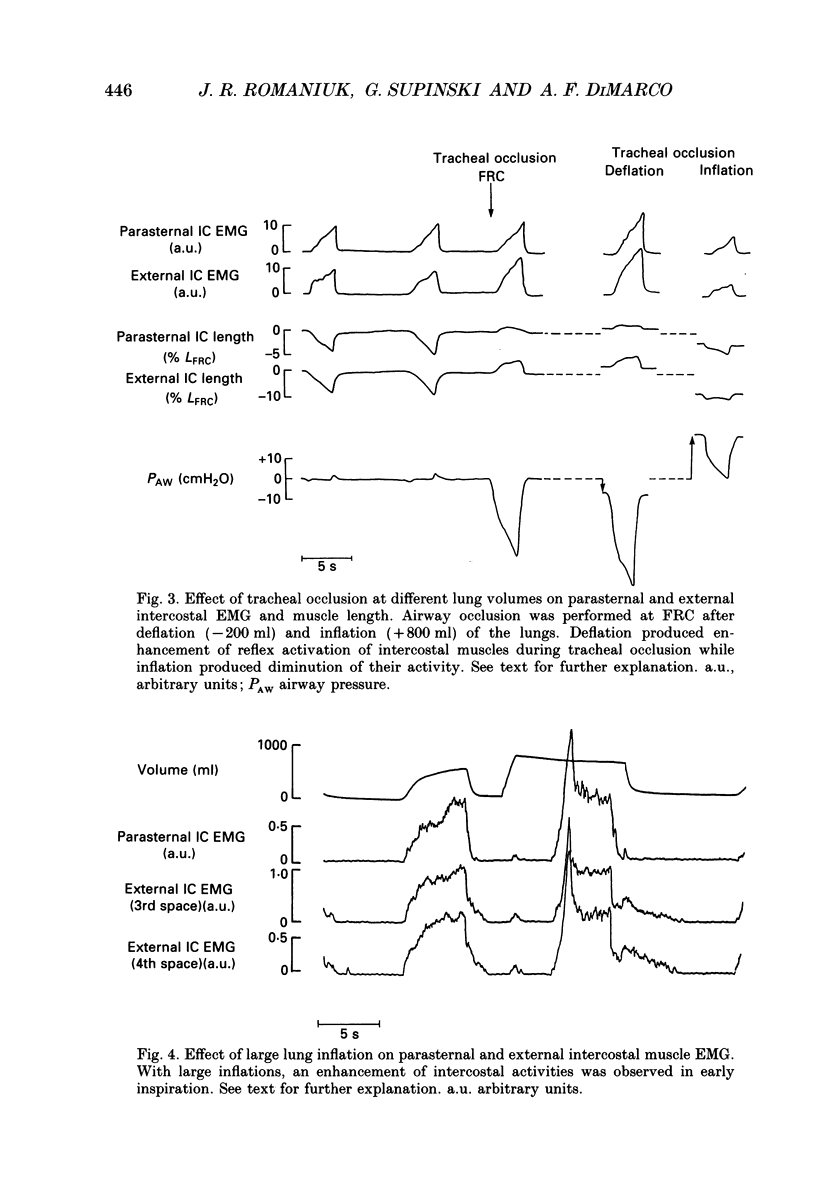
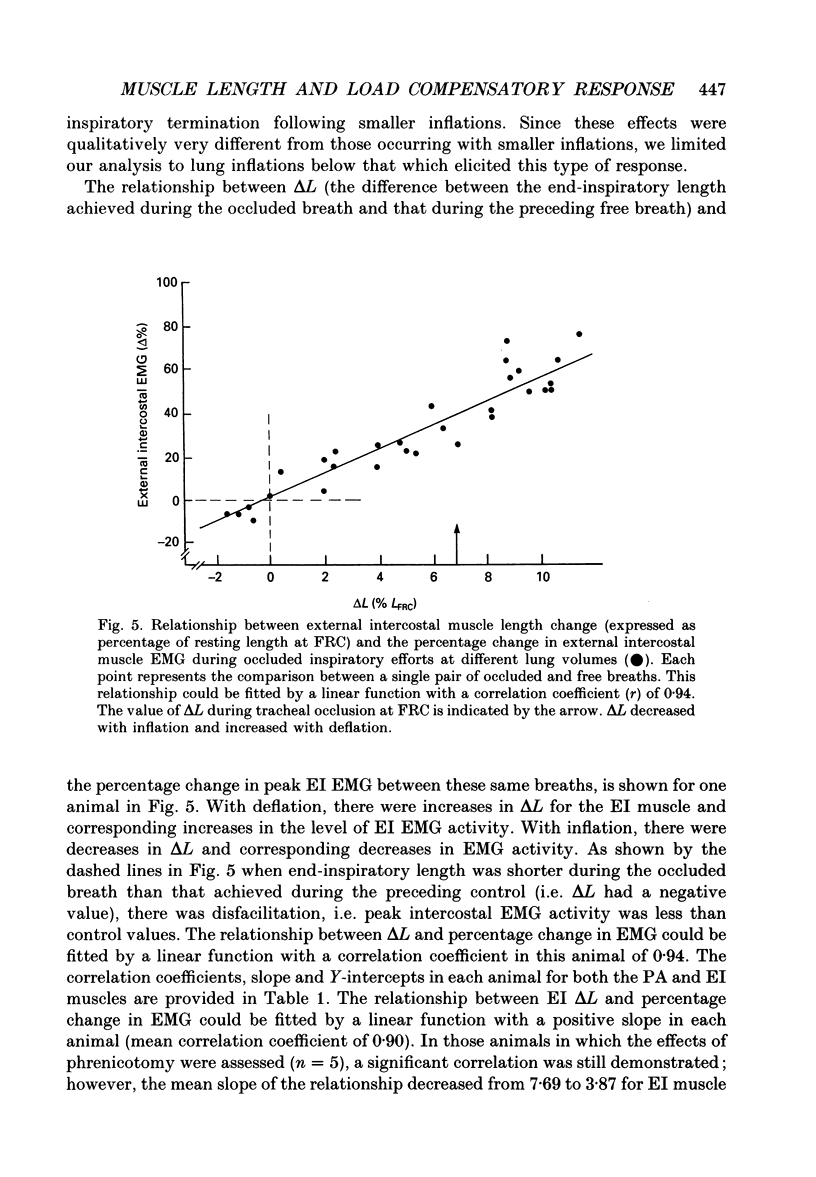
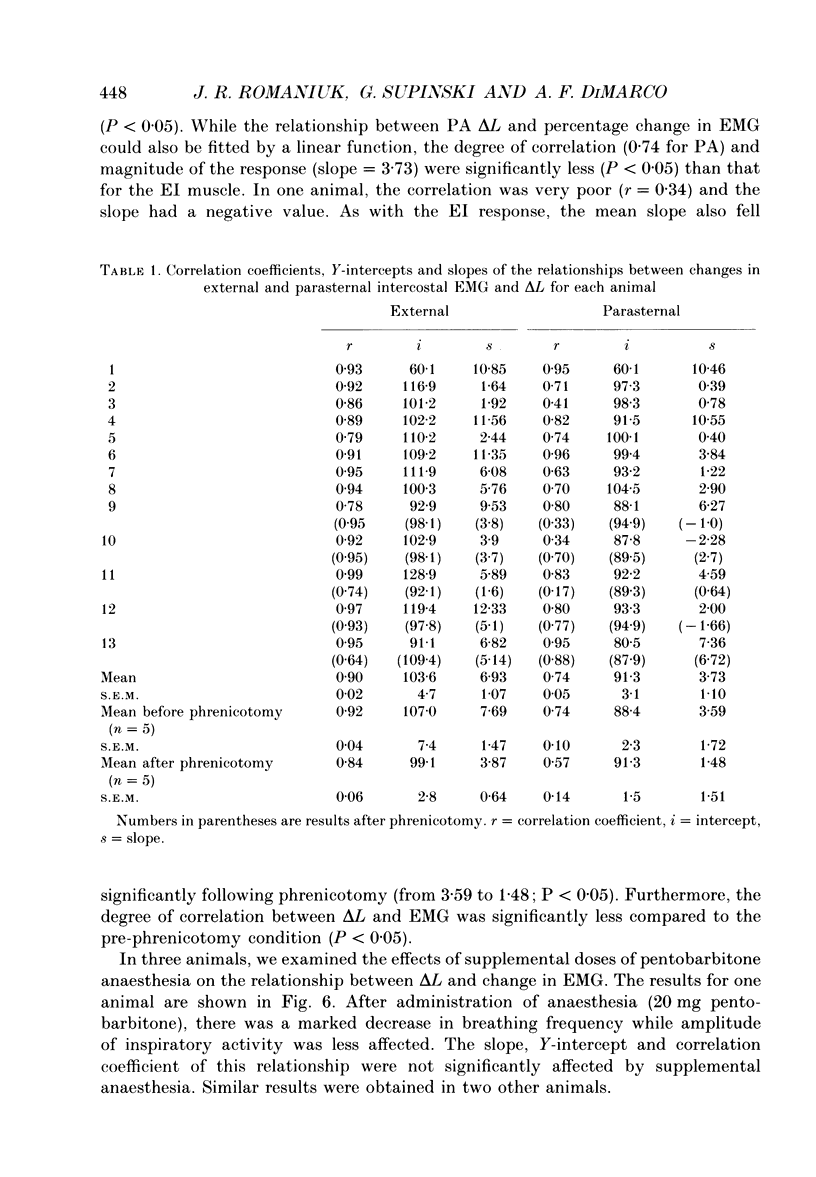
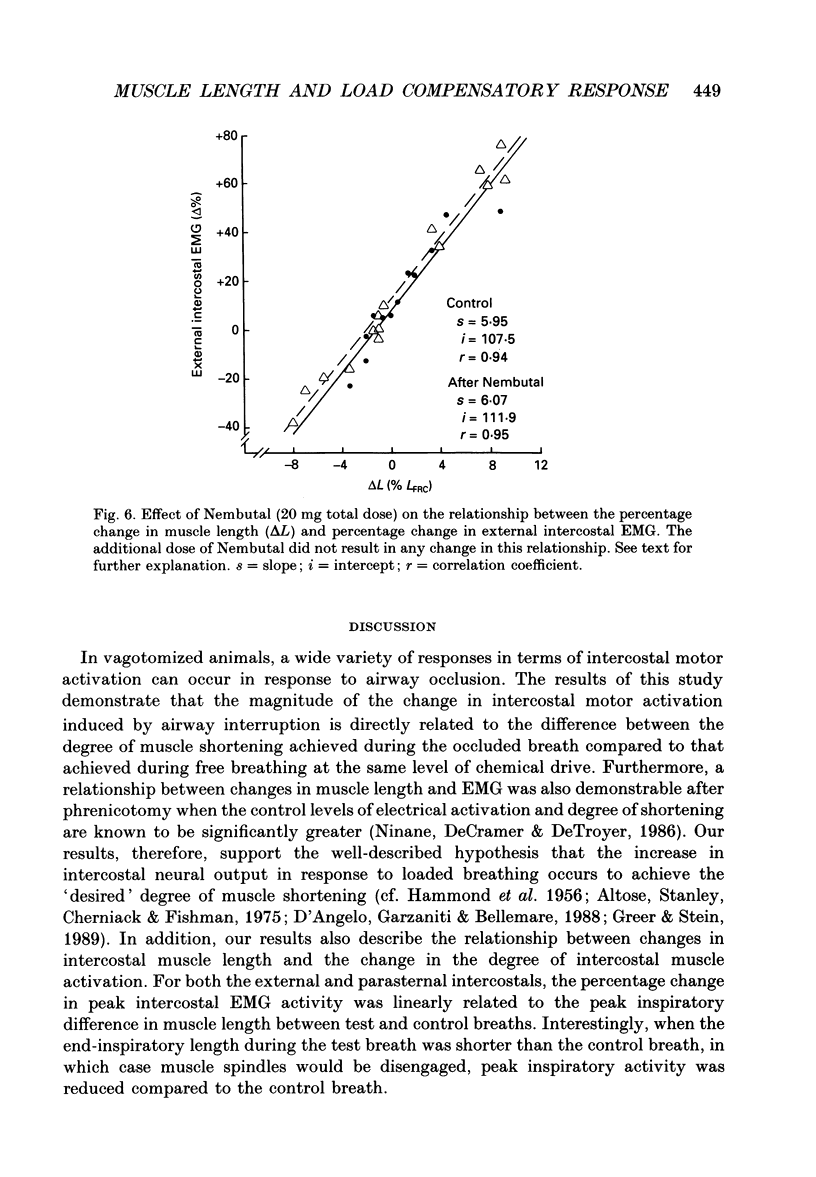
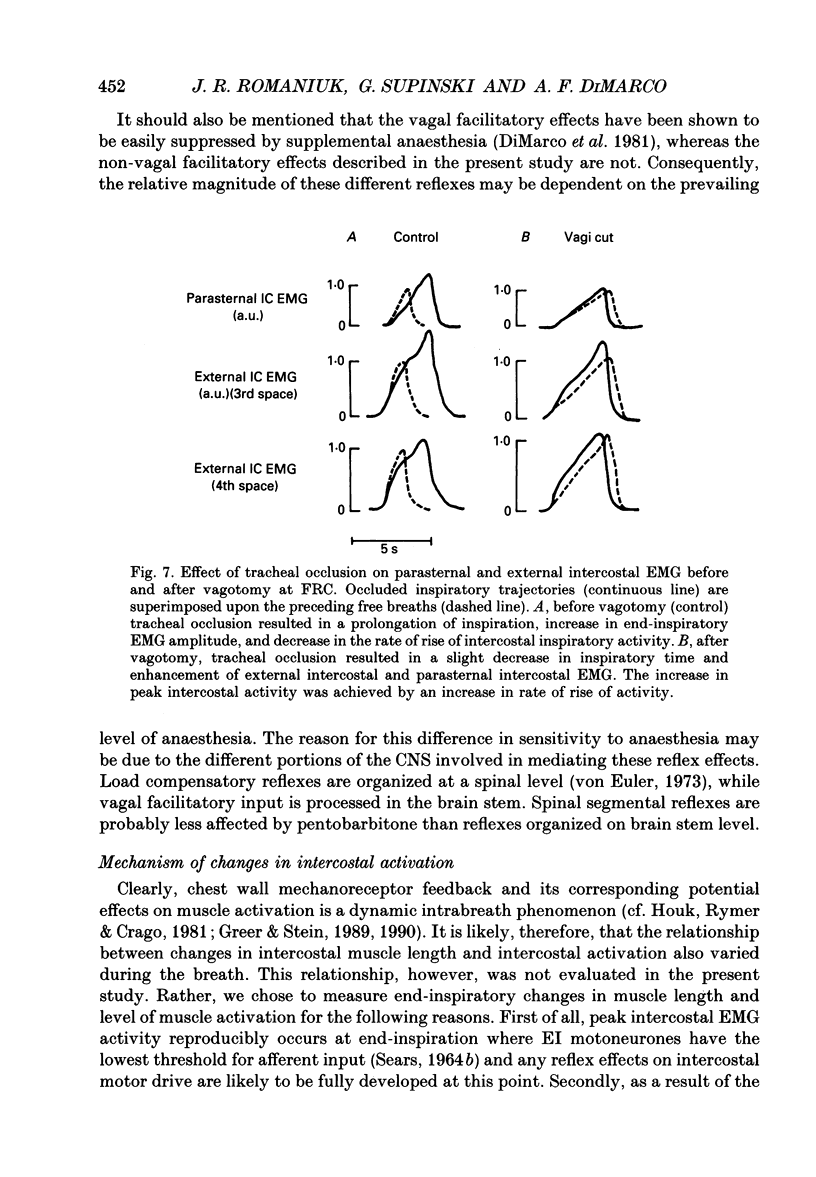
1. The effects of tracheal occlusion on peak parasternal (PA) and external intercostal (EI) (3rd interspace) EMG activities were examined at different end-expiratory lung volumes both above and below functional reserve capacity (FCR) in anaesthetized, vagotomized and spontaneously breathing dogs. 2. Parasternal (PA) and external intercostal (EI) muscle lengths were monitored in situ. The difference in peak EMG activity between free and occluded breaths (test breaths) was related to the coincident peak change in intercostal muscle length (delta L) for each muscle, respectively. 3. At FRC, tracheal occlusion resulted in compensatory augmentation of peak EI, but little change in peak PA EMG activities. At lung volumes below FRC, airway occlusion resulted in augmentation of both PA and EI activities. Responses to airway occlusion at lung volumes above FRC were variable. The magnitude and duration of these changes in EMG, however, could be linearly related to the value of delta L. With delta L = 0, there was no change in peak EI or PA EMG; for values of delta L less than 0, there was attenuation of EI and PA EMG; for delta L greater than 0, there was enhancement of EI and PA EMG activation. 