Abstract
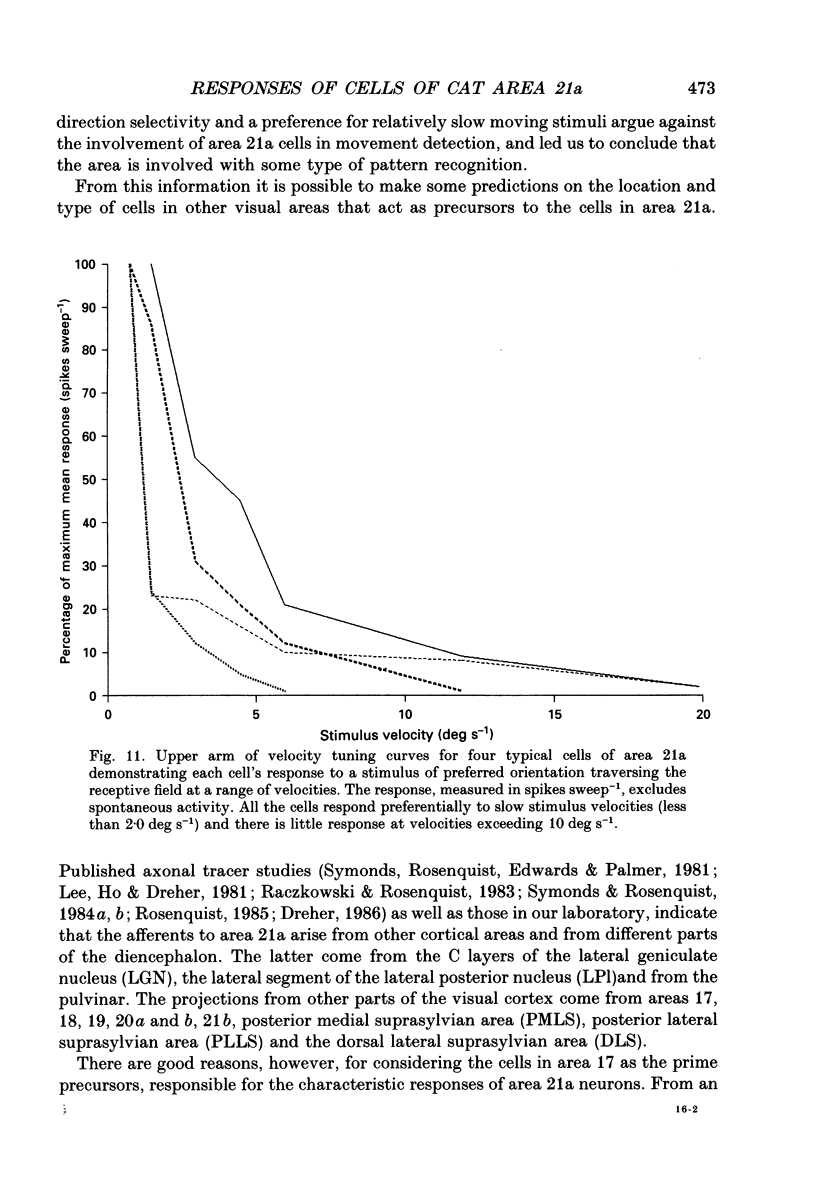
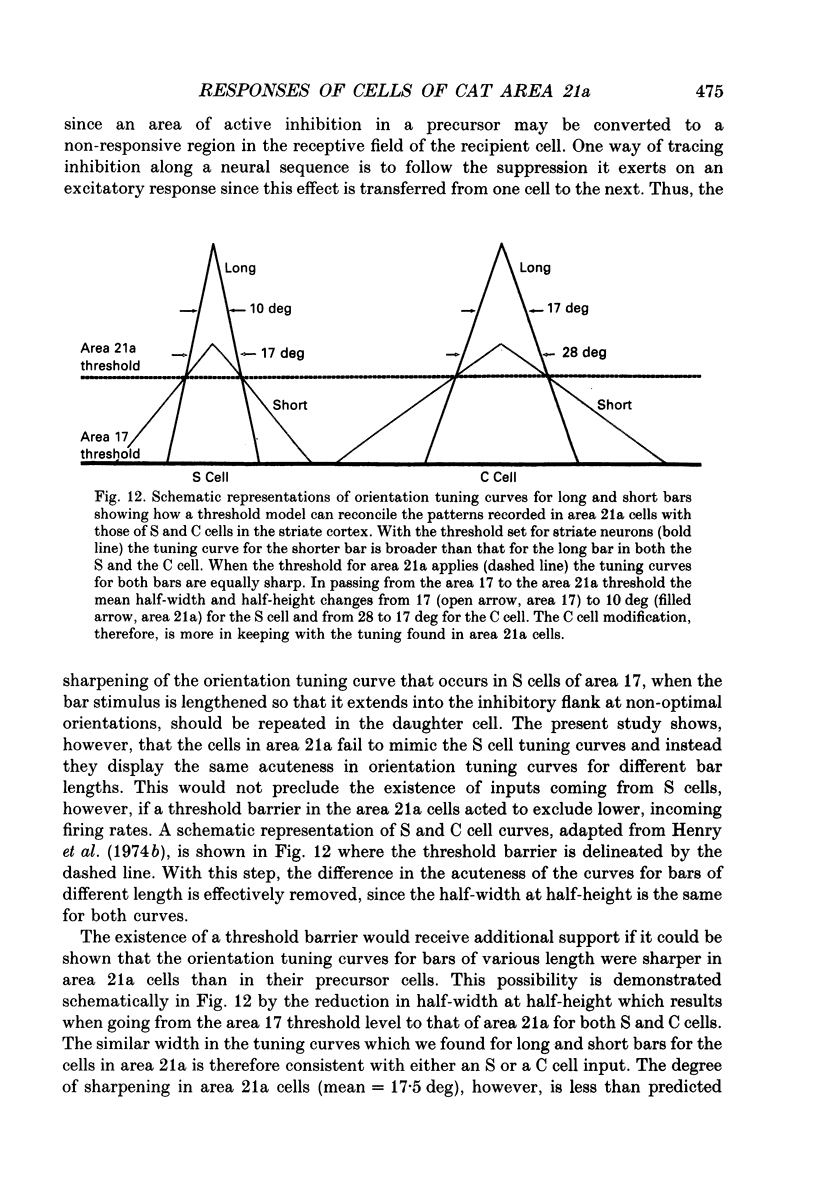
1. Extracellular recording using tungsten-in-glass microelectrodes was conducted on 115 neurons in area 21a of fifteen anaesthetized cats. Quantitative analysis using computer-controlled display and collecting routines were used to investigate the excitatory and inhibitory regions of the receptive field and to see if interaction, within and between these regions, contributed to the response properties of the cells. 2. The responses of the cells in the sample appeared to arise from a single, homogeneous class. All cells had single discharge regions which responded with composite ON/OFF firing to a stationary flashing bar. The same region also responded to moving light and dark bars and edges. There was little evidence of inhibition as measured by the suppression of spontaneous or induced firing. Most cells had relatively small receptive fields (primary width: mean = 2.1 +/- 0.9 deg (S.D.); n = 108), all were binocular and were located within 15.0 deg of the visual axes. 3. All cells responded well to slowly moving stimuli but generally failed to respond to stimuli moving faster than 10.0 deg s-1. All responses were bi-directional and, although many showed evidence of length summation, there was no sign of linear summation. 4. Despite the absence of significant sideband inhibition many cells were acutely tuned for orientation (half-width at half-height: mean = 15.6 +/- 5.3 deg; n = 48). To investigate this property further, cells were analysed to assess the effect of changing the length of a moving bar stimulus on the acuteness of the orientation tuning curve. Short bars, of similar length to the width of the receptive field, had orientation tuning curves of equivalent sharpness to those obtained with longer bars. 5. The equivalence of orientation tuning for long and short bars stands in contrast to the results obtained for both simple (S) and complex (C) cells of the striate cortex where tuning for the longer bar is sharper than that for the shorter. The result from area 21a cells is consistent with the absence of sideband inhibition and can be related to an input from the striate cortex that passes through a threshold barrier. 6. The orientation tuning of cells of area 21a can be explained if it is assumed that they receive their major input from C or complex cells of the striate cortex in which firing must reach a threshold frequency to activate the recipient cell.