4. The magnitude of the changes in EMG activity in response to tracheal occlusion was more prominent for the EI muscle compared to the PA, the latter of which are known to have much fewer muscle spindles than EI muscle. 5. Our results suggest that a difference in end-inspiratory muscle length between the control and occluded breaths is a stimulus for the intercostal response to applied loads implicating muscle spindles as the predominant receptor moderating these responses. We hypothesize that when delta L = 0, no change in EMG occurs since the spindles sense no change in muscle length. When delta L less than 0 (i.e. peak muscle length during the occluded breath is shorter than control) muscle spindles would be disengaged, resulting in a disfacilitation of EMG activity. Where delta L greater than 0 (i.e. peak muscle length during the occluded breath is longer than control), muscle spindles are stimulated, resulting in enhancement of EMG activity. 6. Additional doses of Nembutal (20 mg), which produced significant changes in breathing pattern, did not affect the magnitude of the load compensatory responses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altose M. D., Stanley N. N., Cherniack N. S., Fishman A. P. Effects of mechanical loading and hypercapnia on inspiratory muscle EMG. J Appl Physiol. 1975 Mar;38(3):467–473. doi: 10.1152/jappl.1975.38.3.467. [DOI] [PubMed] [Google Scholar]
- Bartoli A., Cross B. A., Guz A., Huszczuk A., Jeffries R. The effect of varying tidal volume on the associated phrenic motoneurone output:studies of vagal and chemical feedback. Respir Physiol. 1975 Nov;25(2):135–155. doi: 10.1016/0034-5687(75)90093-6. [DOI] [PubMed] [Google Scholar]
- CORDA M., EKLUND G., VON EULER EXTERNAL INTERCOSTAL AND PHRENIC ALPHA-MOTOR RESPONSES TO CHANGES IN RESPIRATORY LOAD. Acta Physiol Scand. 1965 Mar;63:391–400. doi: 10.1111/j.1748-1716.1965.tb04079.x. [DOI] [PubMed] [Google Scholar]
- CRITCHLOW V., VON EULER INTERCOSTAL MUSCLE SPINDLE ACTIVITY AND ITS GAMMA MOTOR CONTROL. J Physiol. 1963 Oct;168:820–847. doi: 10.1113/jphysiol.1963.sp007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman M., Revelette W. R. Phrenic afferent contribution to reflexes elicited by changes in diaphragm length. J Appl Physiol (1985) 1990 Aug;69(2):640–647. doi: 10.1152/jappl.1990.69.2.640. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Garzaniti N., Bellemare F. Inspiratory muscle activity during unloaded and obstructed rebreathing in dogs. J Appl Physiol (1985) 1988 Jan;64(1):90–101. doi: 10.1152/jappl.1988.64.1.90. [DOI] [PubMed] [Google Scholar]
- Da Silva K. M., Sayers B. M., Sears T. A., Stagg D. T. The changes in configuration of the rib cage and abdomen during breathing in the anaesthetized cat. J Physiol. 