Full text
PDF




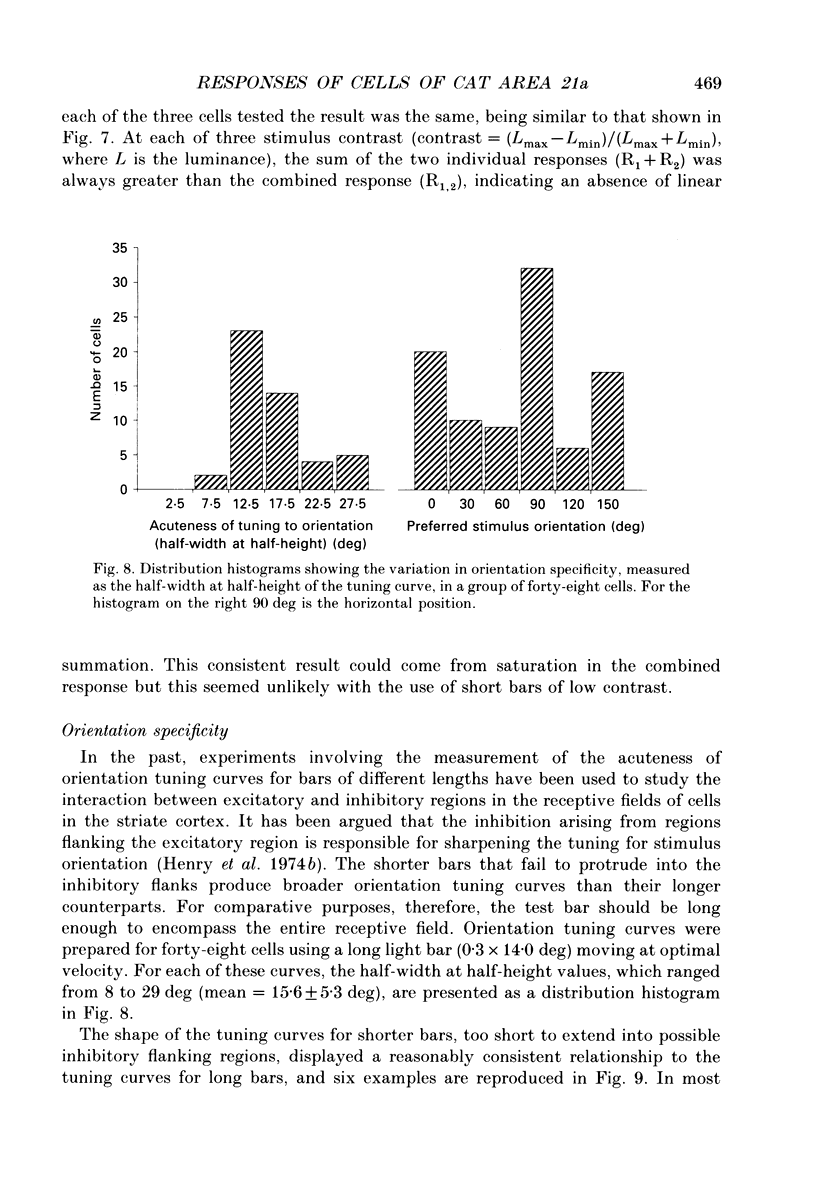
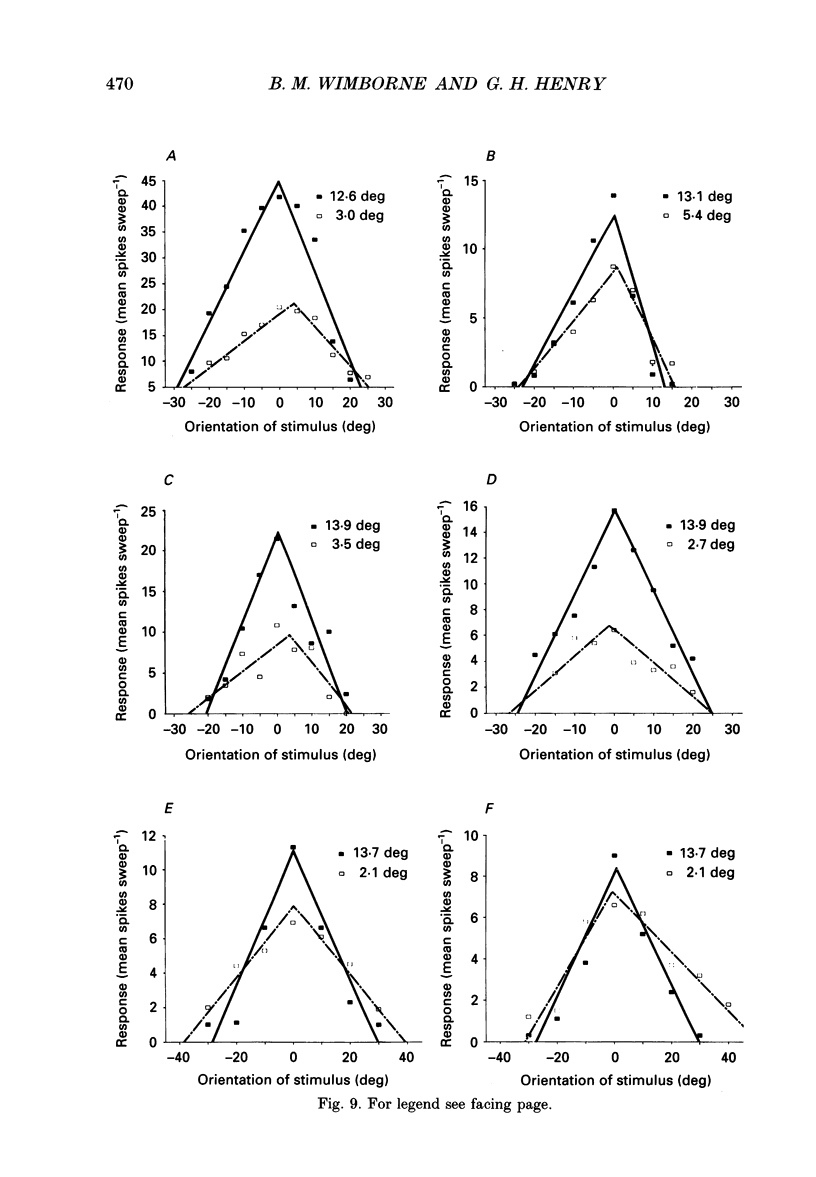





Images in this article
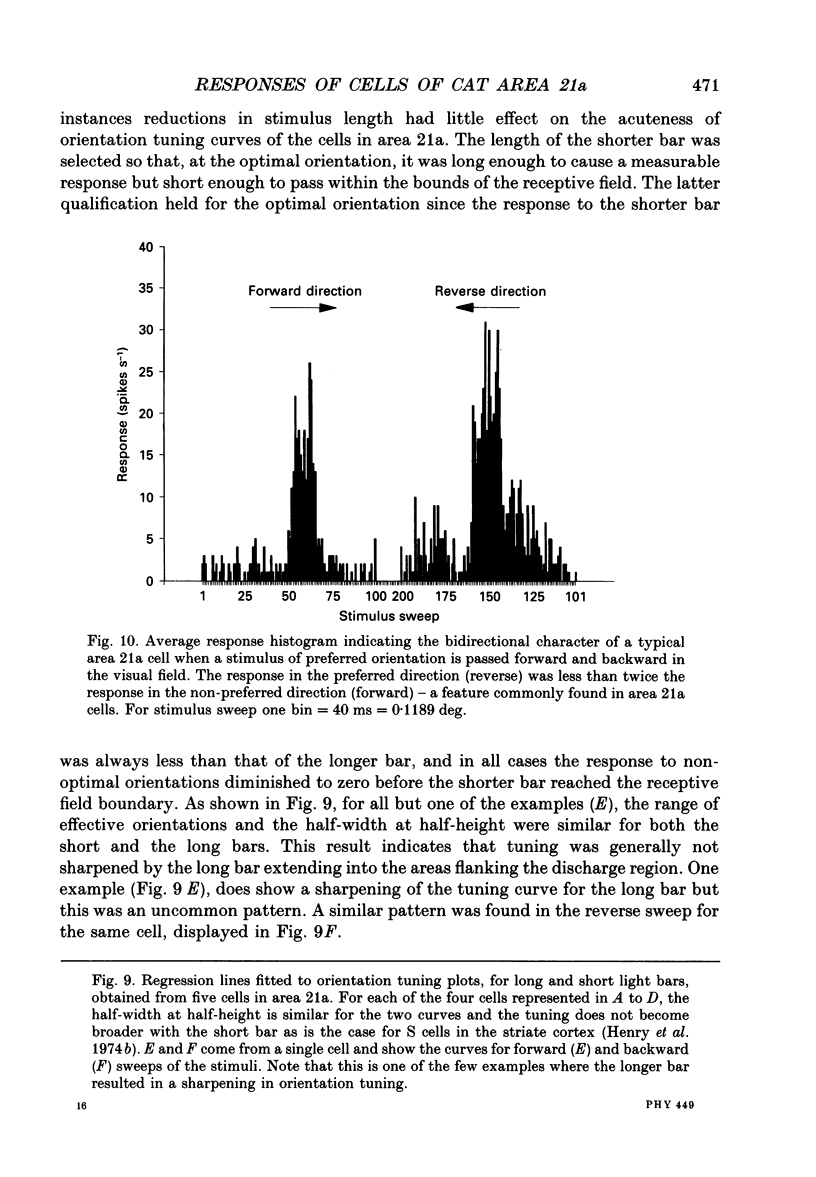
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedek G., Norita M., Creutzfeldt O. D. Electrophysiological and anatomical demonstration of an overlapping striate and tectal projection to the lateral posterior-pulvinar complex of the cat. Exp Brain Res. 1983;52(2):157–169. doi: 10.1007/BF00236624. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Responses to visual contours: spatio-temporal aspects of excitation in the receptive fields of simple striate neurones. J Physiol. 1971 Dec;219(3):625–657. doi: 10.1113/jphysiol.1971.sp009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972 May;11(5):346–354. [PubMed] [Google Scholar]
- Bullier J., Mustari M. J., Henry G. H. Receptive-field transformations between LGN neurons and S-cells of cat-striate cortex. J Neurophysiol. 1982 Mar;47(3):417–438. doi: 10.1152/jn.1982.47.3.417. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H., Bishop P. O. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975 Nov;38(6):1500–1523. doi: 10.1152/jn.1975.38.6.1500. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H. Direction selectivity of complex cells in a comparison with simple cells. J Neurophysiol. 1975 Nov;38(6):1524–1540. doi: 10.1152/jn.1975.38.6.1524. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H. The influence of stimulus velocity on the responses of single neurones in the striate cortex. J Physiol. 1978 Apr;277:467–482. doi: 10.1113/jphysiol.1978.sp012285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Inadequacy of nitrous oxide/oxygen mixtures for maintaining anaesthesia in cats: satisfactory alternatives. Pain. 1978 Aug;5(2):143–151. doi: 10.1016/0304-3959(78)90036-2. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Coombs J. S. Inhibitory and sub-liminal excitatory receptive fields of simple units in cat striate cortex. Vision Res. 1969 Oct;9(10):1289–1296. doi: 10.1016/0042-6989(69)90116-3. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Dreher B. Orientation, axis and direction as stimulus parameters for striate cells. Vision Res. 1974 Sep;14(9):767–777. doi: 10.1016/0042-6989(74)90141-2. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Goodwin A. W., Bishop P. O. Spatial summation of responses in receptive fields of single cells in cat striate cortex. Exp Brain Res. 1978 Jun 19;32(2):245–266. doi: 10.1007/BF00239730. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Kinston W. J., Vadas M. A., Bishop P. O. Multiple projection of the visual field to the medical portion of the dorsal lateral geniculate nucleus and the adjacent nuclei of the thalamus of the cat. J Comp Neurol. 1969 Jul;136(3):295–315. doi: 10.1002/cne.901360304. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Mason R. Differential responsiveness of cells in the visual zones of the cat's LP-pulvinar complex to visual stimuli. Exp Brain Res. 1981;43(1):25–33. doi: 10.1007/BF00238806. [DOI] [PubMed] [Google Scholar]