1977 Apr;266(2):499–521. doi: 10.1113/jphysiol.1977.sp011779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco A. F., Romaniuk J. R., Supinski G. S. Action of the intercostal muscles on the rib cage. Respir Physiol. 1990 Dec;82(3):295–306. doi: 10.1016/0034-5687(90)90099-k. [DOI] [PubMed] [Google Scholar]
- DiMarco A. F., Romaniuk J. R., Supinski G. S. Parasternal and external intercostal muscle shortening during eupneic breathing. J Appl Physiol (1985) 1990 Dec;69(6):2222–2226. doi: 10.1152/jappl.1990.69.6.2222. [DOI] [PubMed] [Google Scholar]
- DiMarco A. F., von Euler C., Romaniuk J. R., Yamamoto Y. Positive feedback facilitation of external intercostal and phrenic inspiratory activity by pulmonary stretch receptors. Acta Physiol Scand. 1981;113(3):375–386. doi: 10.1111/j.1748-1716.1981.tb06910.x. [DOI] [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol Exp (Wars) 1973;33(1):355–380. [PubMed] [Google Scholar]
- Goldman M. D., Loh L., Sears T. A. The respiratory activity of human levator costae muscles and its modification by posture. J Physiol. 1985 May;362:189–204. doi: 10.1113/jphysiol.1985.sp015670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. The functional role of the muscle spindles--facts and hypotheses. Brain. 1975 Dec;98(4):531–556. doi: 10.1093/brain/98.4.531. [DOI] [PubMed] [Google Scholar]
- Greer J. J., Stein R. B. Fusimotor control of muscle spindle sensitivity during respiration in the cat. J Physiol. 1990 Mar;422:245–264. doi: 10.1113/jphysiol.1990.sp017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. J., Stein R. B. Length changes of intercostal muscles during respiration in the cat. Respir Physiol. 1989 Dec;78(3):309–321. doi: 10.1016/0034-5687(89)90106-0. [DOI] [PubMed] [Google Scholar]
- HAMMOND P. H., MERTON P. A., SUTTON G. G. Nervous gradation of muscular contraction. Br Med Bull. 1956 Sep;12(3):214–218. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- Hilaire G. G., Nicholls J. G., Sears T. A. Central and proprioceptive influences on the activity of levator costae motoneurones in the cat. J Physiol. 1983 Sep;342:527–548. doi: 10.1113/jphysiol.1983.sp014867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Rymer W. Z., Crago P. E. Dependence of dynamic response of spindle receptors on muscle length and velocity. J Neurophysiol. 1981 Jul;46(1):143–166. doi: 10.1152/jn.1981.46.1.143. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SEARS T. A. Effects of posterior root section on the activity of some muscles in man. J Neurol Neurosurg Psychiatry. 1960 Feb;23:10–22. doi: 10.1136/jnnp.23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S., Road J., Bellemare F., Clozel J. P., Lavigne C. M., Grassino A. Respiratory muscle length measured by sonomicrometry. J Appl Physiol Respir Environ Exerc Physiol. 1984 Mar;56(3):753–764. doi: 10.1152/jappl.1984.56.3.753. [DOI] [PubMed] [Google Scholar]