- Mason R. Functional organization in the cat's pulvinar complex. Exp Brain Res. 1978 Jan 18;31(1):51–66. doi: 10.1007/BF00235804. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Mizobe K., Itoi M., Kaihara T., Toyama K. Neuronal responsiveness in area 21a of the cat. Brain Res. 1988 Jan 12;438(1-2):307–310. doi: 10.1016/0006-8993(88)91353-4. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Raczkowski D., Rosenquist A. C. Connections of the multiple visual cortical areas with the lateral posterior-pulvinar complex and adjacent thalamic nuclei in the cat. J Neurosci. 1983 Oct;3(10):1912–1942. doi: 10.1523/JNEUROSCI.03-10-01912.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C. Corticocortical connections among visual areas in the cat. J Comp Neurol. 1984 Oct 10;229(1):1–38. doi: 10.1002/cne.902290103. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C., Edwards S. B., Palmer L. A. Projections of the pulvinar-lateral posterior complex to visual cortical areas in the cat. Neuroscience. 1981;6(10):1995–2020. doi: 10.1016/0306-4522(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C. Laminar origins of visual corticocortical connections in the cat. J Comp Neurol. 1984 Oct 10;229(1):39–47. doi: 10.1002/cne.902290104. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A. Retinotopic organization of areas 20 and 21 in the cat. J Comp Neurol. 1980 Sep 1;193(1):147–164. doi: 10.1002/cne.901930110. [DOI] [PubMed] [Google Scholar]