- Ninane V., Decramer M., De Troyer A. Coupling between triangularis sterni and parasternals during breathing in dogs. J Appl Physiol (1985) 1986 Aug;61(2):539–544. doi: 10.1152/jappl.1986.61.2.539. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Marek W., Loeschcke H. H. Altered breathing pattern elicited by stimulation of abdominal visceral afferents. J Appl Physiol (1985) 1985 Jun;58(6):1755–1760. doi: 10.1152/jappl.1985.58.6.1755. [DOI] [PubMed] [Google Scholar]
- Remmers J. E. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol. 1970 Oct;10(3):358–383. doi: 10.1016/0034-5687(70)90055-1. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Ambrogio G., Widdicombe J. G. Respiratory reflexes acting on the diaphragm and inspiratory intercostal muscle of the rabbit. J Physiol. 1965 Oct;180(4):766–779. doi: 10.1113/jphysiol.1965.sp007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E., Cherniack N. S. Electrical and mechanical activity of respiratory muscles during hypercapnia. J Appl Physiol (1985) 1986 Aug;61(2):719–727. doi: 10.1152/jappl.1986.61.2.719. [DOI] [PubMed] [Google Scholar]
- von Euler C. On the neural organization of the motor control of the diaphragm. Am Rev Respir Dis. 1979 Feb;119(2 Pt 2):45–50. doi: 10.1164/arrd.1979.119.2P2.45. [DOI] [PubMed] [Google Scholar]
- von Euler C. The role of proprioceptive afferents in the control of respiratory muscles. Acta Neurobiol Exp (Wars) 1973;33(1):329–341. [PubMed] [Google Scholar]


